Validity of the Japanese Version of the Quick Mild Cognitive Impairment Screen
Abstract
1. Introduction
2. Materials and Methods
2.1. Translation of the Qmci Screen
2.2. Study Settings and Participants
2.3. Data Analysis
2.4. Ethical Considerations
3. Results
3.1. Scenario 1
3.2. Scenario 2 (Using sMMSE-J Age-Adjusted Cut-Offs)
3.3. Scenario 3a (Normative Data Based on sMMSE-J Scores ≥27 Only)
3.4. Scenario 3b (Normative Data Based on sMMSE-J Scores ≥27 in Those Without Cognitive Symptoms or Supportive Features)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef]
- Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prina, A.M.; Winblad, B.; Jonsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levalahti, E.; Ahtiluoto, S.; Antikainen, R.; Backman, L.; Hanninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Straubmeier, M.; Behrndt, E.M.; Seidl, H.; Ozbe, D.; Luttenberger, K.; Graessel, E. Non-Pharmacological Treatment in People With Cognitive Impairment. Dtsch. Ärzteblatt Int. 2017, 114, 815–821. [Google Scholar] [CrossRef]
- Wilson, R.S.; Segawa, E.; Boyle, P.A.; Anagnos, S.E.; Hizel, L.P.; Bennett, D.A. The natural history of cognitive decline in Alzheimer’s disease. Psychol. Aging 2012, 27, 1008–1017. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Sugishita, M.; Hemmi, I.; Takeuchi, T. Reexamination of the validity and reliability of the Japanese version of the Mini-Mental State Examination (MMSE-J). Jpn. J. Cogn. Neurosci. 2016, 18, 168–183. [Google Scholar]
- Yoshida, H.; Terada, S.; Honda, H.; Kishimoto, Y.; Takeda, N.; Oshima, E.; Hirayama, K.; Yokota, O.; Uchitomi, Y. Validation of the revised Addenbrooke’s Cognitive Examination (ACE-R) for detecting mild cognitive impairment and dementia in a Japanese population. Int. Psychogeriatr. 2012, 24, 28–37. [Google Scholar] [CrossRef]
- Lorentz, W.J.; Scanlan, J.M.; Borson, S. Brief screening tests for dementia. Can. J. Psychiatry 2002, 47, 723–733. [Google Scholar] [CrossRef]
- Ministry of Health, Laour and Welfare (Japan). Patient’s Behavior Survey; 2014; Ministry of Health, Labor and Welfare: Tokyo, Japan, 2016; p. 5.
- Meulen, E.F.; Schmand, B.; van Campen, J.P.; de Koning, S.J.; Ponds, R.W.; Scheltens, P.; Verhey, F.R. The seven minute screen: A neurocognitive screening test highly sensitive to various types of dementia. J. Neurol. Neurosurg. Psychiatry 2004, 75, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, J.E.; Simon, S.S.; Bottino, C.M. Cognitive screening for dementia in primary care: A systematic review. Int. Psychogeriatr. 2014, 26, 1783–1804. [Google Scholar] [CrossRef] [PubMed]
- Brodaty, H.; Low, L.F.; Gibson, L.; Burns, K. What is the best dementia screening instrument for general practitioners to use? Am. J. Geriatr. Psychiatry 2006, 14, 391–400. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Gao, Y.; Gallagher, P.F.; Eustace, J.; McGlade, C.; Molloy, D.W. Which part of the Quick mild cognitive impairment screen (Qmci) discriminates between normal cognition, mild cognitive impairment and dementia? Age Ageing 2013, 42, 324–330. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; McGlade, C.; Healy, L.; Gallagher, P.; Timmons, S.; Molloy, D.W. Comparison of the quick mild cognitive impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing 2012, 41, 624–629. [Google Scholar] [CrossRef]
- Bunt, S.; O’Caoimh, R.; Krijnen, W.P.; Molloy, D.W.; Goodijk, G.P.; van der Schans, C.P.; Hobbelen, H.J.S.M. Validation of the Dutch version of the quick mild cognitive impairment screen (Qmci-D). BMC Geriatr. 2015, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.B.; Varan, H.D.; O’Caoimh, R.; Kizilarslanoglu, M.C.; Kilic, M.K.; Molloy, D.W.; Dogrul, R.T.; Karabulut, E.; Svendrovski, A.; Sagir, A.; et al. Validation of the Turkish Version of the Quick Mild Cognitive Impairment Screen. Am. J. Alzheimer’s Dis. 2017, 32, 145–156. [Google Scholar] [CrossRef]
- Xu, Y.F.; Yu, Y.Y.; Li, X.; Chen, Z.M.; Gao, Y.; Molloy, W.; O’Caoimh, R. Development of the Chinese Version of the Quick Mild Cognitive Impairment (Qmci-Cn) Screen. Age Ageing 2017, 46, iii13–iii59. [Google Scholar] [CrossRef]
- Iavarone, A.; Carpinelli Mazzi, M.; Russo, G.; D’Anna, F.; Peluso, S.; Mazzeo, P.; De Luca, V.; De Michele, G.; Iaccarino, G.; Abete, P.; et al. The Italian version of the quick mild cognitive impairment (Qmci-I) screen: Normative study on 307 healthy subjects. Aging Clin. Exp. Res. 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yarnall, K.S.; Pollak, K.I.; Ostbye, T.; Krause, K.M.; Michener, J.L. Primary care: Is there enough time for prevention? Am. J. Public Health 2003, 93, 635–641. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Timmons, S.; Molloy, D.W. Screening for Mild Cognitive Impairment: Comparison of “MCI Specific” Screening Instruments. J. Alzheimer’s Dis. 2016, 51, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.S.; Pesce, A.J. Reference intervals: An update. Clin. Chim. Acta 2003, 334, 5–23. [Google Scholar] [CrossRef]
- Konrad, T.R.; Link, C.L.; Shackelton, R.J.; Marceau, L.D.; von dem Knesebeck, O.; Siegrist, J.; Arber, S.; Adams, A.; McKinlay, J.B. It’s about time: physicians’ perceptions of time constraints in primary care medical practice in three national healthcare systems. Med. Care 2010, 48, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Kasai, M.; Chida, K.; Kato, Y.; Uchishiba, Y.; Takada, J.; Yamamoto, M.; Kumai, K.; Nakamura, K.; Meguro; et al. Construct validity of the Japanese version of Quick Mild Cognitive Impairment. J. Epidemiol. 2018, 28 (Suppl), 121. [Google Scholar]
- O’Caoimh, R.; Timmons, S.; Molloy, D.W. Comparison of the Quick Mild Cognitive Impairment Screen (Qmci) to the Montreal Cognitive Assessment. Ir. J. Med. Sci. 2013, 182, S286. [Google Scholar]
- O’Caoimh, R.; Molloy, W. Brief Dementia Screens in Clinic: Comparison of the Quick Mild Cognitive Impairment (Qmci) Screen and Six Item Cognitive Impairment Test (6CIT). Ir. J. Med. Sci. 2014, 183, S379. [Google Scholar]
- Fujiwara, Y.; Suzuki, H.; Yasunaga, M.; Sugiyama, M.; Ijuin, M.; Sakuma, N.; Inagaki, H.; Iwasa, H.; Ura, C.; Yatomi, N.; et al. Brief screening tool for mild cognitive impairment in older Japanese: Validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr. Gerontol. Int. 2010, 10, 225–232. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; Svendovski, A.; Gallagher, P.; Eustace, J.; Molloy, D.W. Comparing approaches to optimize cut-off scores for short cognitive screening instruments in mild cognitive impairment and dementia. J. Alzheimer’s Dis. 2017, 57, 123–133. [Google Scholar] [CrossRef]
- Maxim, L.D.; Niebo, R.; Utell, M.J. Screening tests: A review with examples. Inhal. Toxicol. 2014, 26, 811–828. [Google Scholar] [CrossRef]
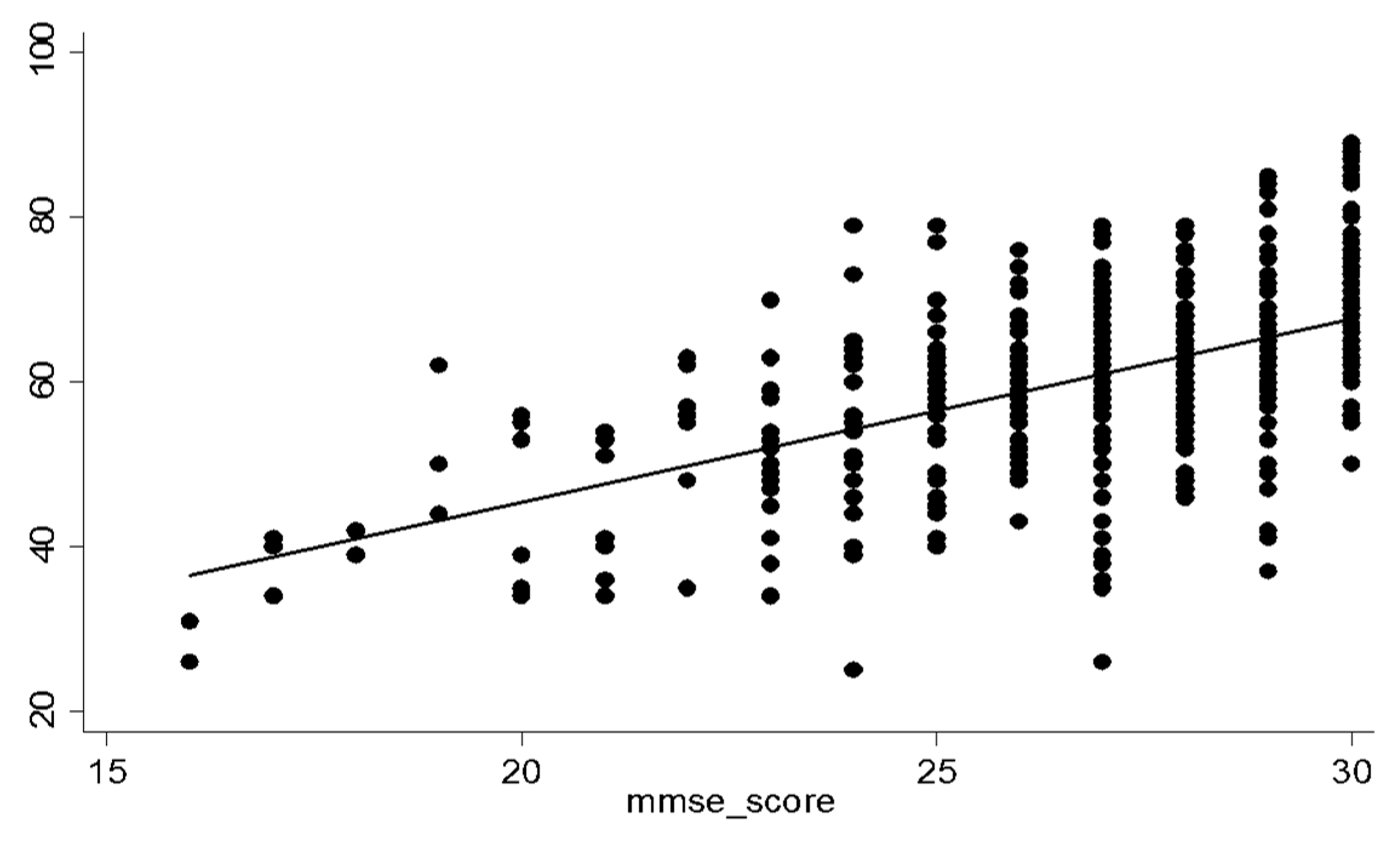
| Total (n = 526) | Suspected Dementia (n = 16) | Suspected MCI (n = 214) | Normal Cognition (n = 351) | p-Value * | |
|---|---|---|---|---|---|
| % or Mean ± SD | % or Mean ± SD | % or Mean ± SD | % or Mean ± SD | ||
| Sex | 0.051 | ||||
| Male | 47.3 | 56.3 | 54.7 | 43.6 | |
| Female | 52.7 | 43.8 | 45.3 | 56.4 | |
| Age (years) | 73.5 ± 5.6 | 78.0 ± 5.3 | 75.2 ± 5.6 | 72.5 ± 5.3 | <0.001 |
| Education | <0.001 | ||||
| Elementary school | 1.3 | 6.3 | 3.8 | 0.0 | |
| Junior high school | 40.3 | 81.3 | 52.2 | 33.1 | |
| High school | 44.7 | 12.5 | 35.2 | 50.4 | |
| Some college/university/graduate school | 13.7 | 0.0 | 8.8 | 16.5 | |
| Visual Impairment | 1.0 | 0.0 | 0.6 | 1.1 | 0.89 |
| Hearing Impairment | 2.7 | 18.8 | 2.5 | 2.0 | <0.001 |
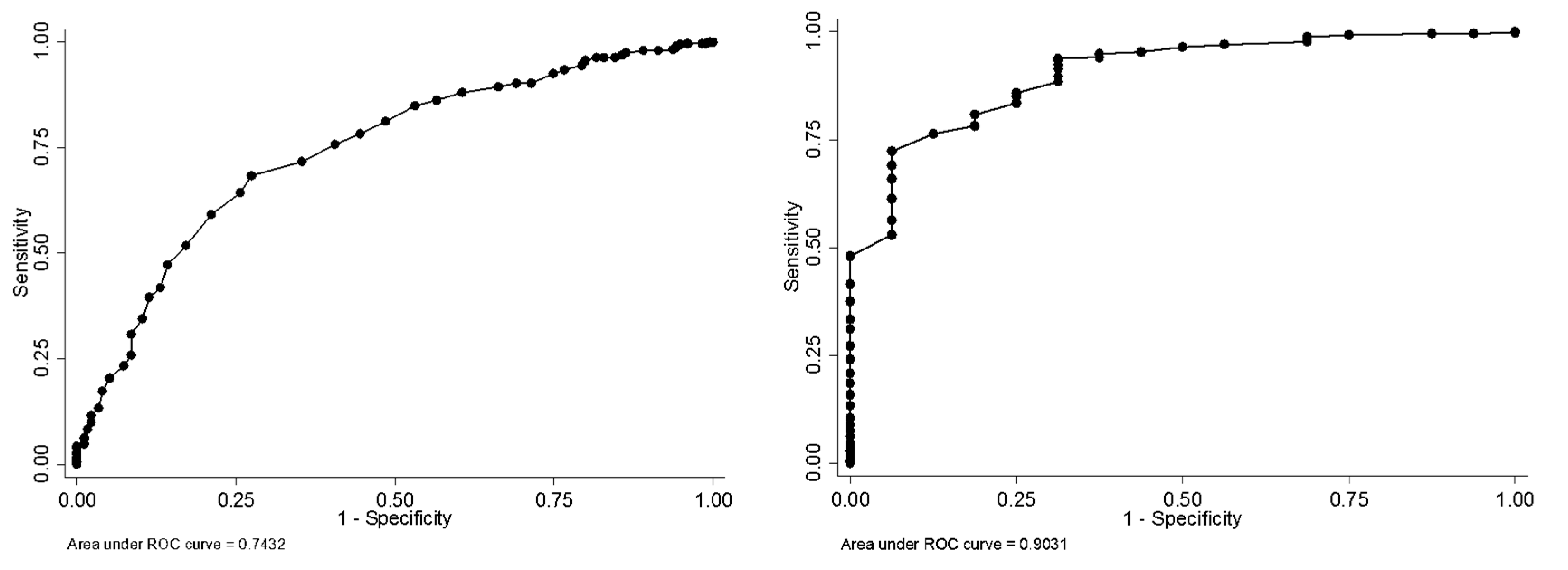
| Cut-off | Youden’s Index (J) | Sen (95%CI) | Spe (95%CI) | PPV (95%CI) | NPV (95%CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 54/55 | 0.29 | 0.43 | 0.36 | 0.51 | 0.86 | 0.82 | 0.90 | 0.61 | 0.52 | 0.70 | 0.75 | 0.71 | 0.80 |
| 55/56 | 0.32 | 0.47 | 0.39 | 0.55 | 0.85 | 0.81 | 0.89 | 0.61 | 0.52 | 0.69 | 0.76 | 0.72 | 0.80 |
| 56/57 | 0.32 | 0.51 | 0.44 | 0.59 | 0.81 | 0.77 | 0.85 | 0.58 | 0.50 | 0.66 | 0.77 | 0.72 | 0.81 |
| 57/58 | 0.33 | 0.55 | 0.48 | 0.63 | 0.78 | 0.74 | 0.83 | 0.56 | 0.48 | 0.64 | 0.78 | 0.73 | 0.82 |
| 58/59 | 0.35 | 0.59 | 0.52 | 0.67 | 0.76 | 0.71 | 0.80 | 0.55 | 0.48 | 0.62 | 0.79 | 0.74 | 0.83 |
| 59/60 | 0.37 | 0.65 | 0.57 | 0.72 | 0.72 | 0.67 | 0.76 | 0.53 | 0.46 | 0.60 | 0.80 | 0.75 | 0.85 |
| 60/61 | 0.41 | 0.73 | 0.65 | 0.79 | 0.68 | 0.63 | 0.73 | 0.53 | 0.47 | 0.60 | 0.83 | 0.79 | 0.87 |
| 61/62 | 0.38 | 0.74 | 0.67 | 0.81 | 0.64 | 0.59 | 0.69 | 0.51 | 0.45 | 0.57 | 0.83 | 0.78 | 0.88 |
| 62/63 | 0.38 | 0.79 | 0.72 | 0.85 | 0.59 | 0.54 | 0.64 | 0.49 | 0.43 | 0.55 | 0.85 | 0.80 | 0.89 |
| 63/64 | 0.35 | 0.83 | 0.76 | 0.88 | 0.52 | 0.47 | 0.57 | 0.46 | 0.41 | 0.52 | 0.86 | 0.80 | 0.90 |
| 64/65 | 0.33 | 0.86 | 0.80 | 0.91 | 0.47 | 0.42 | 0.53 | 0.45 | 0.39 | 0.50 | 0.87 | 0.81 | 0.91 |
| 65/66 | 0.29 | 0.87 | 0.81 | 0.92 | 0.42 | 0.37 | 0.47 | 0.43 | 0.38 | 0.48 | 0.87 | 0.80 | 0.91 |
| Cut-off | Youden’s Index (J) | Sen (95%CI) | Spe (95%CI) | PPV (95%CI) | NPV (95%CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40/41 | 0.47 | 0.50 | 0.25 | 0.75 | 0.97 | 0.95 | 0.98 | 0.31 | 0.14 | 0.52 | 0.98 | 0.97 | 0.99 |
| 41/42 | 0.51 | 0.56 | 0.30 | 0.80 | 0.95 | 0.93 | 0.97 | 0.27 | 0.13 | 0.46 | 0.99 | 0.97 | 0.99 |
| 42/43 | 0.58 | 0.63 | 0.35 | 0.85 | 0.95 | 0.93 | 0.97 | 0.28 | 0.14 | 0.45 | 0.99 | 0.97 | 1.00 |
| 43/44 | 0.57 | 0.63 | 0.35 | 0.85 | 0.94 | 0.92 | 0.96 | 0.25 | 0.13 | 0.41 | 0.99 | 0.97 | 1.00 |
| 44/45 | 0.63 | 0.69 | 0.41 | 0.89 | 0.94 | 0.91 | 0.96 | 0.26 | 0.14 | 0.41 | 0.99 | 0.98 | 1.00 |
| 45/46 | 0.62 | 0.69 | 0.41 | 0.89 | 0.93 | 0.91 | 0.95 | 0.24 | 0.13 | 0.40 | 0.99 | 0.98 | 1.00 |
| 46/47 | 0.61 | 0.69 | 0.41 | 0.89 | 0.92 | 0.90 | 0.95 | 0.22 | 0.12 | 0.36 | 0.99 | 0.98 | 1.00 |
| 47/48 | 0.60 | 0.69 | 0.41 | 0.89 | 0.91 | 0.89 | 0.94 | 0.20 | 0.10 | 0.33 | 0.99 | 0.98 | 1.00 |
| 48/49 | 0.59 | 0.69 | 0.41 | 0.89 | 0.90 | 0.87 | 0.92 | 0.17 | 0.09 | 0.29 | 0.99 | 0.98 | 1.00 |
| 49/50 | 0.57 | 0.69 | 0.41 | 0.89 | 0.88 | 0.85 | 0.91 | 0.16 | 0.08 | 0.26 | 0.99 | 0.98 | 1.00 |
| 50/51 | 0.61 | 0.75 | 0.48 | 0.93 | 0.86 | 0.83 | 0.89 | 0.14 | 0.08 | 0.24 | 0.99 | 0.98 | 1.00 |
| 51/52 | 0.60 | 0.75 | 0.48 | 0.93 | 0.85 | 0.82 | 0.88 | 0.14 | 0.07 | 0.23 | 0.99 | 0.98 | 1.00 |
| 52/53 | 0.59 | 0.75 | 0.48 | 0.93 | 0.84 | 0.80 | 0.87 | 0.13 | 0.07 | 0.21 | 0.99 | 0.98 | 1.00 |
| 53/54 | 0.62 | 0.81 | 0.54 | 0.96 | 0.81 | 0.77 | 0.84 | 0.12 | 0.06 | 0.19 | 0.99 | 0.98 | 1.00 |
| 54/55 | 0.59 | 0.81 | 0.54 | 0.96 | 0.78 | 0.74 | 0.82 | 0.11 | 0.06 | 0.17 | 0.99 | 0.98 | 1.00 |
| 55/56 | 0.64 | 0.88 | 0.62 | 0.98 | 0.76 | 0.72 | 0.80 | 0.10 | 0.06 | 0.17 | 1.00 | 0.98 | 1.00 |
| 56/57 | 0.66 | 0.94 | 0.70 | 1.00 | 0.72 | 0.68 | 0.76 | 0.10 | 0.05 | 0.15 | 1.00 | 0.99 | 1.00 |
| 57/58 | 0.63 | 0.94 | 0.70 | 1.00 | 0.69 | 0.65 | 0.73 | 0.09 | 0.05 | 0.14 | 1.00 | 0.98 | 1.00 |
| 58/59 | 0.60 | 0.94 | 0.70 | 1.00 | 0.66 | 0.62 | 0.70 | 0.08 | 0.05 | 0.13 | 1.00 | 0.98 | 1.00 |
| 59/60 | 0.55 | 0.94 | 0.70 | 1.00 | 0.61 | 0.57 | 0.66 | 0.07 | 0.04 | 0.11 | 1.00 | 0.98 | 1.00 |
| 60/61 | 0.50 | 0.94 | 0.70 | 1.00 | 0.56 | 0.52 | 0.61 | 0.06 | 0.04 | 0.10 | 1.00 | 0.98 | 1.00 |
| 61/62 | 0.47 | 0.94 | 0.70 | 1.00 | 0.53 | 0.49 | 0.57 | 0.06 | 0.03 | 0.10 | 1.00 | 0.98 | 1.00 |
| Education (years) | Age (years) | |||
|---|---|---|---|---|
| 65–69 | 70–74 | 75–79 | 80–84 | |
| 6–9 | 60 (52 to 66) | 63 (57 to 78) | 62.5 (57 to 66) | 61 (55 to 65) |
| 10–12 | 67 (61 to 73) | 66 (61 to 70.5) | 63 (58 to 67) | 58 (53 to 67) |
| 13+ | 70 (63 to 76) | 69 (64.5 to 73) | 66 (60 to 70) | 59 (59 to 70) |
| Education (years) | Age (years) | |||
|---|---|---|---|---|
| 65–69 | 70–74 | 75–79 | 80–84 | |
| 6–9 | 62 (56 to 66) | 63 (56 to 68) | 63 (58 to 66) | 61.5 (54 to 65) |
| 10–12 | 67 (62 to 73) | 67 (62 to 70.5) | 63.5 (58 to 67) | 58 (53 to 67) |
| 13+ | 70 (63 to 75) | 65 (63 to 74) | 63.5 (61.5 to 65.5) | 59 (59 to 70) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, A.; O’Caoimh, R.; Murayama, H.; Molloy, D.W.; Inoue, S.; Shobugawa, Y.; Fujiwara, T. Validity of the Japanese Version of the Quick Mild Cognitive Impairment Screen. Int. J. Environ. Res. Public Health 2019, 16, 917. https://doi.org/10.3390/ijerph16060917
Morita A, O’Caoimh R, Murayama H, Molloy DW, Inoue S, Shobugawa Y, Fujiwara T. Validity of the Japanese Version of the Quick Mild Cognitive Impairment Screen. International Journal of Environmental Research and Public Health. 2019; 16(6):917. https://doi.org/10.3390/ijerph16060917
Chicago/Turabian StyleMorita, Ayako, Rónán O’Caoimh, Hiroshi Murayama, D. William Molloy, Shigeru Inoue, Yugo Shobugawa, and Takeo Fujiwara. 2019. "Validity of the Japanese Version of the Quick Mild Cognitive Impairment Screen" International Journal of Environmental Research and Public Health 16, no. 6: 917. https://doi.org/10.3390/ijerph16060917
APA StyleMorita, A., O’Caoimh, R., Murayama, H., Molloy, D. W., Inoue, S., Shobugawa, Y., & Fujiwara, T. (2019). Validity of the Japanese Version of the Quick Mild Cognitive Impairment Screen. International Journal of Environmental Research and Public Health, 16(6), 917. https://doi.org/10.3390/ijerph16060917







