Adherence and Concordance of Influenza and Pertussis Vaccination Coverage in Pregnant Women in Spain
Abstract
1. Introduction
2. Materials and Methods
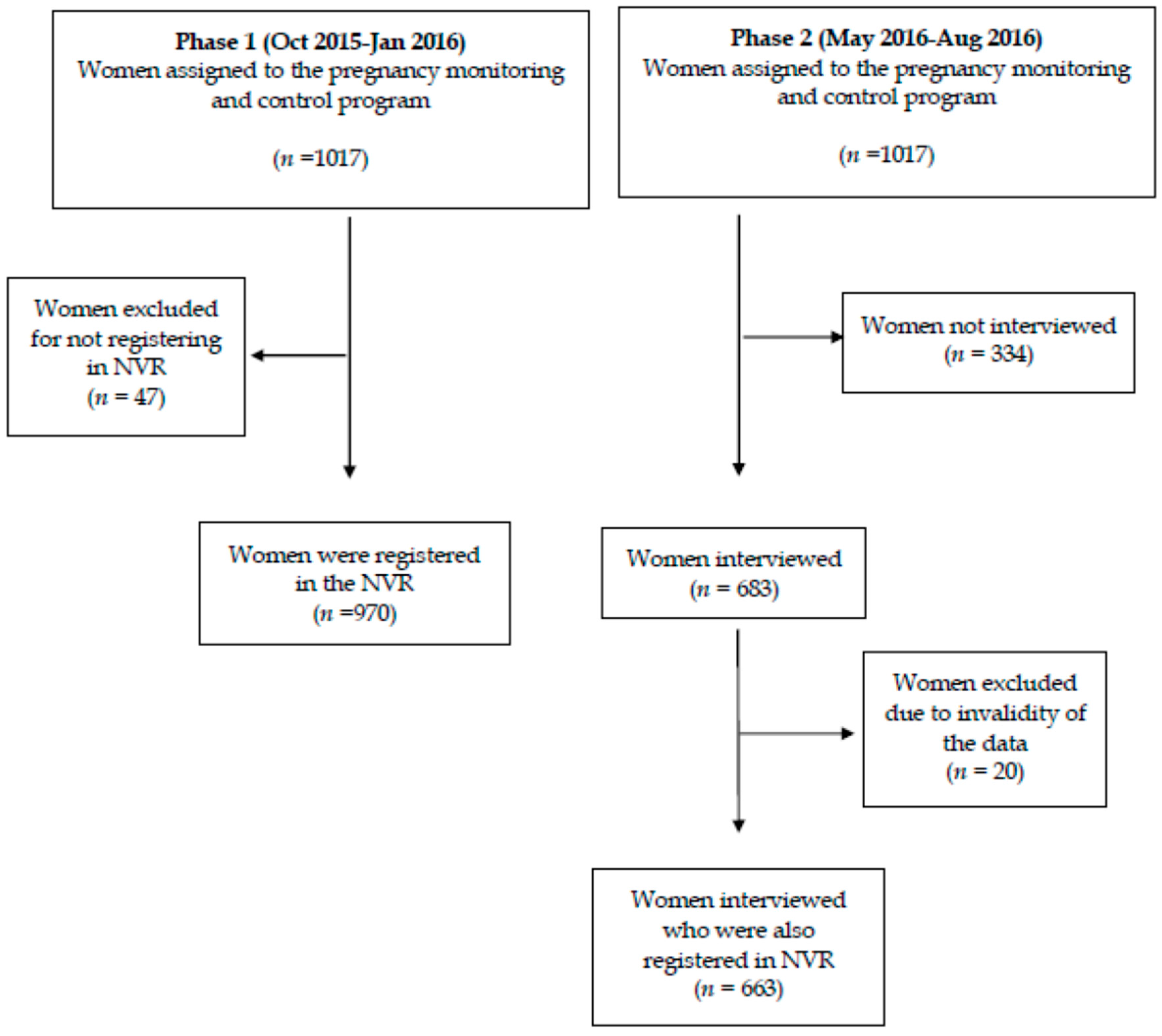
2.1. Design, Population, and Sample
2.2. Data Collection Tools
2.2.1. First Phase of the Study
2.2.2. Second Phase of the Study
2.2.3. Final Analysis of the Study
2.3. Methods of Analysis
3. Results
3.1. First Phase of the Study
3.2. Second Phase of the Study
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VC | Valencian Community |
| ACs | Autonomous Communities |
| MSCBS | Ministerio de Sanidad, Consumo y Bienestar Social (Ministry of Health, Consumer Affairs and Social Welfare) |
| TV | Torrevieja |
| EC | Elx-Crevillent |
| AEMPS | Agencia Española del Medicamento y Productos Sanitarios (Spanish Agency of Medicines and Health Products) |
| NVR | Nominal Vaccination Registry. |
References
- Arriola, C.; Garg, S.; Anderson, E.J.; Ryan, P.A.; George, A.; Zansky, S.M.; Bennett, N.; Reingold, A.; Bargsten, M.; Miller, L.; et al. Influenza vaccination modifies disease severity among community-dwelling adults hospitalized with influenza. Clin. Infect. Dis. 2017, 65, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Tuells, J.; Rodriguez-Blanco, N.; Duro Torrijos, J.L.; Vila-Candel, R.; Nolasco Bonmati, A. Vaccination of pregnant women in the Valencian Community during the 2014–2015 influenza season: A multicentre study. Rev. Esp. Quimioter. 2018, 31, 344–352. [Google Scholar] [PubMed]
- Fernández-Cano, M.I.; Espada-Trespalacios, X.; Reyes-Lacalle, A.; Manresa Domínguez, J.M.; Armadans-Gil, L.; Campins-Martí, M.; Falguera-Puig, G.; Toran Monserrat, P. Vaccination coverage against pertussis in pregnant women of Catalonia in the first year of implementation of the immunisation program. Enferm. Infecc. Microbiol. Clin. 2017, 35, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Bratton, K.N.; Wardle, M.T.; Orenstein, W.A.; Omer, S.B. Maternal influenza immunization and birth outcomes of stillbirth and spontaneous abortion: A systematic review and meta-analysis. Clin. Infect. Dis. 2015, 60, e11–e19. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Aqil, A.R.; Omer, S.B.; Madhi, S.A. The Effects of Influenza Vaccination during Pregnancy on Birth Outcomes: A Systematic Review and Meta-Analysis. Am. J. Perinatol. 2016, 33, 1104–1114. [Google Scholar] [PubMed]
- Mosby, L.G.; Rasmussen, S.A.; Jamieson, D.J. 2009 pandemic influenza A (H1N1) in pregnancy: A systematic review of the literature. Am. J. Obstet. Gynecol. 2011, 205, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.Y.S.; Tarrant, M. Determinants of uptake of influenza vaccination among pregnant women—A systematic review. Vaccine 2014, 32, 4602–4613. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.K.; Mangtani, P.; Leese, J.; Watson, J.M.; Pfeifer, D. Influenza vaccination in pregnancy: Current evidence and selected national policies. Lancet Infect. Dis. 2008, 8, 44–52. [Google Scholar] [CrossRef]
- Barber, A.; Muscoplat, M.H.; Fedorowicz, A. Coverage with Tetanus, Diphtheria, and Acellular Pertussis Vaccine and Influenza Vaccine among Pregnant Women—Minnesota, March 2013–December 2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Vila-Candel, R.; Navarro-Illana, P.; Navarro-Illana, E.; Castro-Sánchez, E.; Duke, K.; Soriano-Vidal, F.J.; Tuells, J.; Díez-Domingo, J. Determinants of seasonal influenza vaccination in pregnant women in Valencia, Spain. BMC Public Health 2016, 16, 1173. [Google Scholar] [CrossRef] [PubMed]
- Vilca Yengle, L.M.; Campins Martí, M.; Cabero Roura, L.; Rodrigo Pendás, J.Á.; Martínez Gómez, X.; Hermosilla Pérez, E.; Vaqué Rafart, J. Influenza vaccination in pregnant women. Coverage, practices and knowledge among obstetricians. Med. Clin. 2010, 134, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Laenen, J.; Roelants, M.; Devlieger, R.; Vandermeulen, C. Influenza and pertussis vaccination coverage in pregnant women. Vaccine 2015, 33, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Black, C.L.; Ball, S.; Donahue, S.; Fink, R.V.; Williams, W.W.; Kennedy, E.D.; Bridges, C.B.; Lu, P.-J.; Kahn, K.E.; et al. Influenza Vaccination Coverage Among Pregnant Women—United States, 2014–2015 Influenza Season. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G.; Campbell, H.; Ribeiro, S.; Fry, N.K.; Ramsay, M.; Miller, E.; Andrews, N. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin. Infect. Dis. 2016, 63, S236–S243. [Google Scholar] [CrossRef] [PubMed]
- Petousis-Harris, H.; Walls, T.; Watson, D.; Paynter, J.; Graham, P.; Turner, N. BMJ open safety of Tdap vaccine in pregnant women: An observational study. BMJ Open 2016, 6, e010911. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, H.; Gbesemete, D.; Gorringe, A.R.; Diavatopoulos, D.A.; Kester, K.E.; Faust, S.N.; Read, R.C. Investigating Bordetella pertussis colonisation and immunity: Protocol for an inpatient controlled human infection model. BMJ Open 2017, 7, e018594. [Google Scholar] [CrossRef]
- Castro-Sánchez, E.; Vila-Candel, R.; Soriano-Vidal, F.J.; Navarro-Illana, E.; Díez-Domingo, J. Influence of health literacy on acceptance of influenza and pertussis vaccinations: A cross-sectional study among Spanish pregnant women. BMJ Open 2018, 8, e022132. [Google Scholar] [CrossRef]
- Segura, A.; Borrell, C.; Garcia-Armesto, S.; Bartoll, X.; Novoa, A.M. Health reports as the centrepiece of public health services. Gac. Sanit. 2016, 30 (Suppl. 1), 31–37. [Google Scholar] [CrossRef]
- Centro Nacional de Epidemiología. Ministerio de Economía, I. y C. Coberturas de Vacunación con dTpa en Embarazadas. Comunidades Autónomas. Años 2016 y 2017. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/CoberturasVacunacion/Tabla11.pdf (accessed on 20 October 2017).
- Centro Nacional de Epidemiología. Ministerio de Economía, I. y C. Coberturas de Vacunación de Gripe en Embarazadas. Comunidades Autónomas. Años 2016 y 2017. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/CoberturasVacunacion/Tabla12.pdf (accessed on 20 October 2017).
- Pérez-Vilar, S.; Díez-Domingo, J.; López-Lacort, M.; Martínez-Úbeda, S.; Martinez-Beneito, M.A. Effectiveness of rotavirus vaccines, licensed but not funded, against rotavirus hospitalizations in the Valencia Region, Spain. BMC Infect. Dis. 2015, 15, 92. [Google Scholar] [CrossRef]
- Pebody, R. Vaccine Registers—Experiences from Europe and Elsewhere Disponible en. Available online: https://www.eurosurveillance.org/content/10.2807/ese.17.17.20159-en (accessed on 13 October 2018).
- Munoz, F.M.; Greisinger, A.J.; Wehmanen, O.A.; Mouzoon, M.E.; Hoyle, J.C.; Smith, F.A.; Glezen, W.P. Safety of influenza vaccination during pregnancy. Am. J. Obstet. Gynecol. 2005, 192, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Naleway, A.L.; Irving, S.A.; Henninger, M.L.; Li, D.-K.; Shifflett, P.; Ball, S.; Williams, J.L.; Cragan, J.; Gee, J.; Thompson, M.G. Safety of influenza vaccination during pregnancy: A review of subsequent maternal obstetric events and findings from two recent cohort studies. Vaccine 2014, 32, 3122–3127. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M. Safety of influenza vaccines in pregnant women. Am. J. Obstet. Gynecol. 2012, 207, s33–s37. [Google Scholar] [CrossRef] [PubMed]
- Leask, J.; Quinn, H.E.; Macartney, K.; Trent, M.; Massey, P.; Carr, C.; Turahui, J. Immunisation attitudes, knowledge and practices of health professionals in regional NSW. Aust. N. Z. J. Public Health 2008, 32, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Conselleria de Sanitat Universal i Salut Pública Generalitat Valenciana. Prevención y Vigilancia de la Gripe en la Comunitat Valenciana. Temporada 2015–2016. Informe de Salud n°149. Available online: http://publicaciones.san.gva.es/publicaciones/documentos/IS149.pdf.%0A (accessed on 20 October 2017).
- Swamy, G.K.; Garcia-Putnam, R. Maternal immunization to benefit the mother, fetus, and infant. Obstet. Gynecol. Clin. N. Am. 2014, 41, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Englund, J.A. Maternal immunization. Clin. Infect. Dis. 2014, 59, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.; Vitale, F. Influenza vaccination in high-risk groups: A revision of existing guidelines and rationale for an evidence-based preventive strategy. J. Prev. Med. Hyg. 2016, 57, e13–e18. [Google Scholar] [PubMed]
- Groom, H.C.; Henninger, M.L.; Smith, N.; Koppolu, P.; Cheetham, T.C.; Glanz, J.M.; Hambidge, S.J.; Jackson, L.A.; Kharbanda, E.O.; Klein, N.P.; et al. Influenza Vaccination during Pregnancy: Influenza Seasons 2002–2012, Vaccine Safety Datalink. Am. J. Prev. Med. 2016, 50, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Maertens, K.; Caboré, R.N.; Huygen, K.; Hens, N.; Van Damme, P.; Leuridan, E. Pertussis vaccination during pregnancy in Belgium: Results of a prospective controlled cohort study. Vaccine 2016, 34, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Vilca, L.M.; Verma, A.; Buckeridge, D.; Campins, M. A population-based analysis of predictors of influenza vaccination uptake in pregnant women: The effect of gestational and calendar time. Prev. Med. (Baltim) 2017, 99, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.E. Promoting healthy behaviors: How do we get the message across? Int. J. Nurs. Stud. 2010, 47, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Maher, L.; Dawson, A.; Wiley, K.; Hope, K.; Torvaldsen, S.; Lawrence, G.; Conaty, S. Influenza vaccination during pregnancy: A qualitative study of the knowledge, attitudes, beliefs, and practices of general practitioners in Central and South-Western Sydney. BMC Fam. Pract. 2014, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.A.; Pollock, W.E.; Nolan, T.; Hay, S.; McDonald, S. Improving influenza vaccination coverage in pregnancy in Melbourne 2010–2011. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Shavell, V.I.; Moniz, M.H.; Gonik, B.; Beigi, R.H. Influenza immunization in pregnancy: Overcoming patient and health care provider barriers. Am. J. Obstet. Gynecol. 2012, 207, S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Wiley, K.E.; Cooper, S.C.; Wood, N.; Leask, J. Understanding pregnant women’s attitudes and behavior toward influenza and pertussis vaccination. Qual. Health Res. 2015, 25, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Dirección General de Investigación y Salud Pública. Embarazo y Salud Perinatal en la Comunitat Valenciana 2011. Available online: http://www.sp.san.gva.es/DgspPortal/docs/Informe_Salud Perinatal_2011.pdf (accessed on 20 October 2017).
- Van Lier, A.; Oomen, P.; de Hoogh, P.; Drifhout, I.; Elsinghorst, B.; Kemmeren, J.; Conyn-van Spaendonck, M.; de Melker, H. Præventis, the immunisation register of the Netherlands: A tool to evaluate the national immunisation programme. Eurosurveillance 2012, 17, 3. [Google Scholar] [CrossRef]
- Chin, L.K.; Crawford, N.W.; Rowles, G.; Buttery, J.P. Australian immunisation registers: Established foundations and opportunities for improvement. Eurosurveillance 2012, 17, 20148. [Google Scholar]
- Regan, A.K.; Mak, D.B.; Hauck, Y.L.; Gibbs, R.; Tracey, L.; Effler, P.V. Trends in seasonal influenza vaccine uptake during pregnancy in Western Australia: Implications for midwives. Women Birth 2016, 29, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, L.; McCarthy, N.L.; Kharbanda, E.O.; Weintraub, E.S.; Vazquez-Benitez, G.; McNeil, M.M.; Li, R.; Klein, N.P.; Hambidge, S.J.; Naleway, A.L.; et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet. Gynecol. 2015, 126, 1069–1074. [Google Scholar] [CrossRef]
| Variables | Influenza Vaccinated n % | Influenza Unvaccinated n % | p * | Tdap Unvaccinated n % | Tdap Unvaccinated n % | p * | Total n | |
|---|---|---|---|---|---|---|---|---|
| Vaccination coverage | 623 64.2 | 347 35.8 | NA | 871 89.8 | 99 10.2 | NA | 970 | |
| Department | TV | 297 73.7 | 106 26.3 | 0.001 | 347 86.1 | 56 13.9 | 0.001 | 403 |
| EC | 326 57.5 | 241 42.5 | 524 92.4 | 43 7.6 | 567 | |||
| Country of origin | Spain | 421 60.8 | 271 39.2 | 0.001 | 635 91.8 | 57 8.2 | 0.001 | 692 |
| Not Spain | 202 72.7 | 76 27.3 | 236 84.9 | 42 15.1 | 278 | |||
| Previous pregnancies | 0 | 21 60.0 | 14 40.0 | 0.003 | 31 88.6 | 4 11.4 | 0.917 | 35 |
| 1 | 602 65.4 | 318 34.6 | 827 89.9 | 93 10.1 | 920 | |||
| ≥2 | 7 33.3 | 8 66.7 | 13 83.3 | 2 16.7 | 15 | |||
| Abortion | No | 613 64.5 | 338 35.5 | 0.048 | 853 89.7 | 98 10.3 | 0.715 | 951 |
| Yes | 10 60.0 | 9 40.0 | 18 93.3 | 1 6.7 | 19 | |||
| Parity | 0 | 18 43.9 | 23 56.1 | 0.019 | 37 90.2 | 4 9.8 | 0.176 | 41 |
| ≥1 | 605 65.1 | 324 34.9 | 834 89.9 | 95 10.1 | 929 | |||
| Visit to the midwife | Oct | 141 71.9 | 55 28.1 | 0.031 | - | - | NA | 196 |
| Nov | 423 63.3 | 245 36.7 | - | - | 668 | |||
| Dec | 43 54.4 | 36 45.6 | - | - | 79 | |||
| Jan | 16 59.3 | 11 40.7 | - | - | 27 | |||
| Intention to be vaccinated | Yes | 596 77.9 | 169 2 2.1 | 0.001 | - | - | NA | 765 |
| No | 27 13.2 | 178 86.8 | - | - | 205 | |||
| Vaccinated Tdap | Yes | 560 57.7 | 311 32.0 | 0.52 | - | - | NA | 871 |
| No | 63 6.5 | 36 3.7 | - | - | 99 | |||
| Age | Mean SD | 30.4 5.6 | 31.4 5.3 | NA | 31.0 5.3 | 29.1 6.6 | NA | 970 |
| Influenza | Tdap | |||||||
|---|---|---|---|---|---|---|---|---|
| Women registered in NVR | vaccinated | unvaccinated | Total | CI (95%) | vaccinated | unvaccinated | Total | CI (95%) |
| 410 (61.8) | 253 (38.2) | 663 (100) | 612 (92.3) | 51 (7.7) | 663 (100) | |||
| Women interviewed | Were you vaccinated during the pregnancy? | |||||||
| Yes | 409 (61.7) | 7 (1.1) | 416 (62.7) | (58.1–67.3) | 612 (92.3) | 7 (1.1) | 619 (93.4) | (91.4–95.3) |
| No | 1 (0.2) | 246 (37.1) | 247 (37.3) | (31.3–43.3) | 0 (0.0) | 44 (6.6) | 44 (6.6) | (0.0–13.9) |
| Influenza Vaccination | n = 421/683 (61.6%) | CI (95%) | |
|---|---|---|---|
| Side effects | Yes | 36/421 (8.6) | (0.0–17.7) |
| No | 385/421 (91.4) | (88.5–94.2) | |
| Type of side effects | Premature birth | 1/36 (2.7) | (0.0–8.0) |
| Pain/Inflammation | 12/36 (33.3) | (18.0–48.7) | |
| Flu | 6/36 (16.7) | (4.5–28.8) | |
| Cold (with no fever) | 17/36 (47.2) | (30.9–63.5) | |
| Pertussis Vaccination | n = 630/683 (92.2%) | CI (95%) | |
| Side effects | Yes | 67/630 (10.6) | (8.2–13.0) |
| No | 563/630 (91.4) | (89.2–93.6) | |
| Type of side effects | Pain/Inflammation | 61/67 (91.0) | (84.1–97.8) |
| Fever at 24 h | 6/67 (8.9) | (2.0–15.7) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Blanco, N.; Tuells, J.; Vila-Candel, R.; Nolasco, A. Adherence and Concordance of Influenza and Pertussis Vaccination Coverage in Pregnant Women in Spain. Int. J. Environ. Res. Public Health 2019, 16, 543. https://doi.org/10.3390/ijerph16040543
Rodríguez-Blanco N, Tuells J, Vila-Candel R, Nolasco A. Adherence and Concordance of Influenza and Pertussis Vaccination Coverage in Pregnant Women in Spain. International Journal of Environmental Research and Public Health. 2019; 16(4):543. https://doi.org/10.3390/ijerph16040543
Chicago/Turabian StyleRodríguez-Blanco, Noelia, José Tuells, Rafael Vila-Candel, and Andreu Nolasco. 2019. "Adherence and Concordance of Influenza and Pertussis Vaccination Coverage in Pregnant Women in Spain" International Journal of Environmental Research and Public Health 16, no. 4: 543. https://doi.org/10.3390/ijerph16040543
APA StyleRodríguez-Blanco, N., Tuells, J., Vila-Candel, R., & Nolasco, A. (2019). Adherence and Concordance of Influenza and Pertussis Vaccination Coverage in Pregnant Women in Spain. International Journal of Environmental Research and Public Health, 16(4), 543. https://doi.org/10.3390/ijerph16040543








