Chemical Composition and Source Apportionment of PM2.5 in Urban Areas of Xiangtan, Central South China
Abstract
1. Introduction
2. Materials and Methods
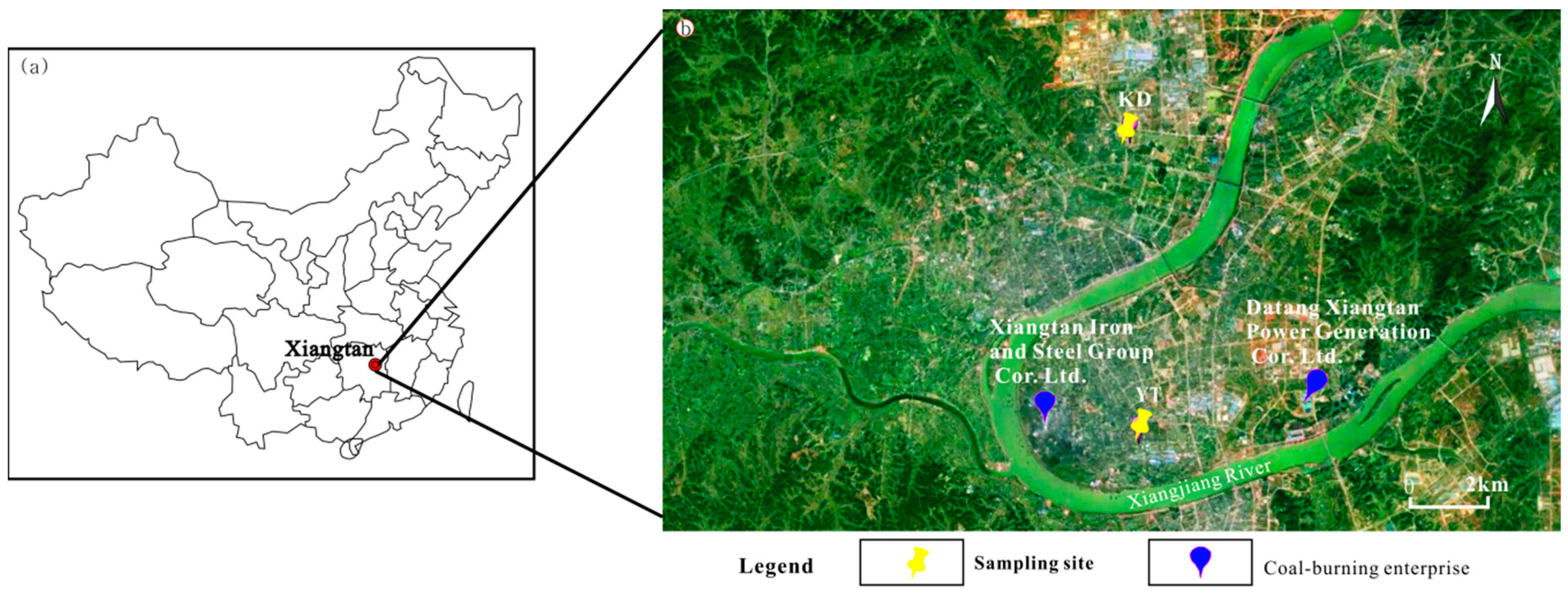
2.1. Sampling Sites
2.2. Sample Collection
2.3. Chemical Analysis
2.3.1. Ions
2.3.2. Carbon
2.3.3. Metals
2.4. PMF Model
3. Results and Discussion
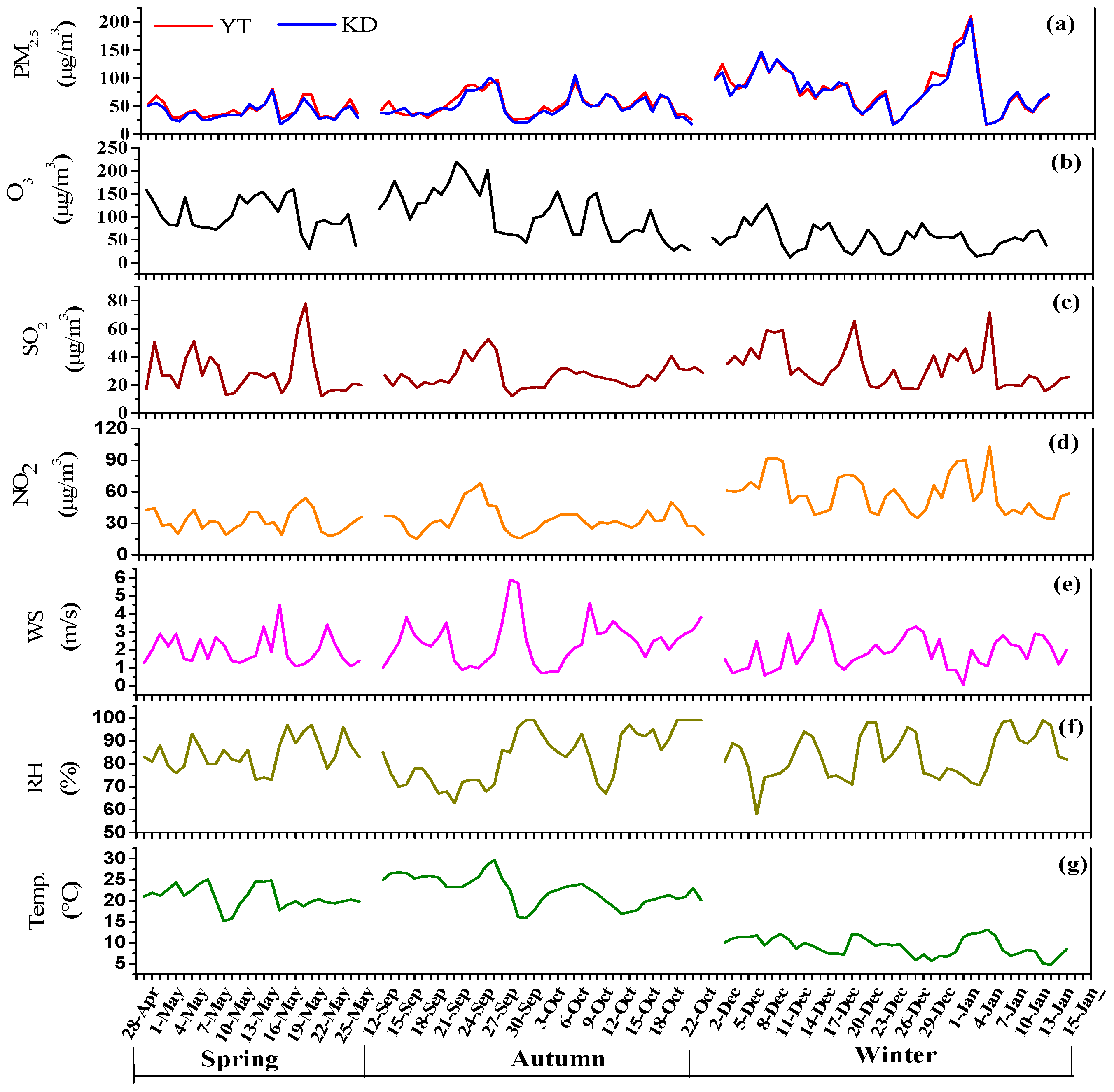
3.1. Mass Concentration of PM2.5
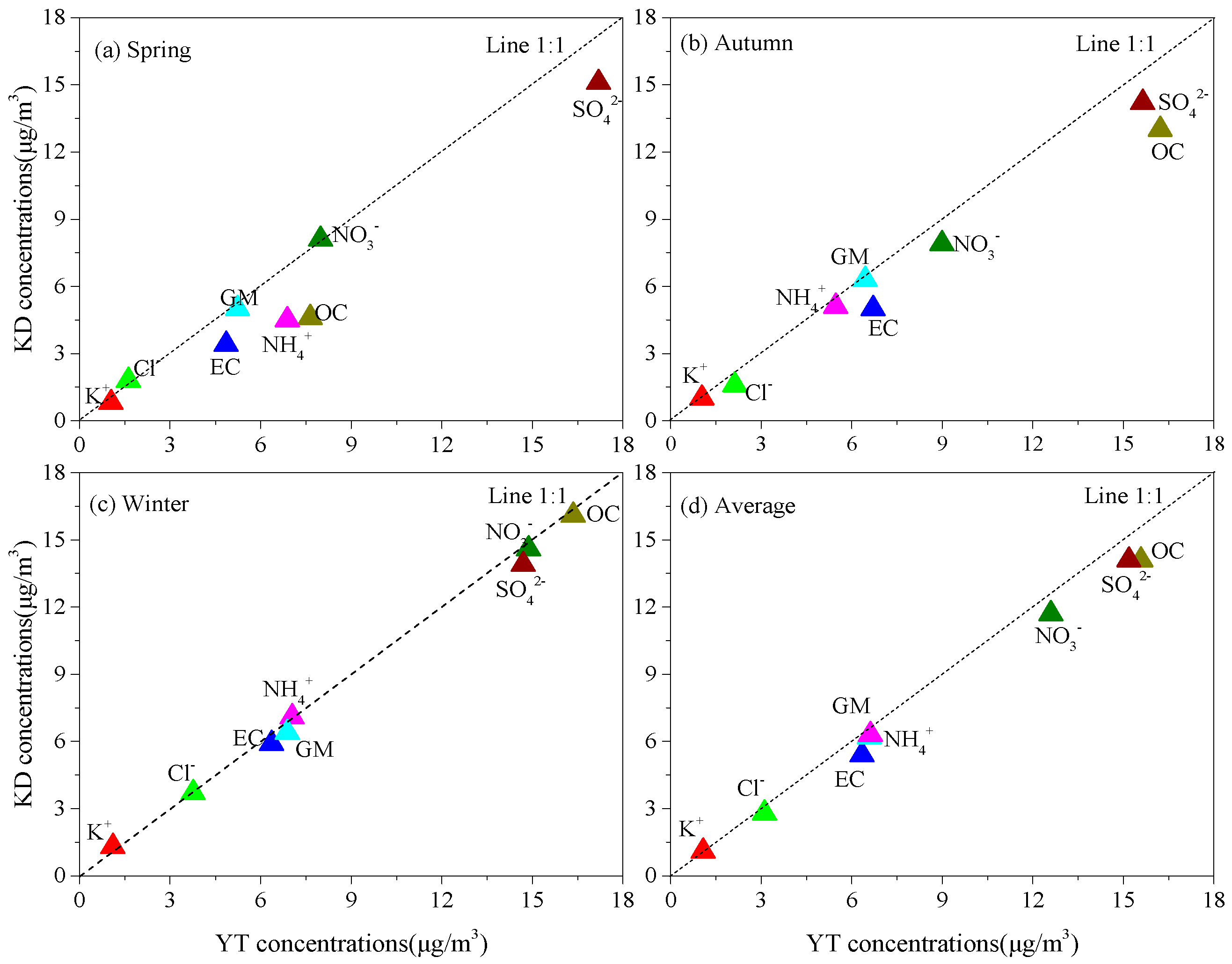
3.2. Chemical Compositions of PM2.5
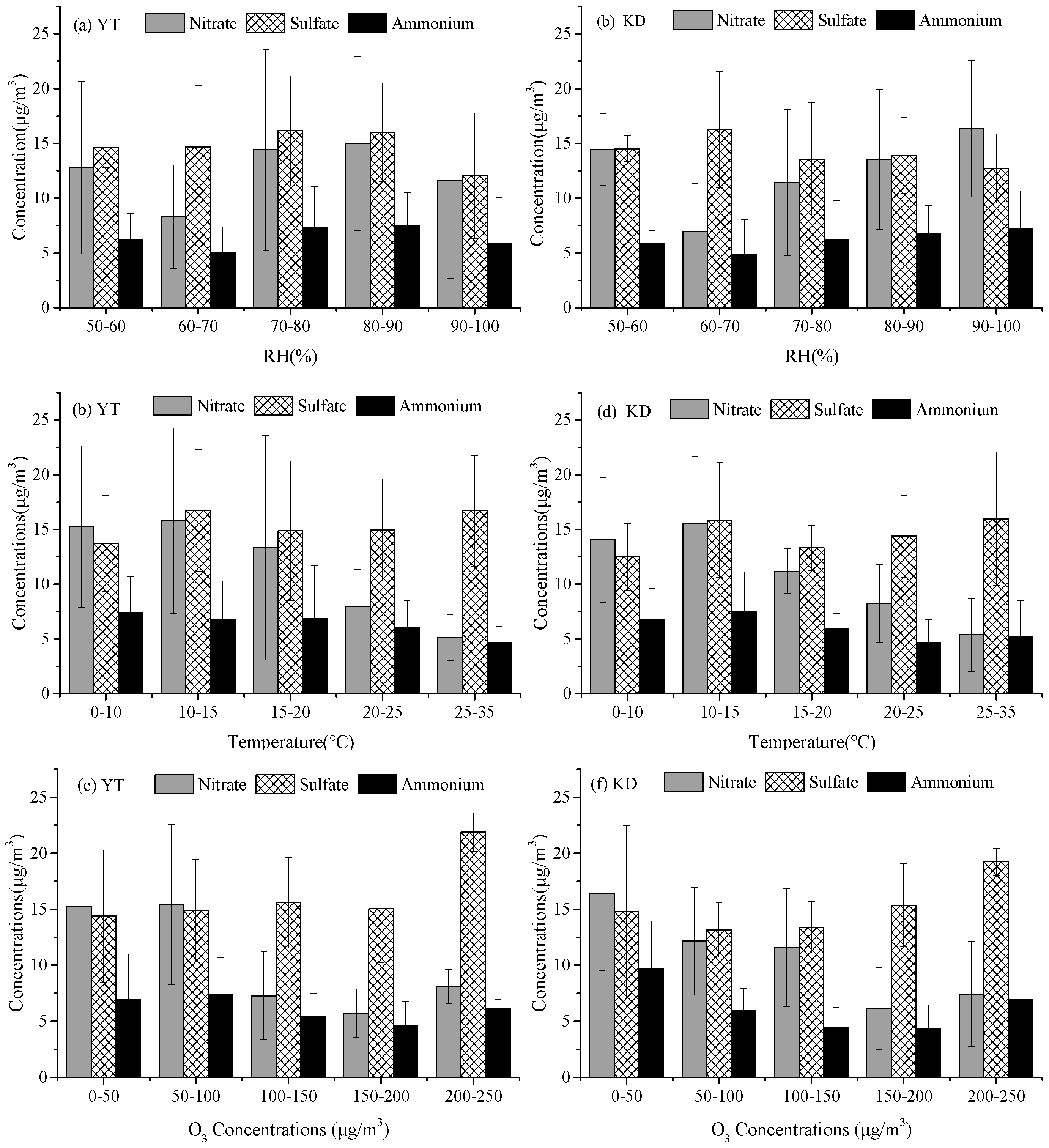
3.2.1. WSIIs
3.2.2. Carbonaceous Species
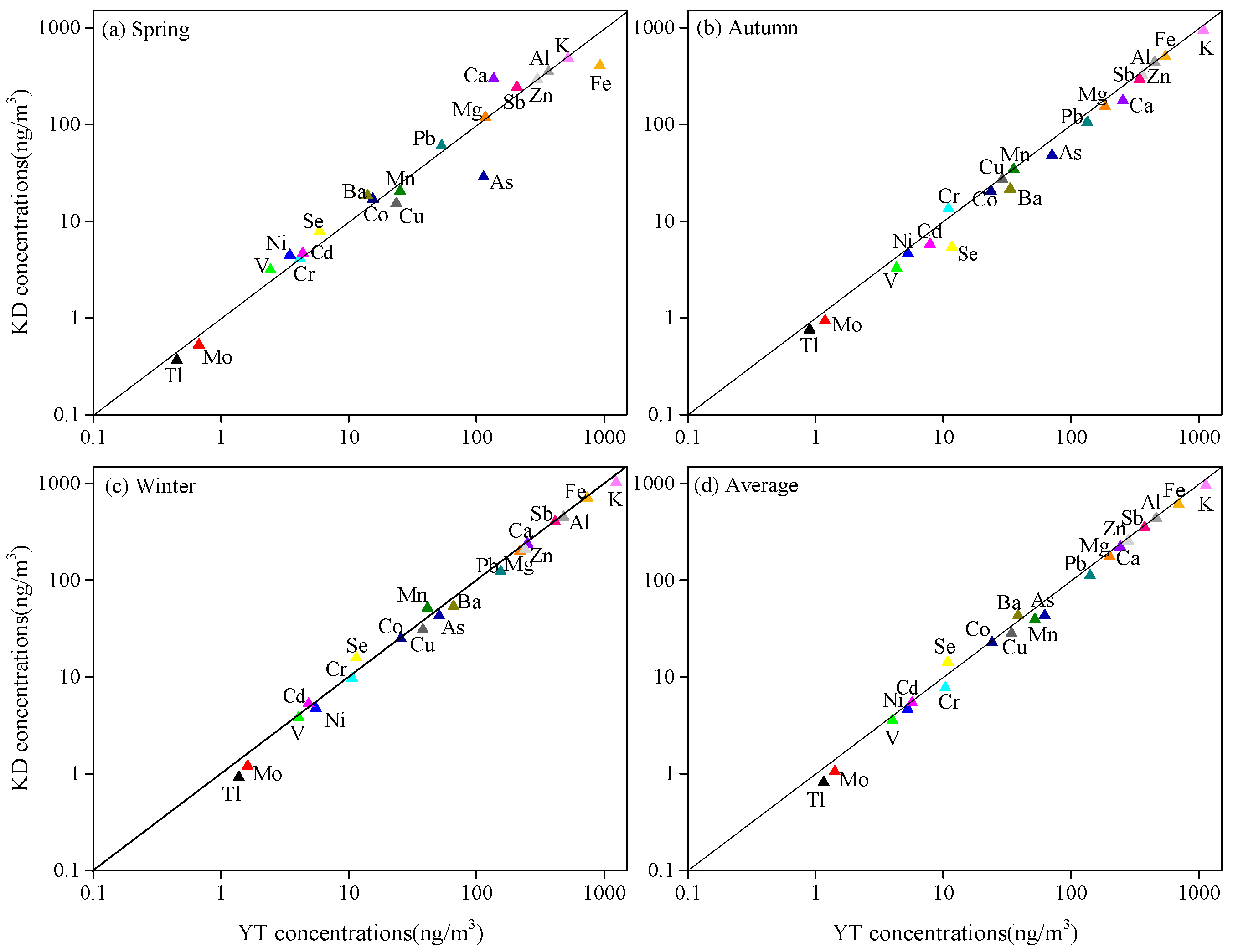
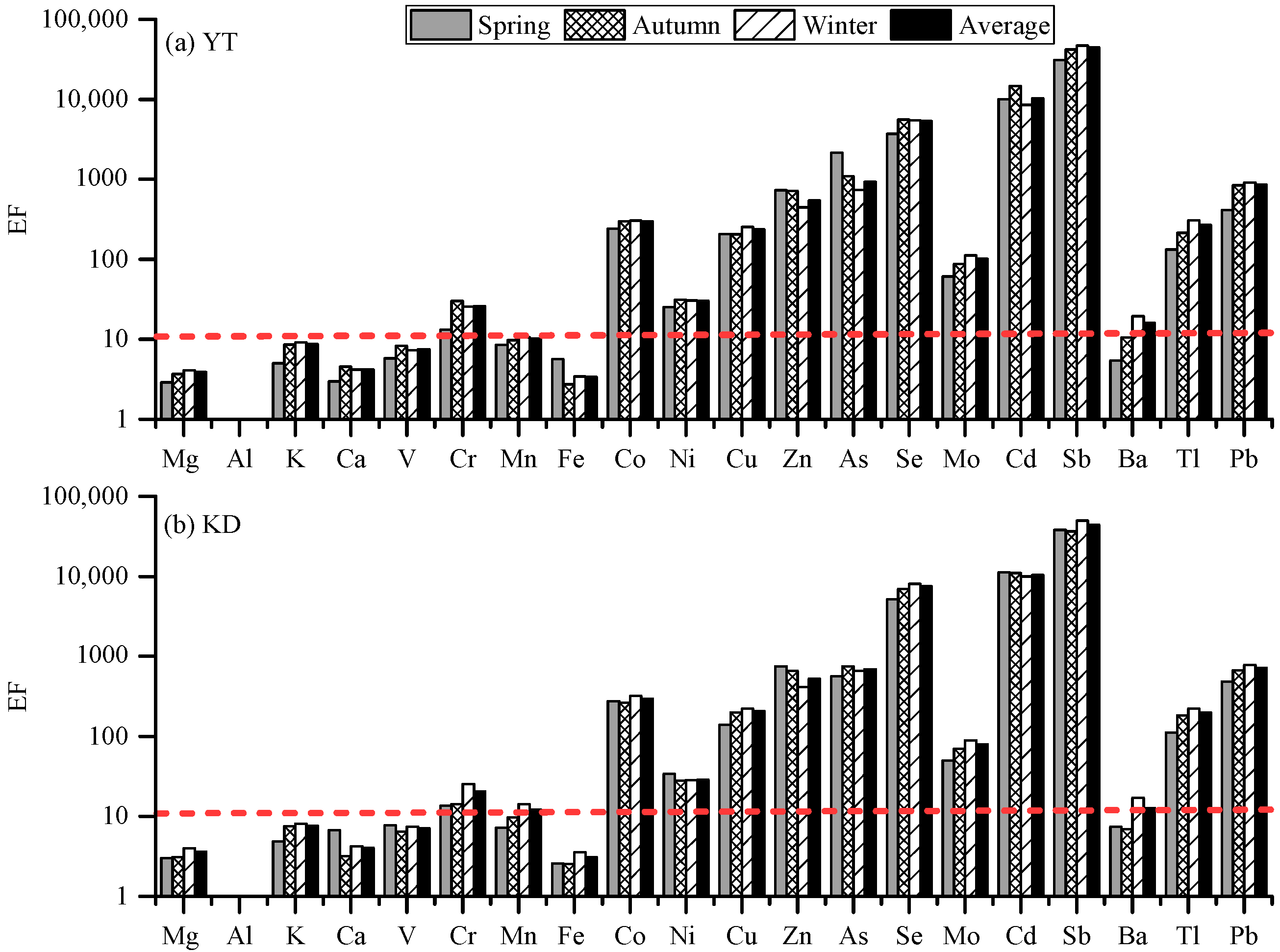
3.2.3. Metals
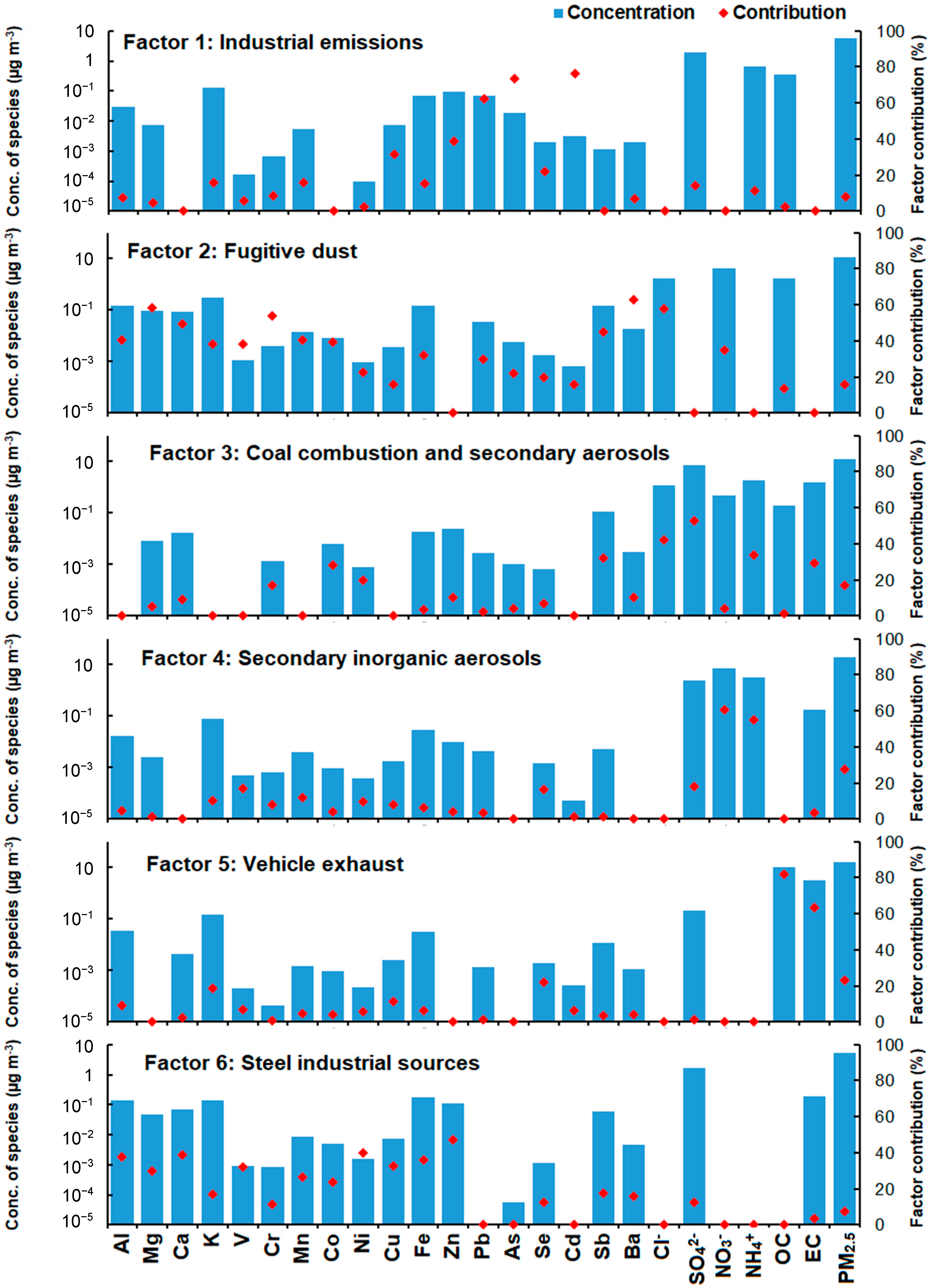
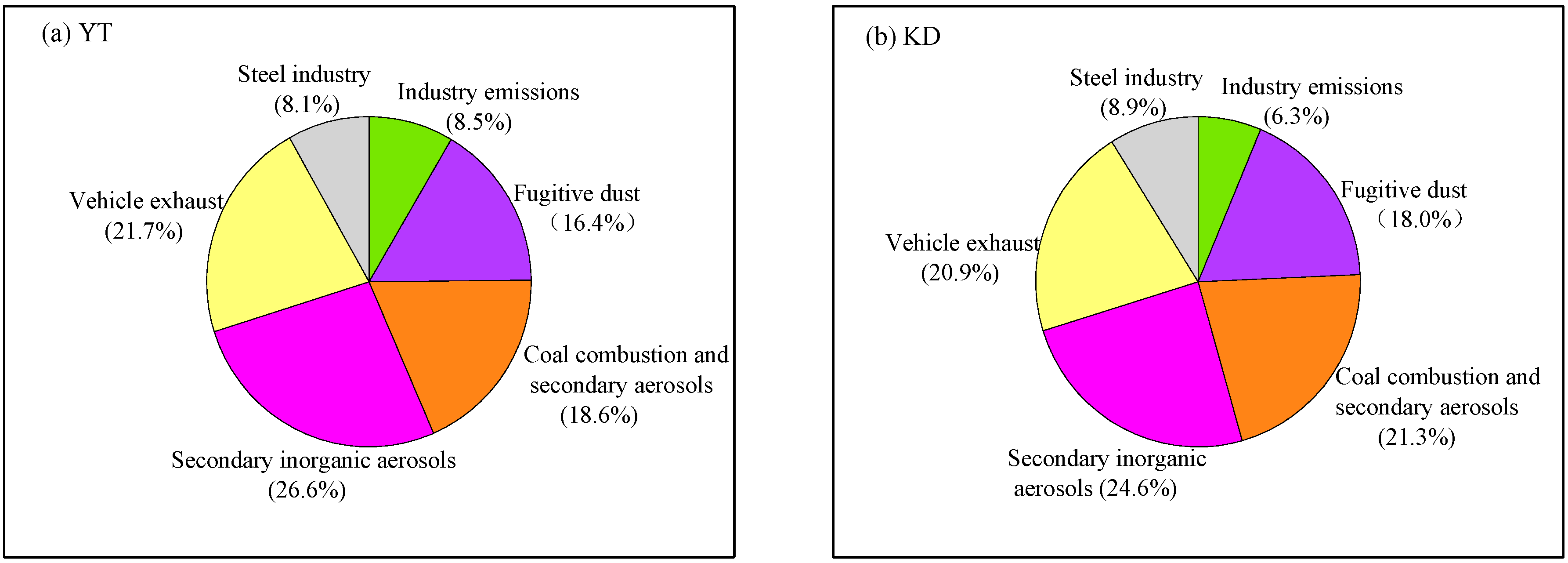
3.3. Source Apportionment Using PMF Models
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiao, B.; Chen, Y.; Tian, M.; Wang, H.; Yang, F.; Shi, G.; Zhang, L.; Peng, C.; Luo, Q.; Ding, S. Characterization of water soluble inorganic ions and their evolution processes during PM2.5 pollution episodes in a small city in southwest China. Sci. Total Environ. 2019, 650, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, L.; Cao, J.; Zhang, R. A review of current knowledge concerning PM2.5 chemical composition, aerosol optical properties and their relationships across China. Atmos. Chem. Phys. 2017, 17, 9485–9518. [Google Scholar] [CrossRef]
- Shi, Z.; Krom, M.D.; Bonneville, S.; Benning, L.G. Atmospheric processing outside clouds increases soluble iron in mineral dust. Environ. Sci. Technol. 2015, 49, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tian, M.; Chen, Y.; Shi, G.; Liu, Y.; Yang, F.; Zhang, L.; Deng, L.; Yu, J.; Peng, C.; et al. Seasonal characteristics, formation mechanisms and source origins of PM2.5 in two megacities in Sichuan Basin, China. Atmos. Chem. Phys. 2018, 18, 865–881. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Cao, J.; Zhong, L.; Chen, D.; Yang, Y.; Chen, D.; Chen, L.; Zhang, Z.; Wu, Y.; et al. Source apportionment of PM2.5 at urban and suburban areas of the Pearl River Delta Region, south China—With emphasis on ship emissions. Sci. Total Environ. 2017, 574, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, W.; Xu, Y.; Yi, K.; Zhou, M.; Tao, S.; Liu, W. Characteristics and oxidative potential of atmospheric PM2.5 in Beijing: Source apportionment and seasonal variation. Sci. Total Environ. 2019, 650, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Jiang, L.; Liu, Y.; Xiong, Q.; Sun, C.; Li, X.; Zhao, W.; Yang, X. Analysis of the characteristics and sources of carbonaceous aerosols in PM2.5 in the Beijing, Tianjin, and Langfang region, China. Int. J. Environ. Res. Public Health 2018, 15, 1483. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, L.; Lou, S.; Zhou, M.; Chen, J.; Wang, Q.; Tao, S.; Chen, C.; Huang, H.; Li, L.; et al. PM2.5 pollution episode and its contributors from 2011 to 2013 in urban Shanghai, China. Atmos. Environ. 2015, 123, 298–305. [Google Scholar] [CrossRef]
- Gao, J.; Tian, H.; Ke, C.; Long, L.; Mei, Z.; Wang, S.; Hao, J.; Wang, K.; Hua, S.; Zhu, C.; et al. The variation of chemical characteristics of PM2.5 and PM10 and formation causes during two haze pollution events in urban Beijing, China. Atmos. Environ. 2015, 107, 1–8. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, X.; Huang, Y.; Shi, A.; Yan, J.; Nie, T.; Yan, X.; Li, X. Secondary inorganic aerosols formation during haze episodes at an urban site in Beijing, China. Atmos. Environ. 2018, 177, 275–282. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Qu, Y.; An, J.; Jiang, R.; Zhang, Y.; Sun, Y.; Wu, Z.; Zhang, F.; Xu, W.; et al. Characteristics and formation mechanism of continuous hazes in China: A case study during the autumn of 2014 in the North China Plain. Atmos. Chem. Phys. 2015, 15, 8165–8178. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.; Tian, Y. Chemical composition and source apportionment of PM2.5—A case study from one year continuous sampling in the Chang-Zhu-Tan urban agglomeration. Atmos. Pollut. Res. 2017, 8, 885–899. [Google Scholar] [CrossRef]
- Zhang, K.; Chai, F.; Zheng, Z.; Yang, Q.; Li, J.; Wang, J.; Zhang, Y. Characteristics of atmospheric particles and heavy metals in winter in Chang-Zhu-Tan city clusters, China. J. Environ. Sci. 2014, 26, 147–153. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Chen, R. Regional distribution characteristics of polycyclic aromatic hydrocarbons in PM2.5 and health risk assessment in winter in Xiangtan, Hu’nan. J. Environ. Health 2016, 33, 703–706, (In Chinese with English abstract). [Google Scholar]
- Xu, L.; Chen, X.; Chen, J. Seasonal variations and chemical compositions of PM2.5 aerosol in the urban area of Fuzhou, China. Atmos. Res. 2012, 104–105, 264–272. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, J.; Chen, J. Pollution characteristics of organic and elemental carbon in PM2.5 in Xiamen, China. J. Environ. Sci. 2011, 23, 1342–1349. [Google Scholar] [CrossRef]
- Liu, B.; Song, N.; Dai, Q.; Mei, R.; Sui, B.; Bi, X.; Feng, Y. Chemical composition and source apportionment of ambient PM2.5 during the non-heating period in Taian, China. Atmos. Res. 2016, 170, 23–33. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Yang, M.; Li, F.; Wang, J.; Sun, Y.; Wang, C. Chemical characterization and source apportionment of PM2.5 aerosols in a megacity of Southeast China. Atmos. Res. 2016, 181, 288–299. [Google Scholar] [CrossRef]
- Liu, B.; Wu, J.; Zhang, J.; Wang, L.; Yang, J.; Liang, D.; Dai, Q.; Bi, X.; Feng, Y.; Zhang, Y.; et al. Characterization and source apportionment of PM2.5 based on error estimation from EPA PMF 5.0 model at a medium city in China. Environ. Pollut. 2017, 222, 10–22. [Google Scholar] [CrossRef]
- Gao, J.; Peng, X.; Chen, G.; Xu, J.; Shi, G.; Zhang, Y.; Feng, Y. Insights into the chemical characterization and sources of PM2.5 in Beijing at a 1-h time resolution. Sci. Total Environ. 2016, 54, 162–171. [Google Scholar] [CrossRef]
- Ming, L.; Jin, L.; Li, J.; Fu, P.; Yang, W.; Liu, D.; Zhang, G.; Wang, Z.; Li, X. PM2.5 in the Yangtze River Delta, China: Chemical compositions, seasonal variations, and regional pollution.events. Environ. Pollut. 2017, 223, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhuang, G.; Wang, J.; Xu, C.; Huang, K.; Li, J.; Hou, B.; Lu, T.; Streets, D. Mechanism of formation of the heaviest pollution episode ever recorded in the Yangtze River Delta, China. Atmos. Environ. 2008, 42, 2023–2036. [Google Scholar] [CrossRef]
- Gao, J.; Wang, K.; Wang, Y.; Liu, S.; Zhu, C.; Hao, J.; Liu, H.; Hua, S.; Tian, H. Temporal-spatial characteristics and source apportionment of PM2.5 as well as its associated chemical species in the Beijing—Tianjin—Hebei region of China. Environ. Pollut. 2018, 233, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, L.; Zhou, X.; Duan, J.; Li, Y.; Hu, J.; He, K. Chemical characteristics and source apportionment of PM2.5 in Lanzhou, China. Sci. Total Environ. 2017, 601–602, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiao, Z.; Chen, K.; Tang, M.; Zheng, N.; Li, P.; Yang, N.; Yang, W.; Deng, X. Spatial and temporal distribution, chemical characteristics, and sources of ambient particulate matter in the Beijing—Tianjin—Hebei region. Sci. Total Environ. 2019, 658, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Al-Naiema, I.M.; Yoon, S.; Wang, Y.; Zhang, Y.; Sheesley, R.J.; Stone, E.A. Source apportionment of fine particulate matter organic carbon in Shenzhen, China by chemical mass balance and radiocarbon methods. Environ. Pollut. 2018, 240, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, S.; Li, J.; Yao, S.; Wang, X. Investigating the aerosol mass and chemical components characteristics and feedback effects on the meteorological factors in the Beijing—Tianjin—Hebei region, China. Environ. Pollut. 2019, 244, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Okita, T.A. Chemical characterization of atmospheric aerosol in Sapporo. Atmos. Environ. 1990, 24, 815–822. [Google Scholar] [CrossRef]
- Zhou, J.; Xing, Z.; Deng, J.; Du, K. Characterizing and sourcing ambient PM2.5 over key emission regions in china Ι: Water-soluble ions and carbonaceous fractions. Atmos. Environ. 2016, 135, 20–30. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Ho, K.F.; Zhang, R.; Lin, Z.; Zhang, Z.; Lin, M.; Cao, J.; Liu, S.; Wang, G. Impact of PM2.5 chemical compositions on aerosol light scattering in Guangzhou—The largest megacity in South China. Atmos. Res. 2014, 135–136, 48–58. [Google Scholar] [CrossRef]
- Cesari, D.; Donateo, A.; Conte, M.; Merico, E.; Giangreco, A.; Giangreco, F.; Contini, D. An inter- comparison of PM2.5 at urban and urban background sites: Chemical characterization and source apportionment. Atmos. Res. 2016, 174, 106–119. [Google Scholar]
- Hsu, C.; Chiang, H.; Chen, M.; Chuang, C.; Tsen, C.; Fang, G.; Tsai, Y.; Chen, N.; Lin, T.; Lin, S.; et al. Ambient PM2.5 in the residential area near industrial complexes: Spatiotemporal variation, source apportionment, and health impact. Sci. Total Environ. 2017, 590–591, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Gerald, J.; Keeler, G.J.; Kamal, A.S.; Wagner, J.G.; Harkema, J.R.; Rohr, A.C. Source identification of ambient PM2.5 for inhalation exposure studies in Steubenville, Ohio using highly time-resolved measurements. Atmos. Environ. 2011, 45, 7688–7697. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, S.K.; Choudhary, N.; Masiwal, R.; Saxena, M.; Sharma, A.; Sharma, C. Chemical characteristics and source apportionment of PM2.5 using PCA/APCS, UNMIX, and PMF at an urban site of Delhi, India. Environ. Sci. Pollut. Res. 2017, 24, 14637–14656. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoshida, Y.; Turpin, B.J.; Hopke, P.K.; Poirot, R.L.; Lioy, P.J.; Oxley, J.C. Identification of Sources Contributing to Mid-Atlantic Regional Aerosol. J. Air Waste Manage. Assoc. 2002, 52, 1186–1205. [Google Scholar] [CrossRef]
- Banerjee, T.; Murari, V.; Kumar, M.; Raju, M.P. Source apportionment of airborne particulates through receptor modeling: Indian scenario. Atmos. Res. 2015, 164, 167–187. [Google Scholar] [CrossRef]
- Yao, L.; Yang, L.; Yuan, Q.; Yan, C.; Dong, C.; Meng, C.; Sui, X.; Yang, F.; Lu, Y.; Wang, W. Sources apportionment of PM2.5 in a background site in the North China Plain. Sci. Total Environ. 2016, 541, 590–598. [Google Scholar] [CrossRef]
- Pian, W.; Cheng, W.; Niu, H.; Fan, J. TEM study of fine particles from coal-fired power plant ambient air. World J. Eng. 2016, 13, 311–316. [Google Scholar] [CrossRef]
- Feng, J.; Yu, H.; Mi, K.; Su, X.; Li, Y.; Li, Q.; Sun, J. One year study of PM2.5 in Xinxiang city, North China: Seasonal characteristics, climate impact and source. Ecotoxicol. Environ. Saf. 2018, 154, 75–83. [Google Scholar]
- Meng, C.; Wang, L.; Zhang, F.; Wei, Z.; Ma, S.; Ma, X.; Yang, J. Characteristics of concentrations and water-soluble inorganic ions in PM2.5 in Handan city, Hebei province, China. Atmos. Res. 2016, 171, 133–146. [Google Scholar] [CrossRef]
- Rengarajan, R.; Sudheer, A.K.; Sarin, M.M. Wintertime PM2.5 and PM10 carbonaceous and inorganic constituents from urban site in western India. Atmos. Res. 2011, 102, 420–431. [Google Scholar] [CrossRef]
- Han, B.; Zhang, R.; Yang, W.; Bai, Z.; Ma, Z.; Zhang, W. Heavy haze episodes in Beijing during January 2013: Inorganic ion chemistry and source analysis using highly time-resolved measurements from an urban site. Sci. Total Environ. 2016, 544, 319–329. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Z.; Zhang, J.; Wen, T.; Ji, D.; Wang, Y. Seasonal variation and secondary formation of size-segregated aerosol water-soluble inorganic ions during pollution episodes in Beijing. Atmos. Res. 2016, 168, 70–79. [Google Scholar] [CrossRef]
| Pollutant and Meteor. Parameters | YT | KD | ||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Winter | Average | Spring | Autumn | Winter | Average | |
| O3 | 134.4 ± 10.6 | 129.4 ± 55.8 | 62.6 ± 30.4 | 108.8 ± 32.3 | 129.5 ± 32.5 | 126.0 ± 10.9 | 58.4 ± 24.8 | 104.6 ± 22.7 |
| SO2 | 27.6 ± 13.5 | 31.0 ±10.6 | 33.3 ± 13.8 | 30.6 ± 12.6 | 26.1 ± 12.7 | 30.7 ± 14.5 | 33.6 ± 12.2 | 30.1 ± 13.1 |
| NO2 | 37.6 ± 7.1 | 41.1 ± 13.4 | 52.4 ± 19.9 | 43.7 ± 13.5 | 36.5 ± 12.5 | 39.7 ± 12.9 | 59.2 ± 18.1 | 45.1 ± 15.2 |
| T (°C) | 20.8 ± 1.1 | 22.1 ± 6.0 | 9.5 ± 3.7 | 17.4 ± 3.6 | 20.6 ± 1.2 | 22.1 ± 6.6 | 9.1 ± 2.3 | 17.3 ± 3.4 |
| RH (%) | 83.8 ± 3.1 | 78.5 ± 10.2 | 79.3 ± 12.3 | 80.5 ± 8.5 | 83.0 ± 4.3 | 78.7 ± 10.7 | 80.4 ± 10.2 | 80.7 ± 98.4 |
| WS (m/s) | 1.4 ± 0.3 | 1.9 ± 0.8 | 1.9 ± 0.9 | 1.7 ± 0.7 | 1.7 ± 0.5 | 2.1 ± 0.8 | 1.8 ± 0.9 | 1.9 ± 0.7 |
| Ions | YT | KD | ||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Winter | Average | Spring | Autumn | Winter | Average | |
| PM2.5 (μg/m3) | 61.7 ± 18.5 | 82.2 ± 30.9 | 93.4 ± 38.5 | 79.1 ± 29.3 | 56.9 ± 19.3 | 68.2 ± 20.2 | 95.6 ± 33.5 | 73.6 ± 24.3 |
| Cl− (μg/m3) | 1.6 ± 0.6 | 2.1 ± 0.8 | 3.8 ± 1.6 | 2.5 ± 1.0 | 1.8 ± 0.8 | 1.6 ± 0.7 | 3.7 ± 1.6 | 2.4 ± 1.0 |
| NO3− (μg/m3) | 8.0 ± 3.9 | 9.0 ± 6.3 | 14.9 ± 8.3 | 10.6 ± 6.1 | 6.3 ± 4.4 | 7.9 ± 4.5 | 14.6 ± 6.0 | 9.6 ± 5.0 |
| SO42− (μg/m3) | 17.2 ± 4.4 | 15.6 ± 4.2 | 14.7 ± 5.4 | 15.9 ± 4.7 | 15.1 ± 4.3 | 14.1 ± 4.3 | 13.9 ± 4.4 | 14.4 ± 4.3 |
| NH4+ (μg/m3) | 6.9 ± 2.4 | 5.5 ± 2.0 | 7.1 ± 3.7 | 6.5 ± 2.7 | 4.5 ± 2.6 | 5.1 ± 2.4 | 7.1 ± 3.3 | 5.6 ± 2.7 |
| Cl−/PM2.5 (%) | 2.6 ± 0.9 | 2.7 ± 1.0 | 4.3 ± 1.6 | 3.2 ± 1.3 | 2.1 ± 0.9 | 1.8 ± 0.8 | 4.3 ± 1.8 | 2.8 ± 1.2 |
| NO3−/PM2.5 (%) | 12.4 ± 2.8 | 11.0 ± 6.6 | 16.0 ± 5.9 | 13.2 ± 5.1 | 9.5 ± 5.11 | 9.3 ± 5.2 | 17.1 ± 7.1 | 12.0 ± 5.8 |
| SO42−/PM2.5 (%) | 28.2 ± 2.1 | 20.3 ± 5.1 | 16.5 ± 4.1 | 21.7 ± 3.8 | 17.7 ± 5.0 | 16.6 ± 5.0 | 16.3 ± 5.1 | 16.9 ± 5.0 |
| NH4+/PM2.5 (%) | 11.1 ± 1.2 | 6.9 ± 2.1 | 7.9 ± 3.5 | 8.6 ± 2.6 | 5.3 ± 3.1 | 6.0 ± 2.8 | 8.3 ± 3.8 | 6.5 ± 3.2 |
| SIAs | 32.1 ± 10.7 | 30.1 ± 10.0 | 36.6 ± 16.3 | 32.9 ± 12.3 | 27.7 ± 10.5 | 27.2 ± 9.1 | 35.6 ± 12.4 | 30.2 ± 10.6 |
| WSIIs | 42.3 ± 12.0 | 41.2 ± 11.9 | 50.3 ± 20.2 | 44.6 ± 14.7 | 36.3 ± 11.2 | 36.1 ± 10.0 | 50.3 ± 16.6 | 40.9 ± 12.6 |
| SIAs/WSIIs (%) | 75.1 ± 4.4 | 72.3 ± 5.7 | 71.5 ± 8.5 | 73.0 ± 6.2 | 75.3 ± 5.7 | 74.7 ± 4.0 | 70.6 ± 6.4 | 73.5 ± 5.4 |
| WSIIs/PM2.5 (%) | 68.9 ± 4.6 | 52.7 ± 12.2 | 56.0 ± 12.4 | 59.2 ± 9.8 | 64.7 ± 9.7 | 53.8 ± 7.7 | 54.7 ± 13.8 | 57.7 ± 10.4 |
| SIAs/PM2.5 (%) | 51.7 ± 4.5 | 38.2 ± 9.4 | 40.4 ± 10.8 | 43.5 ± 8.3 | 49.0 ± 9.9 | 40.2 ± 6.1 | 38.5 ± 9.6 | 42.6 ± 8.5 |
| NO3−/SO42− | 0.4 ± 0.1 | 0.6 ± 0.4 | 1.0 ± 0.5 | 0.7 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.4 | 1.1 ± 0.3 | 0.7 ± 0.3 |
| SOR | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| NOR | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Carbonaceous | YT | KD | ||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Winter | Average | Spring | Autumn | Winter | Average | |
| OC (μg/m3) | 7.7 ± 1.4 | 16.2 ± 7.6 | 16.4 ± 9.3 | 13.4 ± 6.1 | 4.6 ± 2.1 | 13.0 ± 4.2 | 16.1 ± 9.1 | 11.2 ± 5.2 |
| EC (μg/m3) | 4.9 ± 0.8 | 6.7 ± 2.2 | 6.4 ± 2.3 | 6.0 ± 1.8 | 3.4 ± 1.3 | 5.0 ± 1.4 | 5.9 ± 2.2 | 4.8 ± 1.6 |
| OC/EC | 1.6 ± 0.2 | 2.4 ± 0.7 | 2.5 ± 1.0 | 2.1 ± 0.6 | 1.3 ± 0.2 | 2.7 ± 0.8 | 2.7 ± 1.2 | 2.2 ± 0.8 |
| POC (μg/m3) | 4.3 ± 0.7 | 5.9 ± 1.9 | 5.6 ± 2.0 | 5.3 ± 1.5 | 3.5 ± 1.3 | 5.2 ± 1.4 | 6.1 ± 2.2 | 4.9 ± 1.7 |
| SOC (μg/m3) | 3.4 ± 1.0 | 10.3 ± 6.0 | 10.8 ± 7.8 | 8.2 ± 4.9 | 1.1 ± 0.9 | 7.8 ± 3.5 | 10.1 ± 7.5 | 8.5 ± 6.5 |
| OC/PM2.5 (%) | 12.8 ± 2.3 | 19.8 ± 5.4 | 16.5 ± 6.1 | 16.4 ± 4.6 | 8.1 ± 3.2 | 19.3 ± 4.3 | 16.3 ± 6.8 | 14.5 ± 4.8 |
| EC/PM2.5 (%) | 8.2 ± 1.2 | 8.4 ± 1.2 | 7.1 ± 1.8 | 7.9 ± 1.4 | 6.0 ± 1.7 | 7.5 ± 1.3 | 6.3 ± 1.5 | 6.6 ± 1.5 |
| SOC/OC (%) | 43.6 ± 7.2 | 60.7 ± 9.5 | 56.9 ± 20.4 | 53.7 ± 12.4 | 19.8 ± 1.4 | 57.6 ± 12.9 | 53.8 ± 20.8 | 43.7 ± 16.0 |
| SOC/PM2.5 (%) | 5.7 ± 1.8 | 12.4 ± 5.3 | 10.3 ± 6.2 | 9.2 ± 4.4 | 1.9 ± 1.7 | 11.5 ± 4.6 | 9.8 ± 6.6 | 7.7 ± 4.3 |
| Metal | YT | KD | ||||||
|---|---|---|---|---|---|---|---|---|
| Spring | Autumn | Winter | Average | Spring | Autumn | Winter | Average | |
| Mg | 117.7 ± 86.3 | 185.1 ± 126.8 | 219.2 ± 126.3 | 174.0 ± 113.2 | 117.9 ± 74.6 | 152.1 ± 102.3 | 200.4 ± 79.4 | 156.8 ± 85.4 |
| Al | 366.7 ± 300.9 | 451.8 ± 224.5 | 482.3 ± 264.7 | 463.6 ± 263.6 | 351.2 ± 267.0 | 441.8 ± 221.0 | 448.6 ± 225.0 | 413.9 ± 237.7 |
| K | 519.1 ± 301.1 | 1090.2 ± 515.4 | 1244.6 ± 595.9 | 951.3 ± 470.8 | 481.4 ± 329.6 | 938.1 ± 390.0 | 1027.4 ± 433.1 | 815.6 ± 384.2 |
| Ca | 136.4 ± 60.3 | 255.1 ± 171.1 | 250.8 ± 155.6 | 214.1 ± 129.0 | 295.1 ± 279.8 | 175.5 ± 93.5 | 237.3 ± 96.5 | 236.0 ± 156.6 |
| V | 2.4 ± 1.8 | 4.3 ± 1.7 | 4.1 ± 3.1 | 3.6 ± 2.2 | 3.1 ± 1.8 | 3.3 ± 1.3 | 3.8 ± 1.4 | 3.4 ± 1.5 |
| Cr | 4.2 ± 2.5 | 11.7 ± 13.6 | 10.6 ± 4.1 | 8.8 ± 6.7 | 4.1 ± 1.7 | 5.4 ± 2.3 | 9.8 ± 4.1 | 6.4 ± 2.7 |
| Mn | 25.2 ± 18.4 | 35.5 ± 17.4 | 41.5 ± 25.1 | 34.1 ± 20.3 | 20.4 ± 10.5 | 34.6 ± 19.0 | 51.8 ± 27.6 | 35.6 ± 19.0 |
| Fe | 925.7 ± 893.8 | 548.2 ± 246.0 | 734.8 ± 792.7 | 736.2 ± 644.2 | 404.5 ± 297.0 | 502.0 ± 238.5 | 710.7 ± 616.5 | 539.1 ± 384.0 |
| Co | 15.5 ± 5.4 | 23.6 ± 5.3 | 25.6 ± 9.5 | 21.5 ± 6.7 | 16.9 ± 5.4 | 20.4 ± 3.8 | 24.9 ± 7.6 | 20.7 ± 6.0 |
| Ni | 3.5 ± 1.4 | 5.3 ± 2.1 | 5.5 ± 4.0 | 4.8 ± 2.5 | 4.5 ± 2.7 | 4.6 ± 2.4 | 4.8 ± 2.3 | 4.6 ± 2.5 |
| Cu | 23.6 ± 17.8 | 29.0 ± 14.5 | 38.0 ± 33.2 | 30.2 ± 21.8 | 15.3 ± 4.9 | 27.3 ± 20.3 | 30.9 ± 26.7 | 24.5 ± 17.3 |
| Zn | 299.6 ± 224.2 | 361.9 ± 198.0 | 239.9 ± 204.3 | 300.5 ± 208.8 | 294.6 ± 209.9 | 323.3 ± 255.7 | 208.0 ± 168.5 | 275.3 ± 211.3 |
| As | 113.7 ± 105.4 | 71.1 ± 61.2 | 51.1 ± 42.9 | 78.6 ± 69.8 | 28.6 ± 18.5 | 47.8 ± 32.5 | 42.9 ± 34.6 | 39.8 ± 28.6 |
| Se | 6.0 ± 3.8 | 11.0 ± 4.1 | 11.5 ± 6.9 | 9.5 ± 4.9 | 7.9 ± 4.8 | 13.4 ± 4.0 | 15.8 ± 7.4 | 12.4 ± 5.4 |
| Mo | 0.7 ± 0.5 | 1.2 ± 0.7 | 1.6 ± 1.3 | 1.2 ± 0.8 | 0.5 ± 0.3 | 0.9 ± 0.4 | 1.2 ± 0.4 | 0.9 ± 0.4 |
| Cd | 4.4 ± 6.3 | 7.9 ± 7.0 | 4.9 ± 4.2 | 5.7 ± 5.8 | 4.7 ± 5.1 | 5.8 ± 6.7 | 5.3 ± 7.6 | 5.3 ± 6.5 |
| Sb | 207.4 ± 51.9 | 344.2 ± 75.4 | 412.9 ± 146.6 | 321.5 ± 91.3 | 242.9 ± 70.6 | 293.6 ± 46.5 | 404.0 ± 116.5 | 313.5 ± 77.9 |
| Ba | 14.1 ± 7.4 | 33.5 ± 26.5 | 65.9 ± 68.3 | 37.9 ± 34.1 | 18.3 ± 15.6 | 21.4 ± 11.8 | 53.8 ± 48.9 | 31.2 ± 25.4 |
| Tl | 0.5 ± 0.5 | 0.9 ± 0.5 | 1.4 ± 1.1 | 0.9 ± 0.7 | 0.4 ± 0.3 | 0.8 ± 0.7 | 0.9 ± 0.6 | 0.4 ± 0.5 |
| Pb | 53.3 ± 49.0 | 134.4 ± 105.6 | 155.1 ± 118.1 | 114.3 ± 90.9 | 60.4 ± 46.2 | 105.0 ± 109.4 | 123.2 ± 107.3 | 96.2 ± 87.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Xiao, Z.; He, L.; Shi, Z.; Cao, Y.; Tian, Z.; Vu, T.; Liu, J. Chemical Composition and Source Apportionment of PM2.5 in Urban Areas of Xiangtan, Central South China. Int. J. Environ. Res. Public Health 2019, 16, 539. https://doi.org/10.3390/ijerph16040539
Ma X, Xiao Z, He L, Shi Z, Cao Y, Tian Z, Vu T, Liu J. Chemical Composition and Source Apportionment of PM2.5 in Urban Areas of Xiangtan, Central South China. International Journal of Environmental Research and Public Health. 2019; 16(4):539. https://doi.org/10.3390/ijerph16040539
Chicago/Turabian StyleMa, Xiaoyao, Zhenghui Xiao, Lizhi He, Zongbo Shi, Yunjiang Cao, Zhe Tian, Tuan Vu, and Jisong Liu. 2019. "Chemical Composition and Source Apportionment of PM2.5 in Urban Areas of Xiangtan, Central South China" International Journal of Environmental Research and Public Health 16, no. 4: 539. https://doi.org/10.3390/ijerph16040539
APA StyleMa, X., Xiao, Z., He, L., Shi, Z., Cao, Y., Tian, Z., Vu, T., & Liu, J. (2019). Chemical Composition and Source Apportionment of PM2.5 in Urban Areas of Xiangtan, Central South China. International Journal of Environmental Research and Public Health, 16(4), 539. https://doi.org/10.3390/ijerph16040539






