Exposure Characterization of Haloacetic Acids in Humans for Exposure and Risk Assessment Applications: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Enrollment and Water Supplies
2.2. Urine and Drinking Water Sampling
2.3. Laboratory Measurements
2.4. Data Analyses
3. Results
3.1. Comparison of Two Drinking Water Systems Based on Total Organic Halogen
3.2. HAA Concentrations in Urine and Drinking Water
3.3. Correlation of Urinary and Drinking Water TCAA and MCAA
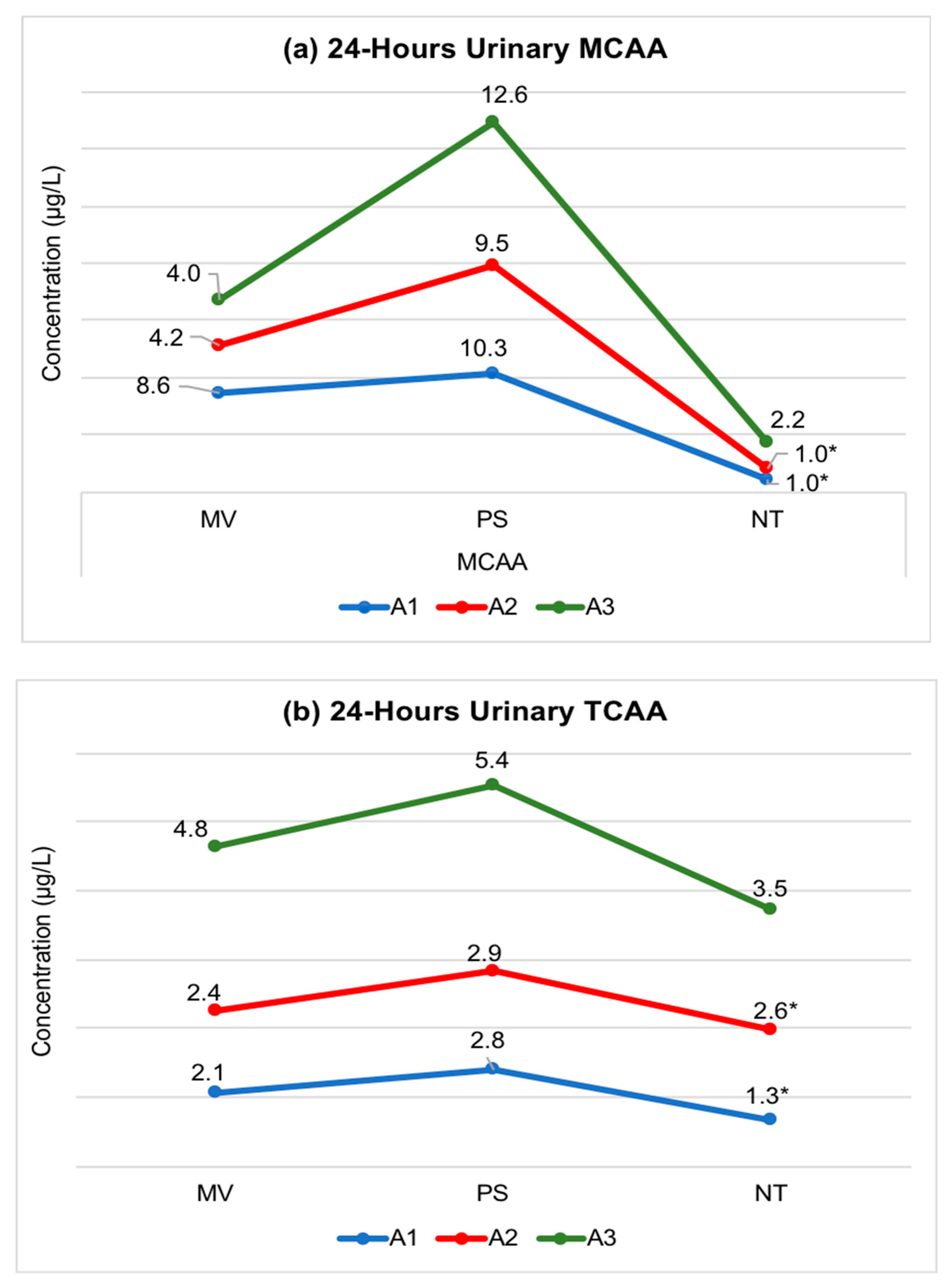
3.4. Longitudinal (Intra-Individual) Exposure Variability of Urinary MCAA and TCAA
4. Discussion
Key Limitations and Future Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BCAA | bromochloroacetic acid |
| CV | coefficient of variance |
| DBAA | dibromoacetic acid |
| DBPs | disinfection byproducts |
| DCAA | dichloroacetic acid |
| EPA | Environmental Protection Agency |
| GSH | glutathione |
| HAAs | haloacetic acids |
| IQR | inter-quartile range |
| LOD | limit of detection |
| MBAA | monobromoacetic acid |
| MCAA | monochloroacetic acid |
| NOM | natural organic matter |
| Q1 | first quartile between 0th-25th percentile |
| Q2 | second quartile between 25th-50th percentile |
| Q3 | third quartile between 50th-75th percentile |
| Q4 | second quartile between 75th-99th percentile |
| SD | standard deviation |
| TCAA | trichloroacetic acid |
| THMs | trihalomethanes |
| TOBr | total organic bromine |
| TOCl | total organic chlorine |
| TOI | total organic iodine |
| TOX | total organic halogen |
References
- Krasner, S.W.; McGuire, M.J.; Jacangelo, J.G.; Patania, N.L.; Reagan, K.M.; Aieta, E.M. The Occurrence of Disinfection By-products in US Drinking Water. Am. Water Work. Assoc. 1989, 81, 41–53. [Google Scholar] [CrossRef]
- Weinberg, H.S.; Krasner, S.W.; Richardson, S.D.; Thruston, A.D. The Occurrence of Disinfection By-Products of Health Concern in Drinking Water: Results of a Nationwide DBP Occurrence Study; EPA: Washington, DC, USA, 2002.
- Singer, P.; Weinberg, H.S.; Brophy, K.; Liang, L.; Roberts, M.; Grisstede, I.; Krasner, S.W.; Baribeau, H.; Arora, H.; Najm, I. Relative Dominance of Haloacetic Acids and Trihalomethanes in Treated Drinking Water; American Water Works Association: Denver, CO, USA, 2002; pp. 1–344. [Google Scholar]
- Kimura, S.Y.; Zheng, W.W.; Hipp, T.N.; Allen, J.M.; Richardson, S.D. Total organic halogen (TOX) in human urine: A halogen-specific method for human exposure studies. J. Environ. Sci. 2017, 58, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Pressman, J.G.; Richardson, S.D.; Speth, T.F.; Miltner, R.J.; Narotsky, M.G.; Hunter, S.E.; Rice, G.E.; Teuschler, L.K.; Mcdonald, A.; Parvez, S.; et al. Concentration, Chlorination, and Chemical Analysis of Drinking Water for Disinfection Byproduct Mixtures Health Effects Research. Environ. Sci. Technol. 2010, 44, 7184–7192. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Rivera-Nunez, Z.; Meyer, A.; Wright, J.M. Temporal Variability in Trihalomethane and Haloacetic Acid Concentrations in Massachusetts Public Drinking Water Systems. Environ. Res. 2011, 111, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.J.; Serodes, J.B.; Levallois, P. Behavior of trihalomethanes and haloacetic acids in a drinking water distribution system. Water Res. 2004, 38, 4367–4382. [Google Scholar] [CrossRef] [PubMed]
- Georgia, A. The Behaviour of Haloacetic Acids in Distribution Zones in Scotland; Cranfield University: Scotland, UK, 2014. [Google Scholar]
- Liang, L.; Singer, P.C. Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environ. Sci. Technol. 2003, 37, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; Demarini, D.M. Occurrence, Genotoxicity, and Carcinogenicity of Regulated and Emerging Disinfection By-Products in Drinking Water: A Review and Roadmap for Research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef] [PubMed]
- Dad, A.; Pals, J.; Wagner, E.; Plewa, M.J. Genotoxic Mechanism of Haloacetic Acid Drinking Water Disinfection Byproducts. Environ. Mol. Mutagen. 2011, 52, S69. [Google Scholar]
- Zhang, S.H.; Miao, D.Y.; Liu, A.L.; Zhang, L.; Wei, W.; Xie, H.; Lu, W.Q. Assessment of the cytotoxicity and genotoxicity of haloacetic acids using microplate-based cytotoxicity test and CHO/HGPRT gene mutation assay. Mutat. Res. 2010, 703, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J. Comparative human cell toxicogenomic analysis of monohaloacetic acid drinking water disinfection byproducts. Environ. Sci. Technol. 2010, 44, 7206–7212. [Google Scholar] [CrossRef]
- Jeong, C.H.; Anduri, S.; Richardson, S.D.; Nieuwenhuijsen, M.J.; Kogevinas, M.; Wagner, E.D.; Plewa, M.J. Cytotoxicity and Genotoxicity of Disinfection By-Products in Drinking Water in Europe: HIWATE. Environ. Mol. Mutagen. 2011, 52, S69. [Google Scholar]
- Muellner, M.G.; Attene-Ramos, M.S.; Hudson, M.E.; Wagner, E.D.; Plewa, M.J. Human cell toxicogenomic analysis of bromoacetic acid: A regulated drinking water disinfection by-product. Environ. Mol. Mutagen. 2010, 51, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Dad, A.; Jeong, C.H.; Wagner, E.D.; Plewa, M.J. Haloacetic Acid Water Disinfection Byproducts Affect Pyruvate Dehydrogenase Activity and Disrupt Cellular Metabolism. Environ. Sci. Technol. 2018, 52, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Larson, J.L.; Bull, R.J. Metabolism and Lipoperoxidative Activity of Trichloroacetate and Dichloroacetate in Rats and Mice. Toxicol. Appl. Pharmacol. 1992, 115, 268–277. [Google Scholar] [CrossRef]
- Escobar-Hoyos, L.F.; Hoyos-Giraldo, L.S.; Londono-Velasco, E.; Reyes-Carvajal, I.; Saavedra-Trujillo, D.; Carvajal-Varona, S.; Sanchez-Gomez, A.; Wagner, E.D.; Plewa, M.J. Genotoxic and clastogenic effects of monohaloacetic acid drinking water disinfection by-products in primary human lymphocytes. Water Res. 2013, 47, 3282–3290. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Gao, L.; Dettro, T.; Wagner, E.D.; Ricke, W.A.; Plewa, M.J.; Flaws, J.A. Monohaloacetic acid drinking water disinfection by-products inhibit follicle growth and steroidogenesis in mouse ovarian antral follicles in vitro. Reprod. Toxicol. 2016, 62, 71–76. [Google Scholar] [CrossRef]
- Plewa, M.J.; Simmons, J.E.; Richardson, S.D.; Wagner, E.D. Mammalian Cell Cytotoxicity and Genotoxicity of the Haloacetic Acids, A Major Class of Drinking Water Disinfection By-Products. Environ. Mol. Mutagen. 2010, 51, 871–878. [Google Scholar] [CrossRef]
- Bove, F.; Shim, Y.; Zeitz, P. Drinking Water Contaminants and Adverse Pregnancy Outcomes. Environ. Health Perspect. 2002, 110, 61–74. [Google Scholar] [CrossRef]
- Rivera-Nunez, Z.; Wright, J.M. Association of Brominated Trihalomethane and Haloacetic Acid Exposure with Fetal Growth and Preterm Delivery in Massachusetts. J. Occup. Environ. Med. 2013, 55, 1125–1134. [Google Scholar] [CrossRef]
- Savitz, D.A.; Andrews, K.W.; Pastore, L.M. Drinking Water and Pregnancy Outcome in Central North Carolina: Source, Amount, and Trihalomethane Levels. Environ. Health Prospect. 1995, 103, 592–596. [Google Scholar] [CrossRef]
- Hoffman, C.S.; Mendola, P.; Savitz, D.A.; Herring, A.H.; Loomis, D.; Hartmann, K.E.; Singer, P.C.; Weinberg, H.S.; Olshan, A.F. Drinking Water Disinfection By-Product Exposure and Fetal Growth. Epidemiology 2008, 19, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Savitz, D.A.; Singer, P.C.; Hartmann, K.E.; Herring, A.H.; Weinberg, H.S.; Makarushka, C.; Hoffman, C.; Chan, R.; Maclehose, R. Drinking Water Disinfection By-Products and Pregnancy Outcome; American Water Works Association: Denver, CO, USA, 2005; pp. 1–244. [Google Scholar]
- Horton, B.J.; Luben, T.J.; Herring, A.H.; Savitz, D.A.; Singer, P.C.; Weinberg, H.S.; Hartmann, K.E. The Effect of Water Disinfection By-products on Pregnancy Outcomes in Two Southeastern U.S. Communities. J. Occup. Environ. Med. 2011, 53, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Savitz, D.A.; Singer, P.C.; Herring, A.H.; Hartmann, K.E.; Weinberg, H.S.; Makarushka, C. Exposure to Drinking Water Disinfection By-Products and Pregnancy Loss. Am. J. Epidemiol. 2006, 164, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Frost, K.; Sundararajan, M. Evaluation of Drinking Water Disinfectant Byproducts Compliance Data as an Indirect Measure for Short-Term Exposure in Humans. Int. J. Environ. Res. Public Health 2017, 14, 548. [Google Scholar] [CrossRef] [PubMed]
- Hinckley, A.F.; Bachand, A.M.; Reif, J.S. Late Pregnancy Exposures to Disinfection By-products and Growth-Related Birth Outcomes. Environ. Health Perspect. 2005, 113, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.K.; Putnam, S.D.; Hunting, K.L.; Riddle, M.R. The effect of trihalomethane and haloacetic acid exposure on fetal growth in a Maryland county. Am. J. Epidemiol. 2005, 162, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.S.; Xu, L.; Xie, S.H.; Li, Y.L.; Li, L.; Zeng, Q.; Du, Y.K.; Lu, W.Q. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci. Total Environ. 2012, 416, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Costet, N.; Garlantezec, R.; Monfort, C.; Rouget, F.; Gagniere, B.; Chevrier, C.; Cordier, S. Environmental and Urinary Markers of Prenatal Exposure to Drinking Water Disinfection By-Products, Fetal Growth, and Duration of Gestation in the Pelagie Birth Cohort (Brittany, France, 2002–2006). Am. J. Epidemiol. 2012, 175, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Reckhow, D.A. Comparison of disinfection byproduct formation from chlorine and alternative disinfectants. Water Res. 2007, 41, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Parvez, S.; Rivera-Nunez, Z.; Wright, M. Analysis of Temporal and Spatial Variability in Disinfection By-product Concentrations for Exposure Assessment Applications. Epidemiology 2011, 22, S97. [Google Scholar] [CrossRef]
- Wright, J.M.; Schwartz, J.; Dockery, D.W. The Effect of Disinfection By-products and Mutagenic Activity on Birth Weight and Gestational Duration. Environ. Health Perspect. 2004, 112, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuijsen, M.J.; Toledano, M.B.; Eaton, N.E.; Fawell, J.; Elliott, P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: A review. Occup. Environ. Med. 2000, 57, 73–85. [Google Scholar] [CrossRef] [PubMed]
- King, W.D.; Dodds, L.; Armson, B.A.; Allen, A.C.; Fell, D.B.; Nimrod, C. Exposure Assessment in Epidemiologic Studies of Adverse Pregnancy Outcomes and Disinfection Byproducts. J. Expo. Anal. Environ. Epidemiol. 2004, 14, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Graves, C.G.; Matanoski, G.M.; Tardiff, R.G. Weight of evidence for an association between adverse reproductive and developmental effects and exposure to disinfection by-products: A critical review. Regul. Toxicol. Pharm. 2001, 34, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.; Gallego, M. Alveolar Air and Urine Analysis As Biomarkers of Exposure to Trihalomethanes in an Indoor Swimming Pool. Environ. Sci. Technol. 2008, 42, 5002–5007. [Google Scholar] [CrossRef] [PubMed]
- Cardador, M.J.; Gallego, M. Haloacetic acids in swimming pools: Swimmer and worker exposure. Environ. Sci. Technol. 2011, 45, 5783–5790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gabos, S.; Schopflocher, D.; Li, X.F.; Gati, W.P.; Hrudey, S.E. Validation of urinary trichloroacetic acid as a biomarker of exposure to drinking water disinfection by-products. J. Water Health 2009, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Haltmeier, P.; Klotz, J.B.; Weisel, C.P. Evaluation of Biomarkers of Environmental Exposures Urinary Haloacetic Acids Associated with Ingestion of Chlorinated Drinking Water. Environ. Res. Sect. A 1999, 80, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bader, E.L.; Hrudey, S.E.; Froese, K.L. Urinary excretion half life of trichloroacetic acid as a biomarker of exposure to chlorinated drinking water disinfection by-products. Occup. Environ. Med. 2004, 61, 715–716. [Google Scholar] [CrossRef]
- Plewa, M.J.; Kargalioglu, Y.; Vankerk, D.; Minear, R.A.; Wagner, E.D. Mammalian Cell Cytotoxicity and Genotoxicity Analysis of Drinking Water Disinfection By-Products. Environ. Mol. Mutagen. 2002, 40, 134–142. [Google Scholar] [CrossRef]
- Krasner, S.W. The Formation and Control of Emerging Disinfection By-Products of Health Concern. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 4077–4095. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.D.; Thruston, A.D., Jr.; Rav-Acha, C.; Groisman, L.; Popilevsky, I.; Juraev, O.; Glezer, V.; McKague, A.B.; Plewa, M.J.; Wagner, E.D. Tribromopyrrole, brominated acids, and other disinfection byproducts produced by disinfection of drinking water rich in bromide. Environ. Sci. Technol. 2003, 37, 3782–3793. [Google Scholar] [CrossRef] [PubMed]
- EPA (US Environmental Protection Agency). 1995b Method 552.2: Determination of Haloacetic Acids and Dalapon in Drinking Water by Liquid–Liquid Extraction, Derivatization, and Gas Chromatography with Electron Capture Detection; US EPA: Cincinnati, OH, USA, 1995.
- Zeng, Q.; Wang, Y.X.; Xie, S.H.; Xu, L.; Chen, Y.Z.; Li, M.; Yue, J.; Li, Y.F.; Liu, A.L.; Lu, W.Q. Drinking Water Disinfection By-Products and Semen Quality: A Cross-Sectional Study in China. Environ. Health Perspect. 2014, 122, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Mage, D.T.; Allen, R.H.; Kodali, A. Creatinine corrections for estimating children’s and adult’s pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tang, W.; Lu, Y.; Tu, X. Rank regression: An alternative regression approach for data with outliers. Shanghai Arch. Psychiatry 2014, 26, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med. Res. Methodol. 2005, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable value. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Malliarou, E.; Collins, C.; Graham, N.; Nieuwenhuijsen, M.J. Haloacetic acids in drinking water in the United Kingdom. Water Res. 2005, 39, 2722–2730. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Caudill, S.P.; Ashley, D.L. Urinary Levels of Trichloroacetic Acid, a Disinfection By-Product in Chlorinated Drinking Water, in a Human Reference Population. Environ. Health Perspect. 2002, 111, 151–154. [Google Scholar] [CrossRef]
- Reimann, S.; Grob, K.; Frank, H. Environmental chloroacetic acids in foods analyzed by GC-ECD. Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene 1996, 87, 212–222. [Google Scholar]
- WHO (World Health Organization). Monochloroacetic Acid in Drinking-Water; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Yllner, S. Metabolism of 1,1,2-trichloroethane-1,2–14C in the mouse. Acta Pharmacol. Toxicol. 1971, 30, 248–256. [Google Scholar] [CrossRef]
- Saghir, S.A.; Rozman, K.K. Kinetics of monochloroacetic acid at subtoxic and toxic doses in rats after single oral and dermal administrations. Toxicol. Sci. 2003, 76, 51–64. [Google Scholar] [CrossRef] [PubMed]
- James, M.O.; Yan, Z.; Cornett, R.; Jayanti, V.M.K.M.; Henderson, G.N.; Davydova, N.; Katovich, M.J.; Pollock, B.; Stacpoole, P.W. Pharmacokinetics and metabolism of [14C] dichloroacetate in male Sprague-Dawley rats. Identification of glycine conjugates, including hippurate, as urinary metabolites of dichloroacetate. Drug Metab. Dispos. 1998, 26, 1134–1143. [Google Scholar] [PubMed]
- Lin, E.L.C.; Mattox, J.K.; Daniel, F.B. Tissue distribution excretion and urinary metabolites of dichloroacetic acid in the male Fisher 344 rat. J. Toxicol. Environ. Health 1993, 38, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.H.; Lorenz, A.; Chu, P.-I.; Limacher, M.; Stacpoole, P.W. Disposition and pharmacodynamics of dichloroacetate (DCA) and oxalate following oral DCA doses. Biopharm. Drug Dispos. 1991, 12, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Schultz, I.R.; Merdink, J.L.; Gonzalez-Leon, A.; Bull, R.J. Comparative toxicokinetics of chlorinated and brominated haloacetates in F344 rats. Toxicol. Appl. Pharmacol. 1999, 158, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Board, P.G.; Anders, M.W. Glutathione transferase Zeta catalyses the oxygenation of the carcinogen dichloroacetic acid to glyoxylic acid. Biochem. J. 1998, 331, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Weisel, C.P.; Kim, H.; Haltmeier, P.; Klotz, J.B. Exposure Estimates to Disinfection By-Products of Chlorinated Drinking Water. Environ. Health Prospect. 1999, 107, 103–110. [Google Scholar] [CrossRef]
- Xu, X.; Weisel, C.P. Inhalation Exposure to Haloacetic Acids and Haloketones During Showering. Environ. Sci. Technol. 2003, 37, 569–576. [Google Scholar] [CrossRef]
- Xu, X.; Weisel, C.P. Dermal uptake of chloroform and haloketones during bathing. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 289–296. [Google Scholar] [CrossRef]
- Xu, X.; Weisel, C.P. Human respiratory uptake of chloroform and haloketones during showering. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, G.G.; Dubinina, O.N. Materials of experimental substantiation of maximally permissible concentration of monochloroacetic acid in the air of production area. Gig. Tr. Prof. Zabol. 1974, 9, 32–35. [Google Scholar]
- Kulling, P.; Anderson, H.; Bostrom, K.; Johansson, L.; Lindstrom, B.; Nystrom, B. Fatal systemic poisoning after skin exposure to monochloroacetic acid. Clin. Toxicol. 1992, 30, 643–652. [Google Scholar]
- Kusch, G.D.; McCarty, L.P.; Lanham, J.M. Monochloroacetic acid exposure: A case report. Pol. J. Occup. Med. 1990, 3, 409–414. [Google Scholar] [PubMed]
- Millischer, R.J.; Jouglard, J.; Vincenti, M.; Ruty, J.; Contassot, J.C. Monochloroacetic acid: Seven worldwide cases of systemic poisoning resulting from accidental skin contact. In Proceedings of the International Congress on Occupational Health, Sydney, Australia, 23 April 1987. [Google Scholar]
- Arbuckle, T.E.; Hrudey, S.E.; Krasner, S.W.; Nuckols, J.R.; Richardson, S.D.; Singer, P.; Mendola, P.; Dodds, L.; Weisel, C.; Ashley, D.L.; et al. Assessing exposure in epidemiologic studies to disinfection by-products in drinking water: Report from an international workshop. Environ. Health Perspect. 2002, 110, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Froese, K.L.; Sinclair, M.I.; Hrudey, S.E. Trichloroacetic Acid as a Biomarker of Exposure to Disinfection By-Products in Drinking Water: A Human Exposure Trial in Adelaide, Australia. Environ. Health Perspect. 2002, 110, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.B.; Nieuwenhuijsen, M.J.; Wright, J.; Raynor, P.; Cocker, J.; Jones, K.; Kostopoulou-Karadanelli, M.; Toledano, M.B. Validation of trichloroacetic acid exposure via drinking water during pregnancy using a urinary TCAA biomarker. Environ. Res. 2013, 126, 145–151. [Google Scholar] [CrossRef]
- Rivera-Nunez, Z.; Wright, J.M.; Blount, B.C.; Silva, L.K.; Jones, E.; Chan, R.L.; Pegram, R.A.; Singer, P.C.; Savitz, D.A. Comparison of Trihalomethanes in Tap Water and Blood: A Case Study in the United States. Environ. Health Prospect. 2012, 120, 661–667. [Google Scholar] [CrossRef]
- Symanski, E.; Savitz, D.A.; Singer, P.C. Assessing spatial fluctuations, temporal variability, and measurement error in estimated levels of disinfection by-products in tap water: Implications for exposure assessment. Occup. Environ. Med. 2004, 61, 65–72. [Google Scholar]

| Site | Number of Participants | Specimen Type | Specimens | |
|---|---|---|---|---|
| Longitudinal | Cross-Sectional | |||
| Site-A | 7 | UR | 11 | 18 |
| DW | 7 | |||
| Site-B | 10 | UR | 10 | |
| DW | 10 | |||
| HAAs | N (%) | Q1 | Q2 | Q3 | Q4 | IQR | Mean | SD | Min | Max | CV | <LOD (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCAA | 20 (100) | 1.0 | 1.0 | 4.6 | 10.7 | 3.6 | 3.2 | 3.1 | 1.0 | 12.6 | 0.97 | 11 (56) |
| Site A | 10 (50) | 1.0 | 1.0 | 4.3 | 6.3 | 3.3 | 2.6 | 2.1 | 1.0 | 6.3 | 0.8 | 6 (59) |
| Site B | 10 (50) | 1.0 | 1.7 | 4.7 | 10.7 | 3.7 | 3.7 | 3.8 | 1.0 | 10.7 | 1.0 | 5 (50) |
| TCAA | 20 (100) | 1.6 | 2.5 | 3.9 | 7.6 | 2.3 | 2.9 | 2.0 | 0.5 | 7.6 | 0.7 | 3 (11) |
| Site A | 10 (50) | 2.2 | 2.5 | 3.5 | 7.0 | 1.3 | 3.1 | 1.6 | 1.6 | 7.0 | 0.5 | 0 (0) |
| Site B | 10 (50) | 0.6 | 2.3 | 3.9 | 7.6 | 3.2 | 2.7 | 2.3 | 0.5 | 7.6 | 0.9 | 3 (30) |
| HAAs | N (%) | Q1 | Q2 | Q3 | Q4 | IQR | Mean | SD | Min | Max | CV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DCAA | 17 (100) | 14.8 | 17.4 | 18.8 | 20.9 | 4.0 | 15.8 | 4.9 | 2.3 | 20.9 | 0.3 |
| Site A | 7 (41) | 9.4 | 14.8 | 16.1 | 17.4 | 6.7 | 12.2 | 5.8 | 2.3 | 17.4 | 0.5 |
| Site B | 10 (59) | 17.5 | 18.7 | 19.4 | 20.9 | 1.9 | 18.4 | 1.8 | 14.8 | 20.9 | 0.1 |
| TCAA | 17 (100) | 6.5 | 7.4 | 7.9 | 11.0 | 1.4 | 6.6 | 3.4 | 0.5 | 11.0 | 0.5 |
| Site A | 7 (41) | 0.9 | 2.9 | 10.7 | 11.0 | 9.8 | 5.4 | 5.1 | 0.5 | 11.0 | 1.0 |
| Site B | 10 (59) | 7.1 | 7.5 | 7.6 | 9.6 | 0.4 | 7.5 | 0.9 | 6.5 | 9.6 | 0.1 |
| DBAA | 17 (100) | 0.5 | 0.5 | 0.5 | 1.8 | 0.0 | 0.6 | 0.4 | 0.5 | 1.8 | 0.6 |
| Site A | 7 (41) | 0.5 | 0.5 | 1.1 | 1.8 | 0.6 | 0.8 | 0.6 | 0.5 | 1.8 | 0.7 |
| Site B | 10 (59) | 0.5 | 0.5 | 0.5 | 0.5 | 0.0 | 0.5 | 0.0 | 0.5 | 0.5 | 0.0 |
| BCAA | 17 (100) | 3.7 | 4.4 | 4.7 | 4.9 | 1.0 | 4.2 | 0.7 | 2.2 | 4.9 | 0.2 |
| Site A | 7 (41) | 3.5 | 3.6 | 4.3 | 4.7 | 0.8 | 3.7 | 0.9 | 2.2 | 4.7 | 0.2 |
| Site B | 10 (59) | 4.4 | 4.5 | 4.7 | 4.9 | 0.3 | 4.5 | 0.3 | 3.7 | 4.9 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvez, S.; Ashby, J.L.; Kimura, S.Y.; Richardson, S.D. Exposure Characterization of Haloacetic Acids in Humans for Exposure and Risk Assessment Applications: An Exploratory Study. Int. J. Environ. Res. Public Health 2019, 16, 471. https://doi.org/10.3390/ijerph16030471
Parvez S, Ashby JL, Kimura SY, Richardson SD. Exposure Characterization of Haloacetic Acids in Humans for Exposure and Risk Assessment Applications: An Exploratory Study. International Journal of Environmental Research and Public Health. 2019; 16(3):471. https://doi.org/10.3390/ijerph16030471
Chicago/Turabian StyleParvez, Shahid, Jeffrey L. Ashby, Susana Y. Kimura, and Susan D. Richardson. 2019. "Exposure Characterization of Haloacetic Acids in Humans for Exposure and Risk Assessment Applications: An Exploratory Study" International Journal of Environmental Research and Public Health 16, no. 3: 471. https://doi.org/10.3390/ijerph16030471
APA StyleParvez, S., Ashby, J. L., Kimura, S. Y., & Richardson, S. D. (2019). Exposure Characterization of Haloacetic Acids in Humans for Exposure and Risk Assessment Applications: An Exploratory Study. International Journal of Environmental Research and Public Health, 16(3), 471. https://doi.org/10.3390/ijerph16030471





