Determinants of Erythrocyte Lead Levels in 454 Adults in Florence, Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Data Collection
2.3. Analytical Determzination of Lead
2.4. Reconstruction of Participants’ Residential and Occupational History
2.5. Statistical Methods
2.6. Ethical Aspects and Informed Consent
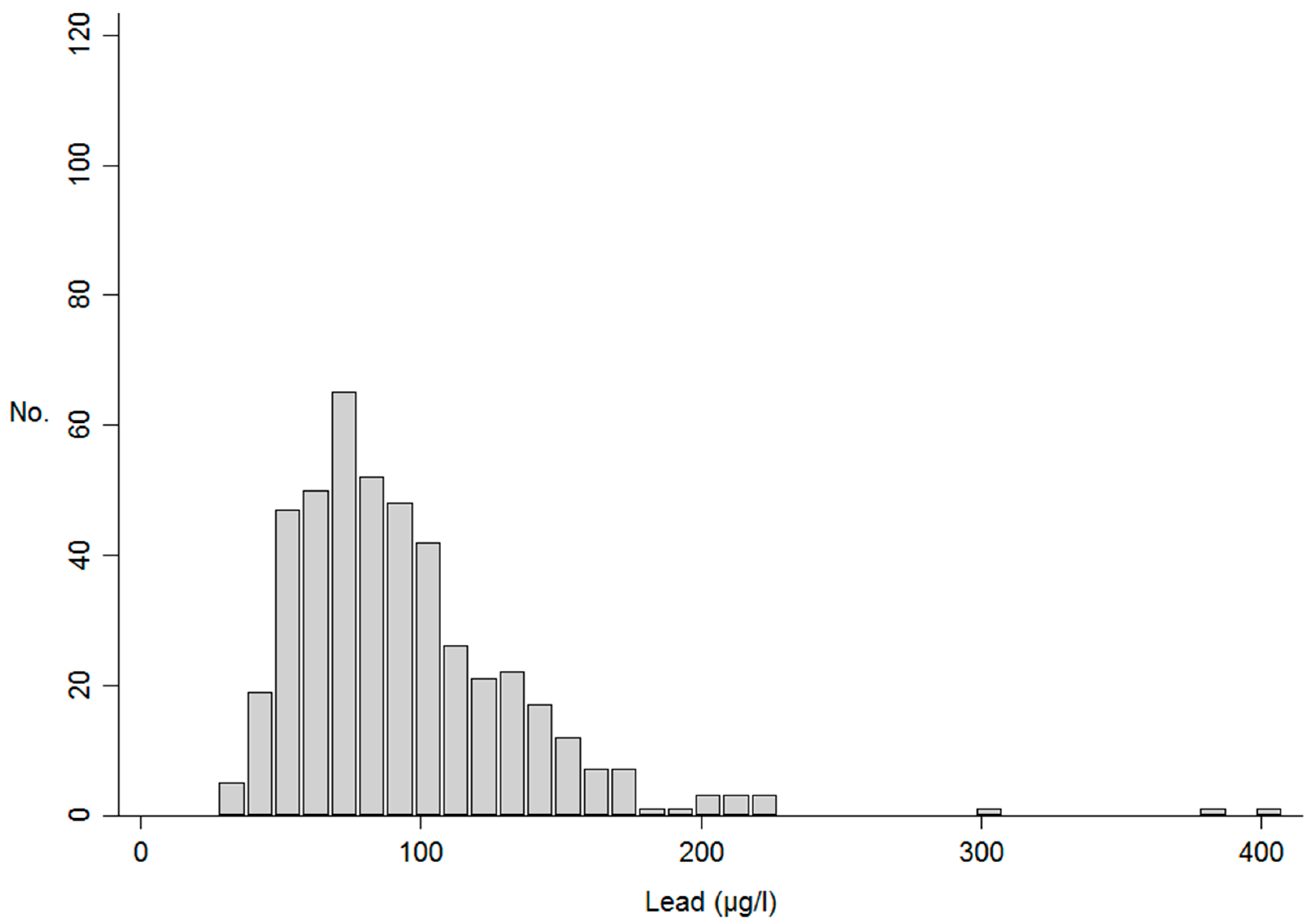
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Jang, T.W.; Chae, H.J.; Choi, W.J.; Ha, M.N.; Ye, B.J.; Kim, B.G.; Jeon, M.J.; Kim, S.Y.; Hong, Y.S. Evaluation and management of lead exposure. Ann. Occup. Environ. Med. 2015, 27, 30. [Google Scholar] [CrossRef] [PubMed]
- Ekong, E.B.; Jaar, B.G.; Weaver, V.M. Lead-related nephrotoxicity: A review of the epidemiologic evidence. Kidney Int. 2006, 70, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borné, Y.; Engström, G.; Barregard, L. Blood lead levels and decreased kidney function in a population-based cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program (NTP), U.S. Department of Health and Human Services. NTP Monograph on Health Effects of Low-Level Lead. Available online: https://ntp.niehs.nih.gov/ntp/ohat/lead/final/monographhealtheffectslowlevellead_newissn_508.pdf (accessed on 4 August 2018).
- Obeng-Gyasi Armijos, R.X.; Weigel, M.M.; Filippelli, G.; Sayegh, M.A. Hepatobiliary-related outcomes in US adults exposed to lead. Environments 2018, 5, 46. [Google Scholar] [CrossRef]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Lead exposure and oxidative stress—A life course approach in U.S. adults. Toxics 2018, 6, 42. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Caricnogenic Risks to Humans. Volume 87. Available online: https://monographs.iarc.fr/ENG/Monographs/vol87/mono87.pdf (accessed on 5 June 2018).
- Riboli, E.; Kaaks, R. The EPIC Project: Rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J. Epidemiol 1997, 26 (Suppl. 1), S6–S14. [Google Scholar] [CrossRef]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.R.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef]
- Palli, D.; Berrino, F.; Vineis, P.; Tumino, R.; Panico, S.; Masala, G.; Saieva, C.; Salvini, S.; Ceroti, M.; Pala, V.; et al. A molecular epidemiology project on diet and cancer: The EPIC-Italy Prospective Study. Design and baseline characteristics of participants. Tumori J. 2003, 89, 586–593. [Google Scholar] [CrossRef]
- Kelly, R.S.; Lundh, T.; Porta, M.; Bergdahl, I.A.; Palli, D.; Johansson, A.S.; Botsivali, M.; Vineis, P.; Vermeulen, R.; Kyrtopoulos, S.A.; et al. Blood erythrocyte concentrations of cadmium and lead and the risk of B-cell non-Hodgkin’s lymphoma and multiple myeloma: A nested case-control study. PLoS ONE 2013, 8, e81892. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Shin, J.H.; Han, H.S.; Chung, J.H. In vivo effects of lead on erythrocytes following chronic exposure through drinking water. Arch. Pharm. Res. 2006, 29, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Barany, E.; Bergdahl, I.A.; Schütz, A.; Skerfving, S.; Oskarsson, A. Inductively coupled plasma mass spectrometry for direct multielement analysis of diluted human blood and serum. J. Anal. At. Spectrom. 1997, 12, 1005–1009. [Google Scholar] [CrossRef]
- International Labour Organization (ILO). International Standard Classification of Occupations (ISCO), Version ISCO-68. Available online: http://www.ilo.org/public/english/bureau/stat/isco/ (accessed on 3 June 2018).
- Cornell University—Cornell Statistical Consulting Unit. StatNews#83: Interpreting Coefficients in Regression with Log-Transformed Variables. June 2012. Available online: https://www.cscu.cornell.edu/news/statnews/stnews83.pdf (accessed on 3 January 2019).
- Forte, G.; Madeddu, R.; Tolu, P.; Asara, Y.; Marchal, J.A.; Bocca, B. Reference intervals for blood Cd and Pb in the general population of Sardinia (Italy). Int. J. Hyg. Environ. Health 2011, 214, 102–109. [Google Scholar] [CrossRef]
- Sakellari, A.; Karavoltsos, S.; Kalogeropoulos, N.; Theodorou, D.; Dedoussis, G.; Chrysohoou, C.; Dassenakis, M.; Scoullos, M. Predictors of cadmium and lead concentrations in the blood of residents from the metropolitan area of Athens (Greece). Sci. Total Environ. 2016, 568, 263–270. [Google Scholar] [CrossRef]
- Fløtre, C.H.; Varsi, K.; Helm, T.; Bolann, B.; Bjørke-Monsen, A.L. Predictors of mercury, lead, cadmium and antimony status in Norwegian never-pregnant women of fertile age. PLoS ONE 2017, 12, e0189169. [Google Scholar] [CrossRef]
- Tsoi, M.F.; Cheung, C.L.; Cheung, T.T.; Cheung, B.M. Continual decrease in blood lead level in Americans: United States National Health Nutrition and Examination Survey 1999–2014. Am. J. Med. 2016, 129, 1213–1218. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Nie, X.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; et al. Blood lead level and its association with body mass index and obesity in China—Results from SPECT-China study. Sci. Rep. 2015, 5, 18299. [Google Scholar] [CrossRef]
- Scinicariello, F.; Buser, M.C.; Mevissen, M.; Portier, C.J. Blood lead level association with lower body weight in NHANES 1999–2006. Toxicol. Appl. Pharmacol. 2013, 273, 516–523. [Google Scholar] [CrossRef]
- Richmond-Bryant, J.; Meng, Q.; Davis, J.A.; Cohen, J.; Svendsgaard, D.; Brown, J.S.; Tuttle, L.; Hubbard, H.; Rice, J.; Kirrane, E.; et al. A multi-level model of blood lead as a function of air lead. Sci. Total Environ. 2013, 461-462, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Pawlas, N.; Strömberg, U.; Carlberg, B.; Cerna, M.; Harari, F.; Harari, R.; Horvat, M.; Hruba, F.; Koppova, K.; Krskova, A.; et al. Cadmium, mercury and lead in the blood of urban women in Croatia, the Czech Republic, Poland, Slovakia, Slovenia, Sweden, China, Ecuador and Morocco. Int. J. Occup. Med. Environ. Health 2013, 26, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Dix-Cooper, L.; Kosatsky, T. Blood mercury, lead and cadmium levels and determinants of exposure among newcomer South and East Asian women of reproductive age living in Vancouver, Canada. Sci. Total Environ. 2018, 619, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, A.; Brodowski, J.; Grochans, E.; Karakiewicz, B.; Szkup-Jabłońska, M.; Wieder-Huszla, S.; Mroczek, B.; Włoszczak-Szubzda, A.; Grzywacz, A. Effect of menopausal hormone therapy on the levels of magnesium, zinc, lead and cadmium in post-menopausal women. Ann. Agric. Environ. Med. 2013, 20, 147–151. [Google Scholar] [PubMed]
- Eum, K.D.; Weisskopf, M.G.; Nie, L.H.; Hu, H.; Korrick, S.A. Cumulative lead exposure and age at menopause in the Nurses‘ Health Study cohort. Environ. Health Perspect. 2014, 122, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, Y. Sex-specific profiles of blood metal levels associated with metal-iron interactions. Saf. Health Work 2014, 5, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.; Anderson, G.J.; Collins, J.F. Mechanistic and regulatory aspects of intestinal iron absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G397–G409. [Google Scholar] [CrossRef] [PubMed]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, B.K. Iron deficiency increases blood manganese level in the Korean general population according to KNHANES 2008. Neurotoxicology 2011, 32, 247–254. [Google Scholar] [CrossRef]
- Suh, Y.J.; Lee, J.E.; Lee, D.H.; Yi, H.G.; Lee, M.H.; Kim, C.S.; Nah, J.W.; Kim, S.K. Prevalence and relationships of iron deficiency anemia with blood cadmium and vitamin D levels in Korean women. J. Korean Med. Sci. 2016, 31, 25–32. [Google Scholar] [CrossRef]
- Oulhote, Y.; Mergler, D.; Bouchard, M.F. Sex- and age-differences in blood manganese levels in the U.S. general population: National health and nutrition examination survey 2011–2012. Environ. Health 2014, 13, 87. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, Y.; Kim, N.S.; Lee, B.K. Gender difference in blood cadmium concentration in the general population: Can it be explained by iron deficiency? J. Trace Elem. Med. Biol. 2014, 28, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Kim, Y. Effects of menopause on blood manganese levels in women: Analysis of 2008–2009 Korean National Health and Nutrition Examination Survey data. Neurotoxicology 2012, 33, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Gunier, R.B.; Horn-Ross, P.L.; Canchola, A.J.; Duffy, C.N.; Reynolds, P.; Hertz, A.; Garcia, E.; Rull, R.P. Determinants and within-person variability of urinary cadmium concentrations among women in northern California. Environ. Health Perspect. 2013, 121, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Bendinelli, B.; Masala, G.; Saieva, C.; Lundh, T.; Kyrtopoulos, S.A.; Palli, D. Predictors of erythrocyte cadmium levels in 454 adults in Florence, Italy. Sci. Total Environ. 2018, 644, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bressler, J.P.; Olivi, L.; Cheong, J.H.; Kim, Y.; Bannona, D. Divalent metal transporter 1 in lead and cadmium transport. Ann. N. Y. Acad. Sci. 2004, 1012, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kayaaltı, Z.; Akyüzlü, D.K.; Söylemezoğlu, T. Evaluation of the effect of divalent metal transporter 1 gene polymorphism on blood iron, lead and cadmium levels. Environ. Res. 2015, 137, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Lin-Tan, D.T.; Wang, M.L.; Huang, H.Y.; Lee, C.L.; Wang, H.S.; Soong, Y.K.; Lin, J.L. Lead level in seminal plasma may affect semen quality for men without occupational exposure to lead. Reprod. Biol. Endocrinol. 2012, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.P.; Kolstad, H. Fertility of Danish battery workers exposed to lead. Int. J. Epidemiol. 1997, 26, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Joffe, M.; Bisanti, L.; Apostoli, P.; Kiss, P.; Dale, A.; Roeleveld, N.; Lindbohm, M.L.; Sallmén, M.; Vanhoorne, M.; Bonde, J.P. Time to pregnancy and occupational lead exposure. Occup. Environ. Med. 2003, 60, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Kaplowitz, S.A.; Perlstadt, H.; Dziura, J.D.; Post, L.A. Behavioral and environmental explanations of elevated blood lead levels in immigrant children and children of immigrants. J. Immigr. Minor. Health 2016, 18, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; LeTertre, A.; Etchevers, A.; Le Bot, B.; Lucas, J.P.; Mandin, C.; Le Strat, Y.; Lanphear, B.; Glorennec, P. Implications of different residential lead standards on children’s blood lead levels in France: Predictions based on a national cross-sectional survey. Int. J. Hyg. Environ. Health 2013, 216, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Spanier, A.J.; Wilson, S.; Ho, M.; Hornung, R.; Lanphear, B.P. The contribution of housing renovation to chidren’s blood lead levels: A cohort study. Environ. Health 2013, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E. Low level environmental lead exposure—A continuing challenge. Clin. Biochem. Rev. 2008, 29, 63–70. [Google Scholar] [PubMed]
- Pain, D.J.; Cromie, R.L.; Newth, J.; Brown, M.J.; Crutcher, E.; Hardman, P.; Hurst, L.; Mateo, R.; Meharg, A.A.; Moran, A.C.; et al. Potential hazard to human health from exposure to fragments of lead bullets and shot in the tissues of game animals. PLoS ONE 2010, 5, e10315. [Google Scholar] [CrossRef] [PubMed]
- Ertl, K.; Kitzer, R.; Goessler, W. Elemental composition of game meat from Austria. Food Addit. Contam. Part B Surveill. 2016, 9, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Blumenthal, W.; Kennedy, C.; Yip, F.Y.; Pickard, S.; Flanders, W.D.; Loringer, K.; Kruger, K.; Caldwell, K.L.; Brown, M.J. Hunting with lead: Association between blood lead levels and wild game consumption. Environ. Res. 2009, 109, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, A.; Sallsten, G.; Borné, Y.; Forsgard, N.; Hedblad, B.; Nilsson, P.; Fagerberg, B.; Engström, G.; Barregard, L. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ. Res. 2016, 149, 157–163. [Google Scholar] [CrossRef] [PubMed]
| Participants’ Characteristics | No. | % | Lead (μg/L) | |
|---|---|---|---|---|
| Median (IQR) | p-Value (a) | |||
| Total | 454 | 100.0% | 86.07 (65.53–111.93) | - |
| Gender | ||||
| Male | 26 | 5.7% | 104.45 (85.09–137.54) | |
| Female | 428 | 94.3% | 84.07 (64.05–110.57) | 0.002 |
| Age | ||||
| <45 years | 107 | 23.6% | 69.20 (55.61–82.46) | |
| 45–55 years | 164 | 36.1% | 88.63 (66.70–111.38) | |
| >55 years | 183 | 40.3% | 94.96 (74.54–123.18) | <0.001 |
| Education level | ||||
| None/primary school | 129 | 28.5% | 94.42 (71.04–116.50) | |
| Technical/professional school | 83 | 18.3% | 87.30 (62.24–110.73) | |
| Secondary school | 174 | 38.4% | 80.60 (62.23–113.58) | |
| University or higher degree | 67 | 14.8% | 75.05 (61.89–103.94) | 0.003 |
| Work condition | ||||
| Housewife | 114 | 25.1% | 92.80 (65.10–119.70) | |
| Retired | 75 | 16.5% | 92.37 (73.09–118.39) | |
| Unemployed | 7 | 1.5% | 100.55 (68.48–216.21) | |
| Professional, technical and related | 78 | 17.2% | 75.19 (63.04–105.54) | |
| Administrative, manager, clerical | 101 | 22.2% | 75.49 (59.19–95.11) | |
| Sales workers | 29 | 6.4% | 99.23 (75.45–122.80) | |
| Service workers | 17 | 3.7% | 80.34 (69.91–133.16) | |
| Production, transport, labourers | 33 | 7.3% | 90.42 (73.63–134.20) | 0.011 |
| Smoking habits | ||||
| Never smoker | 209 | 46.0% | 80.34 (62.23–104.73) | |
| Former smoker | 118 | 26.0% | 87.89 (68.87–115.63) | 0.025 |
| Current smoker | 127 | 28.0% | 92.88 (70.87–128.58) | 0.004 |
| Pack years | ||||
| Former smoker, 1st tertile | 40 | 34.8% | 81.72 (58.35–96.30) | |
| 2nd tertile | 37 | 32.2% | 82.96 (68.34–112.48) | |
| 3rd tertile | 38 | 33.0% | 108.08 (78.80–139.11) | 0.003 |
| Current smoker, 1st tertile | 42 | 33.9% | 73.17 (62.70–93.26) | |
| 2nd tertile | 41 | 33.1% | 100.35 (70.87–134.33) | |
| 3rd tertile | 41 | 33.1% | 101.15 (82.72–139.58) | 0.001 |
| Body mass index | ||||
| <25 | 248 | 54.9% | 80.43 (62.06–105.39) | |
| 25–30 | 161 | 35.6% | 93.26 (70.95–124.96) | |
| >30 | 43 | 9.5% | 80.34 (60.36–102.21) | 0.092 |
| Living in Florence (anytime during the study period) | ||||
| Yes | 329 | 72.5% | 91.25 (68.34–115.63) | |
| No | 125 | 27.5% | 73.96 (59.47–95.16) | 0.001 |
| Working in Florence (anytime during the study period) | ||||
| Yes | 170 | 76.6% | 80.50 (63.47–107.65) | |
| No | 52 | 23.4% | 70.99 (55.89–89.52) | 0.024 |
| Driving to workplace | ||||
| No | 102 | 43.6% | 82.43 (66.60–105.54) | |
| Yes | 132 | 56.4% | 73.97 (61.01–100.06) | 0.334 |
| By train to workplace | ||||
| No | 221 | 94.4% | 80.40 (63.04–103.46) | |
| Yes | 13 | 5.6% | 56.68 (48.21–78.03) | 0.010 |
| Walking to workplace | ||||
| No | 175 | 74.8% | 75.49 (59.24–103.90) | |
| Yes | 59 | 25.2% | 81.45 (72.01–101.49) | 0.176 |
| Women (n = 428) Age at menarche | ||||
| ≤12 years | 223 | 52.2% | 80.25 (60.77–110.71) | |
| ≥13 years | 204 | 47.8% | 89.47 (68.49–109.69) | 0.071 |
| Menopausal status | ||||
| Pre- or peri-menopausal | 196 | 45.8% | 71.07 (55.76–91.14) | |
| Post-menopausal | 232 | 54.2% | 97.66 (77.23–126.49) | <0.001 |
| Full-term pregnancies | ||||
| None | 20 | 4.7% | 79.14 (59.39–102.30) | |
| 1 | 138 | 32.2% | 80.92 (62.29–109.18) | |
| 2 | 179 | 41.8% | 82.47 (63.46–110.73) | |
| ≥3 | 91 | 21.3% | 93.03 (68.10–113.25) | 0.132 |
| Breastfeeding | ||||
| Never | 334 | 81.9% | 82.59 (63.04–107.65) | |
| Ever | 74 | 18.1% | 93.10 (72.15–113.58) | 0.064 |
| Oral contraceptives | ||||
| Never | 230 | 53.7% | 86.05 (68.26–106.64) | |
| Ever | 198 | 46.3% | 80.60 (61.89–112.00) | 0.355 |
| Hormones for menopause | ||||
| Never | 175 | 75.4% | 99.64 (78.01–130.59) | |
| Ever | 57 | 24.6% | 93.03 (76.41–112.13) | 0.128 |
| Selected Foods and Food Groups | Lead (μg/L), Median Values (IQR) | |||
|---|---|---|---|---|
| 1st Tertile | 2nd Tertile | 3rd Tertile | p-Value (a) | |
| Vegetables | 91.12 (72.44–118.46) | 82.72 (62.23–108.33) | 80.60 (62.24–109.44) | 0.021 |
| Olive oil | 90.45 (69.46–113.46) | 81.45 (64.65–110.39) | 84.77 (63.55–110.73) | 0.306 |
| Fruit | 90.10 (67.06–118.14) | 83.68 (65.42–108.76) | 81.35 (65.02–110.44) | 0.172 |
| Legumes | 85.55 (66.80–120.21) | 89.79 (69.73–112.35) | 81.45 (62.23-106.38) | 0.095 |
| Pasta and rice | 88.63 (69.52–106.71) | 87.60 (63.55–125.36) | 79.92 (62.70–106.73) | 0.173 |
| Mushrooms | 86.69 (69.20–113.25) | 87.54 (66.80–116.36) | 82.24 (60.36–101.35) | 0.204 |
| Milk and dairy products | 91.28 (71.07–123.91) | 81.32 (62.64–107.65) | 83.04 (63.83–107.05) | 0.016 |
| White meat | 89.68 (68.78–113.27) | 82.85 (66.23–109.52) | 86.28 (61.88–111.66) | 0.247 |
| Red meat | 78.46 (62.06–108.89) | 87.91 (67.71–112.00) | 88.84 (66.80–116.50) | 0.168 |
| Processed meat | 88.61 (65.97–110.67) | 85.09 (66.60–112.48) | 83.23 (63.55–111.66) | 0.652 |
| Fish | 88.84 (70.63–113.58) | 88.78 (68.41–111.19) | 78.13 (60.77–110.71) | 0.038 |
| Crustaceans and molluscs | 84.95 (68.87–112.35) | 86.06 (65.23–109.27) | 86.09 (61.23–113.25) | 0.489 |
| Alcohol | 76.28 (58.71–101.49) | 80.40 (63.46–103.90) | 100.35 (78.13–135.85) | <0.001 |
| Energy intake | 88.61 (68.67–110.67) | 86.14 (68.01–121.46) | 81.97 (61.67–110.73) | 0.271 |
| Participants’ Characteristics | Erythrocyte Lead Levels (μg/L) | ||
|---|---|---|---|
| Percent Change | 95% CI | p-Value (for Trend) | |
| All study sample (n = 454) | |||
| Gender | |||
| Female | ref | ||
| Male | 22.4% | (5.4%, 42.0%) | 0.008 |
| Age | |||
| <45 years | ref | ||
| 45–55 years | 20.5% | (10.3%, 31.6%) | |
| >55 years | 31.8% | (19.1%, 45.8%) | <0.001 |
| Smoking habits | |||
| Never smoker | ref | ||
| Former smoker, 1st tertile PY | 2.7% | (−8.8%, 15.6%) | |
| 2nd tertile PY | 7.3% | (−5.0%, 21.1%) | |
| 3rd tertile PY | 22.2% | (7.8%, 38.5%) | 0.006 |
| Current smoker, 1st tertile PY | −1.9% | (−12.5%, 10.0%) | |
| 2nd tertile PY | 21.8% | (8.5%, 36.6%) | |
| 3rd tertile PY | 23.0% | (9.5%, 38.1%) | <0.001 |
| Alcohol intake | |||
| 1st tertile | ref | ||
| 2nd tertile | 12.2% | (3.7%, 21.4%) | |
| 3rd tertile | 40.8% | (29.8%, 52.7%) | <0.001 |
| Living in Florence during the study period | |||
| Ever | ref | ||
| Never | −8.7% | (−15.3%, −1.5%) | 0.018 |
| Work condition | |||
| Housewife | ref | ||
| Retired | −10.4% | (−19.2%, −0.6%) | 0.039 |
| Unemployed | 22.1% | (−6.0%, 58.6%) | 0.135 |
| Professional, technical and related | −13.0% | (−21.4%, −3.7%) | 0.007 |
| Administrative, manager, clerical | −11.9% | (−19.9%, −3.1%) | 0.009 |
| Sales workers | 1.7% | (−12.1%, 17.6%) | 0.819 |
| Service workers | 6.5% | (−10.6%, 26.9%) | 0.480 |
| Production, transport, labourers | −1.4% | (−14.3%, 13.4%) | 0.846 |
| Women (n = 428) | |||
| Menopausal status | |||
| Pre- or peri-menopausal | ref | ||
| Post-menopausal | 27.1% | (16.2%, 40.5%) | <0.001 |
| Use of hormones for menopause | |||
| Never | ref | ||
| Ever | −13.9% | (−21.3%, −4.9%) | 0.004 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caini, S.; Bendinelli, B.; Masala, G.; Saieva, C.; Assedi, M.; Querci, A.; Lundh, T.; Kyrtopoulos, S.A.; Palli, D. Determinants of Erythrocyte Lead Levels in 454 Adults in Florence, Italy. Int. J. Environ. Res. Public Health 2019, 16, 425. https://doi.org/10.3390/ijerph16030425
Caini S, Bendinelli B, Masala G, Saieva C, Assedi M, Querci A, Lundh T, Kyrtopoulos SA, Palli D. Determinants of Erythrocyte Lead Levels in 454 Adults in Florence, Italy. International Journal of Environmental Research and Public Health. 2019; 16(3):425. https://doi.org/10.3390/ijerph16030425
Chicago/Turabian StyleCaini, Saverio, Benedetta Bendinelli, Giovanna Masala, Calogero Saieva, Melania Assedi, Andrea Querci, Thomas Lundh, Soterios A. Kyrtopoulos, and Domenico Palli. 2019. "Determinants of Erythrocyte Lead Levels in 454 Adults in Florence, Italy" International Journal of Environmental Research and Public Health 16, no. 3: 425. https://doi.org/10.3390/ijerph16030425
APA StyleCaini, S., Bendinelli, B., Masala, G., Saieva, C., Assedi, M., Querci, A., Lundh, T., Kyrtopoulos, S. A., & Palli, D. (2019). Determinants of Erythrocyte Lead Levels in 454 Adults in Florence, Italy. International Journal of Environmental Research and Public Health, 16(3), 425. https://doi.org/10.3390/ijerph16030425







