New Polymer Inclusion Membrane Containing β-Cyclodextrin Polymer: Application for Pharmaceutical Pollutant Removal from Waste Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Insoluble Β-Cyclodextrins Polymer
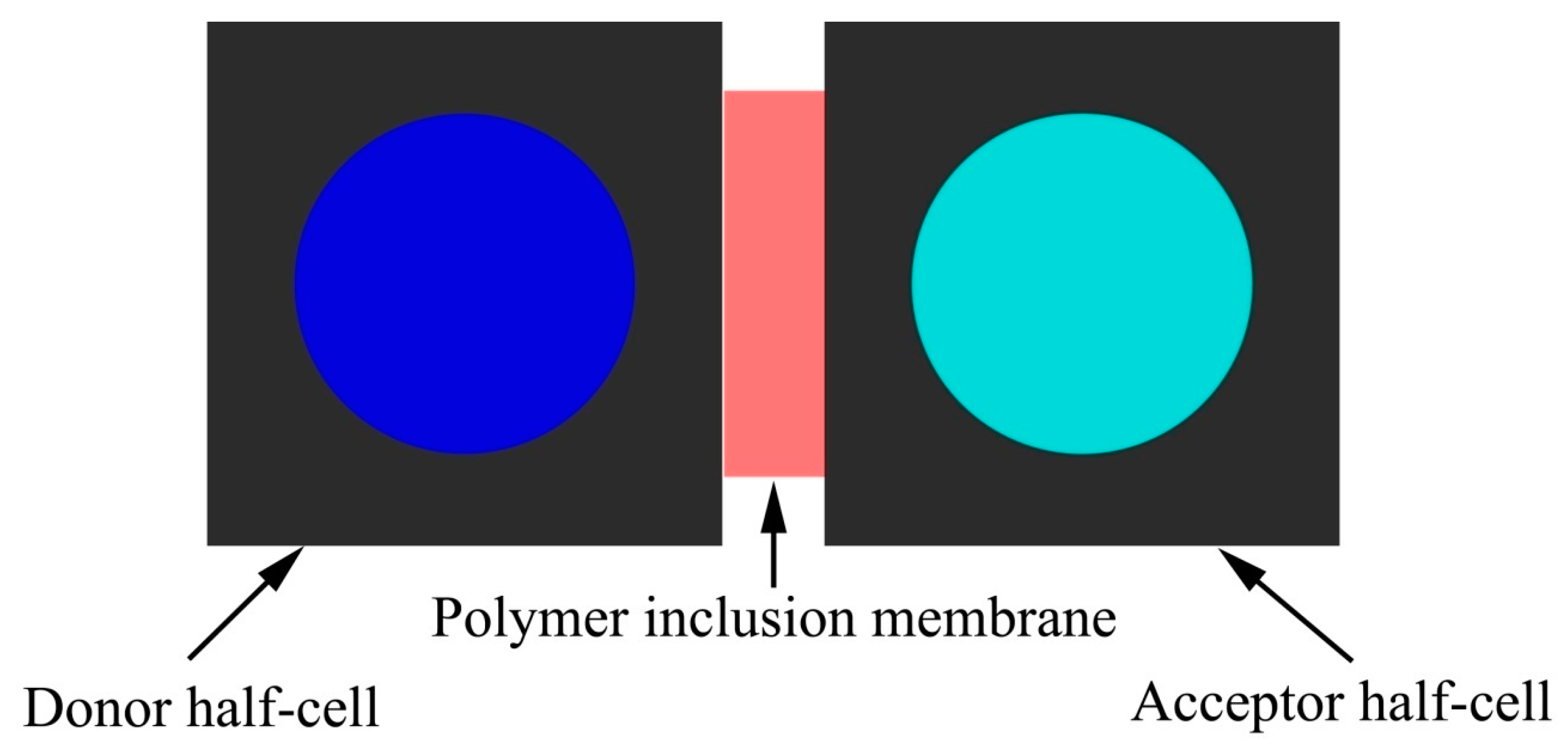
2.3. Preparation of the Polymer Inclusion Membrane
2.4. Membrane Characterization
2.4.1. Fourier Transform Infrared Spectroscopy
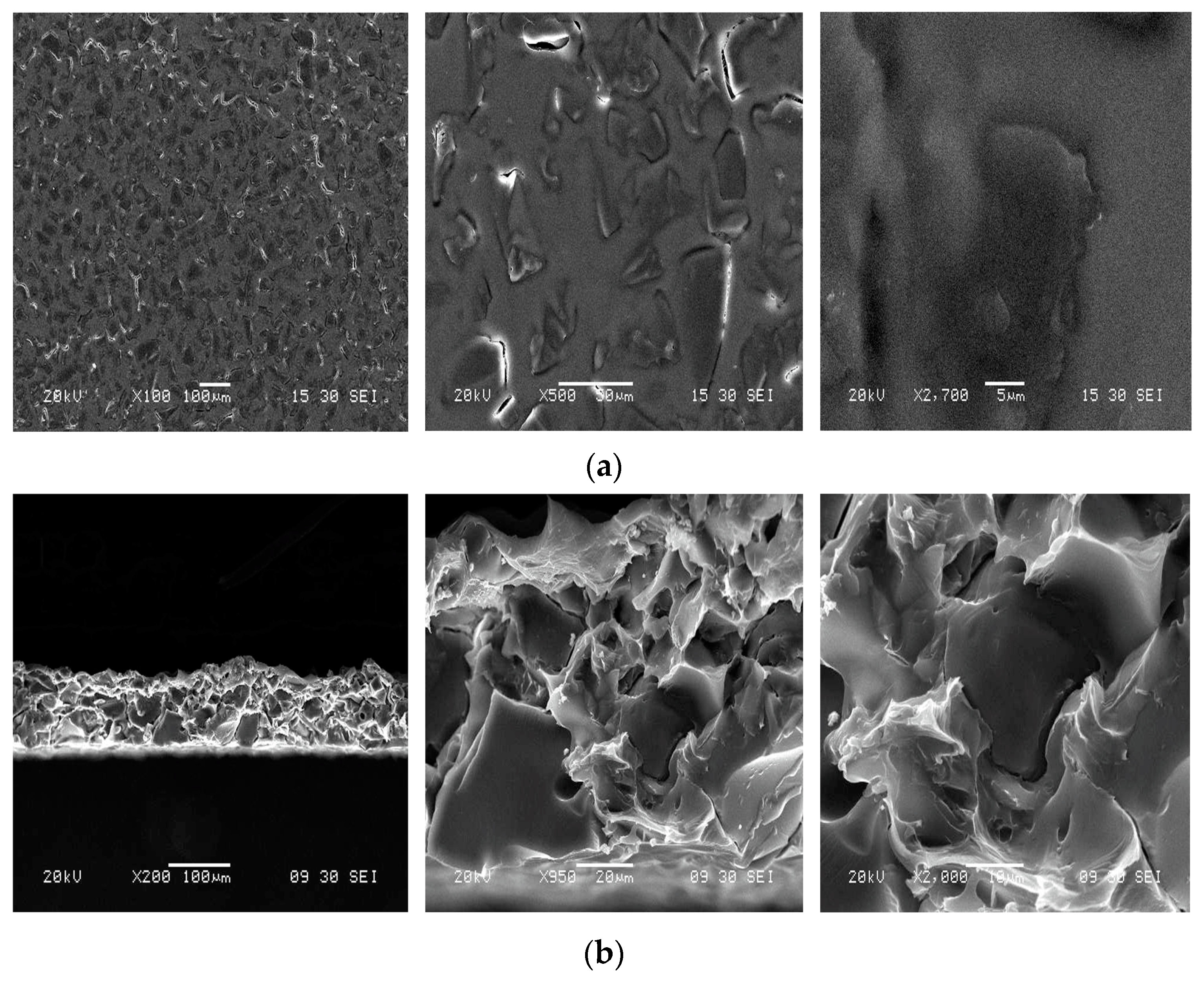
2.4.2. Scanning Microscopy Analysis
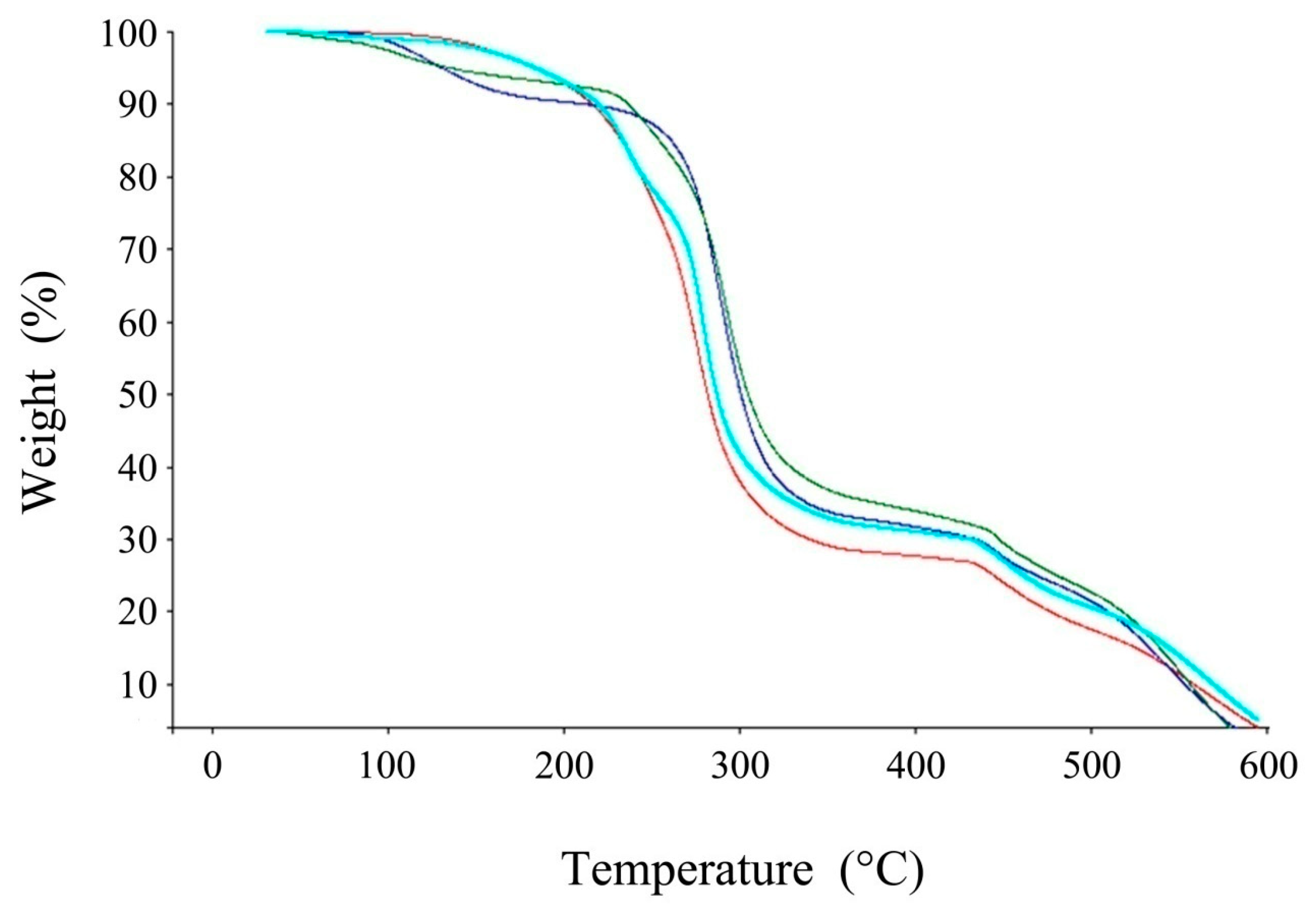
2.4.3. Thermogravemetric Analysis
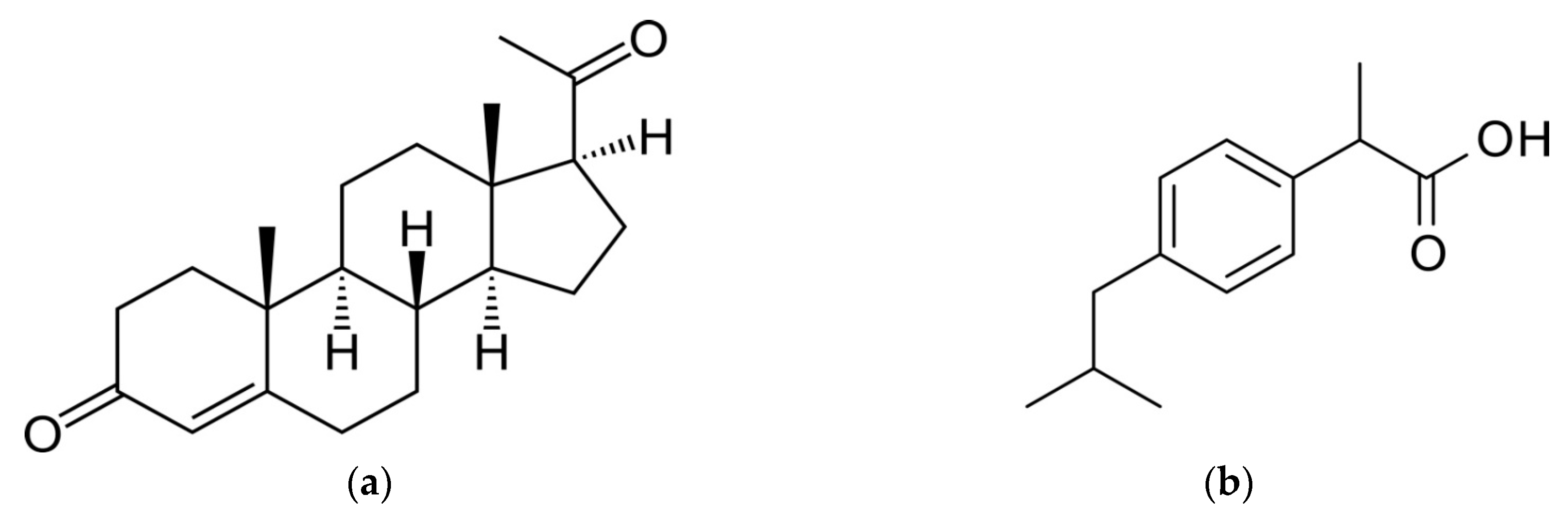
2.5. Application of Membrane to Pharmaceuticals Removal
3. Results and Discussion
3.1. Characterization of Polymer Inclusion Membranes
3.1.1. Scanning Electronic Microscopy
3.1.2. Thermogravimetric Analysis
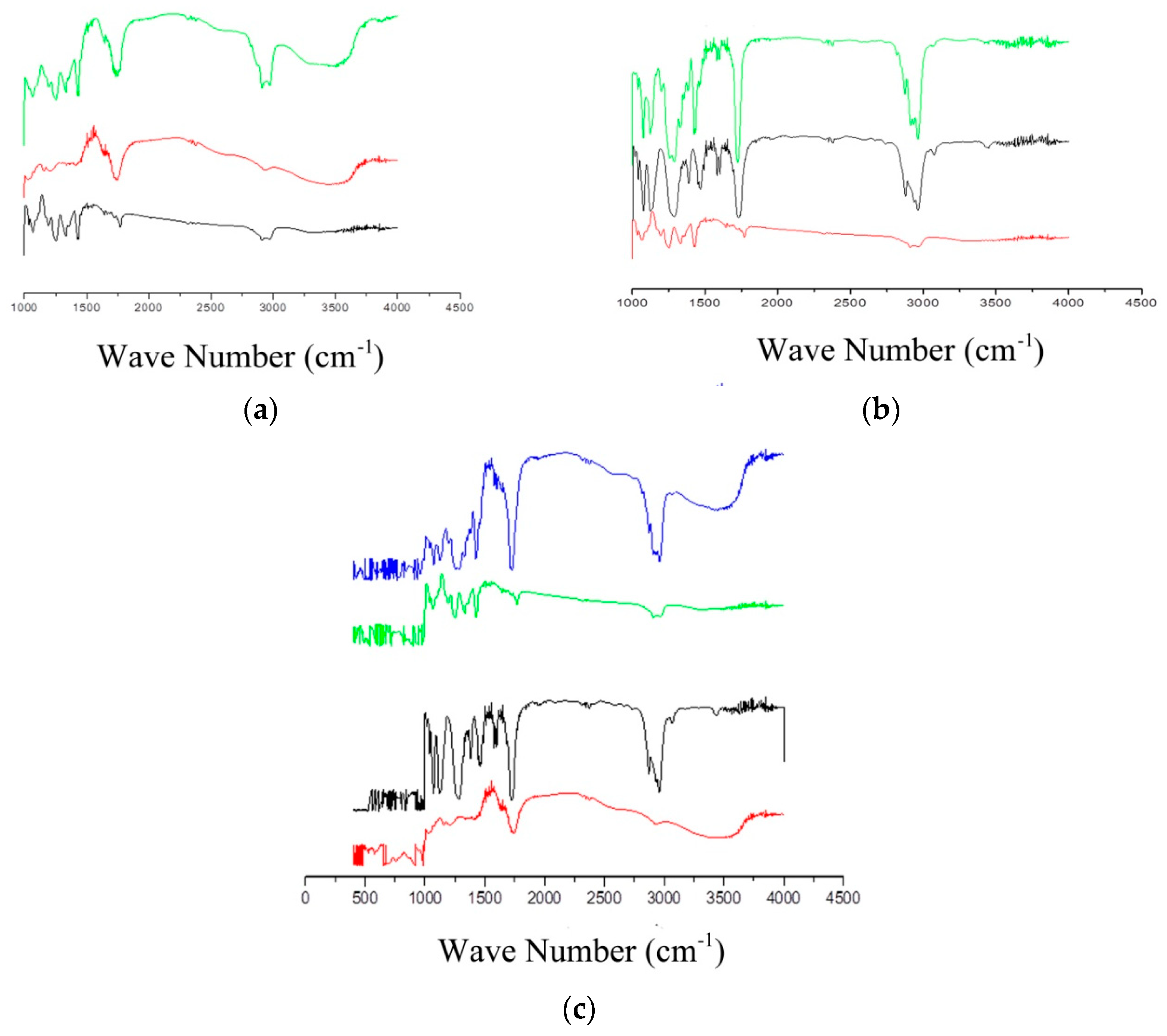
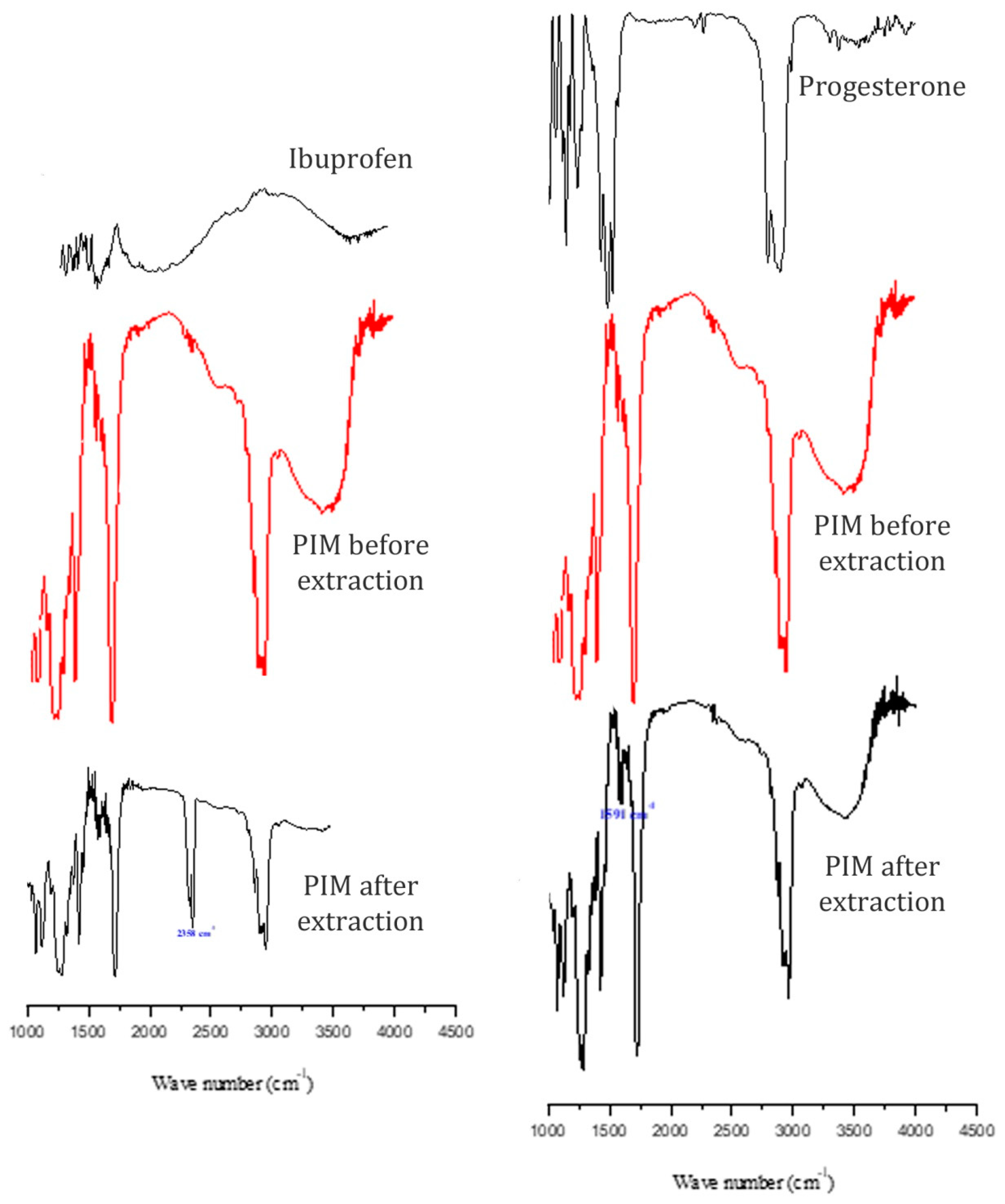
3.1.3. FT-IR Spectroscopy
3.1.4. Polymer Inclusion Membranes Stability Study
3.2. Removal of Pharmaceuticals and Effect of Operating Parameters
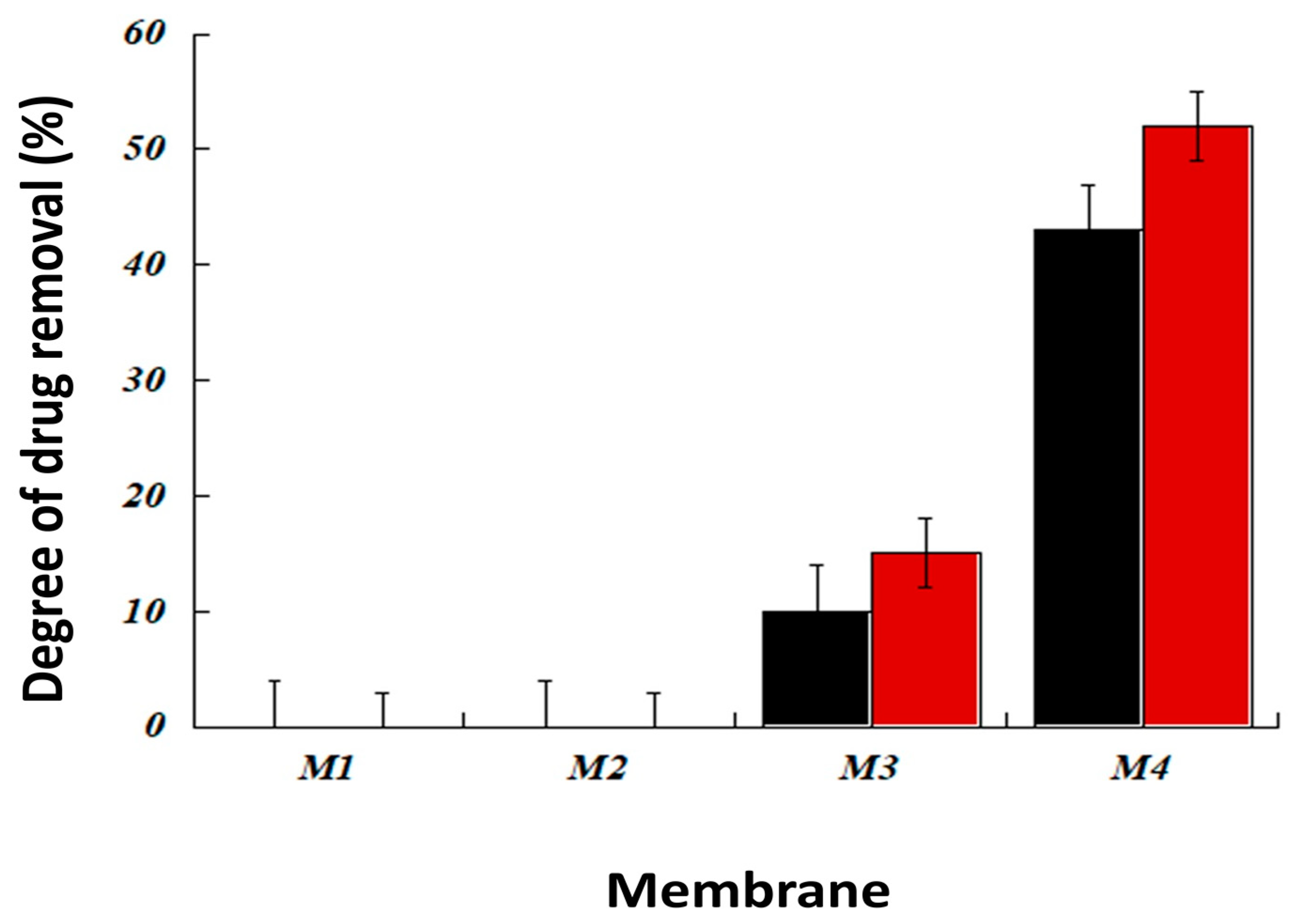
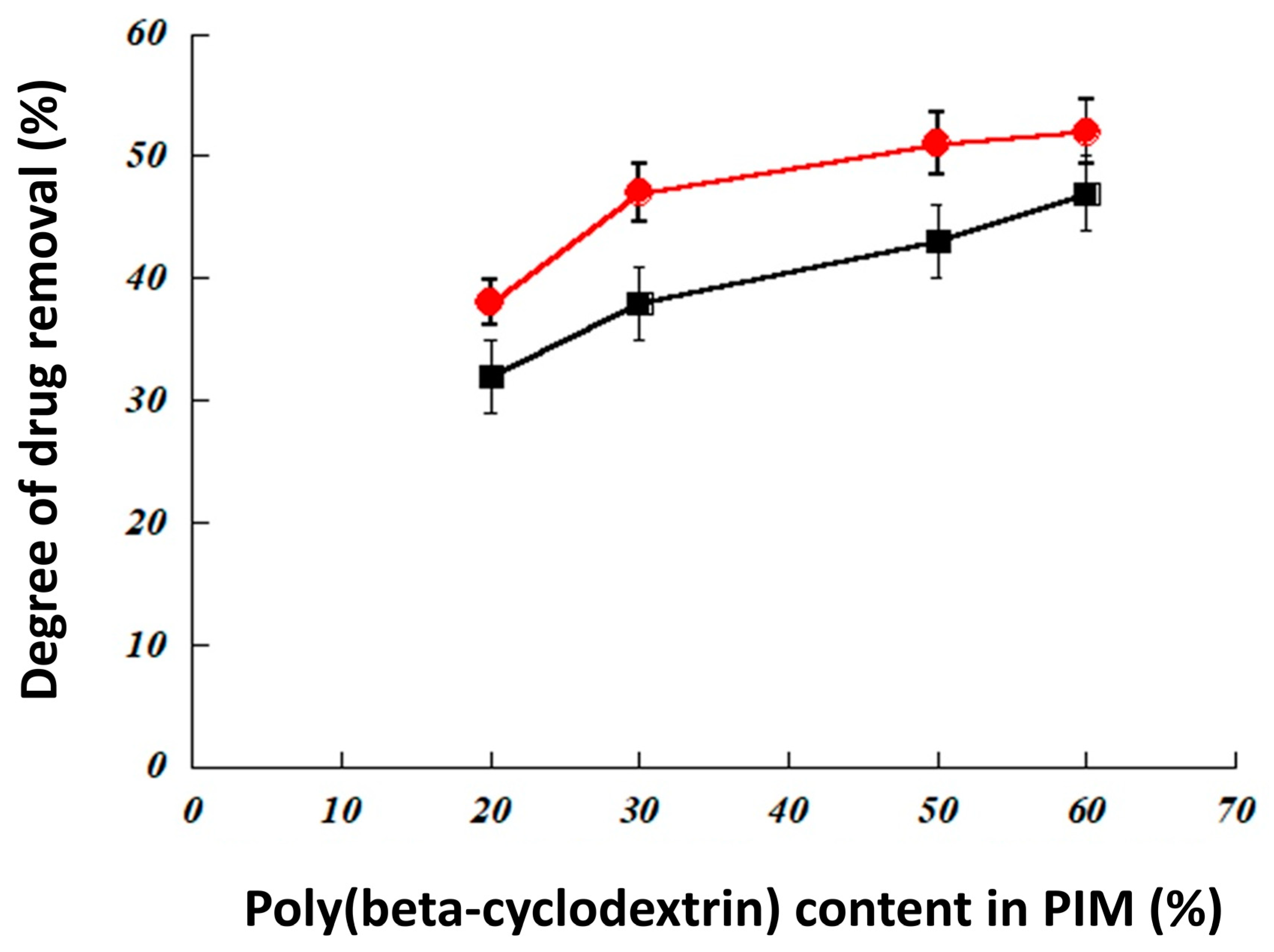
3.2.1. Effect of the Type and Composition of The Membrane for Drug Extraction
3.2.2. Effect of pH
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klamerth, N.; Rizzo, L.; Malato, S.; Maldonado, M.I.; Agüera, A.; Fernández-Alba, A.R. Degradation of fifteen emerging contaminants at microg L−1 initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res. 2010, 44, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I.; Arikan, O.A.; Wiesner, M.R.; Rice, C. Removal of hormones and antibiotics by nanofiltration membranes. J. Membr. Sci. 2008, 309, 94–101. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Broséus, R.; Vincent, S.; Aboulfadl, K.; Daneshvar, A.; Sauvé, S.; Barbeau, B.; Prévost, M. Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment. Water Res. 2009, 43, 4707–4717. [Google Scholar] [CrossRef] [PubMed]
- Banasiak, L.J.; Schäfer, A.I. Sorption of steroidal hormones by electrodialysis membranes. J. Membr. Sci. 2010, 365, 198–205. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Pre-treatment of hospital wastewater by coagulation-flocculation and flotation. Bioresour Technol 2009, 100, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Onac, C.; Surucu, A.; Karapinar, E.; Alpoguz, H.K.; Tabakci, B. Preparation of CTA-based polymer inclusion membrane using calix[4]arene derivative as a carrier for Cr(VI) transport. J. Incl. Phenom. Macrocycl. Chem. 2013, 79, 103–111. [Google Scholar] [CrossRef]
- Ghuzlaan, A.; Omari, M.M.A.; Al-Sou’od, K.A. Prednisone/Cyclodextrin Inclusion Complexation: Phase Solubility, Thermodynamic, Physicochemical and Computational Analysis. J. Solut. Chem. 2008, 38, 83–94. [Google Scholar] [CrossRef]
- Sata, T.; Kawamura, K. Permeation behavior of anions through poly(vinyl alcohol) membranes containing α-cyclodextrin or supramolecules composed of α-cyclodextrin and poly(ethylene glycol) in electrodialysis. J. Membr. Sci. 2000, 171, 97–104. [Google Scholar] [CrossRef]
- Sata, T.; Kawamura, K.; Higa, M.; Matsusaki, K. Electrodialytic transport properties of cation exchange membranes in the presence of cyclodextrins. J. Membr. Sci. 2001, 183, 201–212. [Google Scholar] [CrossRef]
- Nakata, K.; Sakamoto, M.; Taguchi, S.; Yoshida, N.; Shimazu, K. Facile formation of water-insoluble cyclodextrin/Nafion composite films on solid surfaces. J. Colloid Interface Sci. 2005, 288, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Touil, S.; Tingry, S.; Bouchtalla, S.; Deratani, A. Selective pertraction of isomers using membranes having fixed cyclodextrin as molecular recognition sites. Desalination 2006, 193, 291–298. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowiak, W.; Girek, T. Modified cyclodextrin polymers as selective ion carriers for Pb(II) separation across plasticized membranes. J. Membr. Sci. 2008, 310, 312–320. [Google Scholar] [CrossRef]
- Zha, F.; Li, S.; Chang, Y.; Yan, J. Preparation and adsorption kinetics of porous γ-glycidoxypropyltrimethoxysilane crosslinked chitosan–β-cyclodextrin membranes. J. Membr. Sci. 2008, 321, 316–323. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Wang, J.; Li, L.; Guo, X. Spherical particles of α-, β- and γ-cyclodextrin polymers and their capability for phenol removal. Mater. Lett. 2012, 79, 156–158. [Google Scholar] [CrossRef]
- Bhaskar, M.; Aruna, P.; Ganesh Jeevan, R.J.; Radhakrishnan, G. β-Cyclodextrin-polyurethane polymer as solid phase extraction material for the analysis of carcinogenic aromatic amines. Anal. Chim. Acta 2004, 509, 39–45. [Google Scholar] [CrossRef]
- Skiba, M.; Lahiani-Skiba, M. Novel method for preparation of cyclodextrin polymers: Physico-chemical characterization and cytotoxicity. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 341–349. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Zhu, C.; Tian, Z.; Shen, X. Synthesis and properties of water-insoluble β-cyclodextrin polymer crosslinked by citric acid with PEG-400 as modifier. Carbohydr. Polym. 2009, 78, 125–130. [Google Scholar] [CrossRef]
- Crini, G. Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour. Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Ozmen, E.Y.; Yilmaz, M. Use of β-cyclodextrin and starch based polymers for sorption of Congo red from aqueous solutions. J. Hazard. Mater. 2007, 148, 303–310. [Google Scholar] [CrossRef] [PubMed]
- ANSM. ANSES Campagne Nationale D’analyse des Résidus de Médicaments Dans L’eau: Des Résultats Conformes Aux Attentes; ANSM: Saint-Denis, France, 2011.
- Saussereau, E.; Lacroix, C.; Guerbet, M.; Cellier, D.; Spiroux, J.; Goullé, J.-P. Determination of Levels of Current Drugs in Hospital and Urban Wastewater. Bull. Environ. Contam. Toxicol. 2013, 91, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Spongberg, A.L.; Witter, J.D.; Acuña, J.; Vargas, J.; Murillo, M.; Umaña, G.; Gómez, E.; Perez, G. Reconnaissance of selected PPCP compounds in Costa Rican surface waters. Water Res. 2011, 45, 6709–6717. [Google Scholar] [CrossRef] [PubMed]
- Bendz, D.; Paxéus, N.A.; Ginn, T.R.; Loge, F.J. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Höje River in Sweden. J. Hazard. Mater. 2005, 122, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; González-Doncel, M.; Pro, J.; Carbonell, G.; Tarazona, J.V. Occurrence of pharmaceutically active compounds in surface waters of the Henares-Jarama-Tajo river system (Madrid, Spain) and a potential risk characterization. Sci. Total Environ. 2010, 408, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Ryu, J.; Oh, J.; Choi, B.-G.; Snyder, S.A. Occurrence of endocrine disrupting compounds, pharmaceuticals, and personal care products in the Han River (Seoul, South Korea). Sci. Total Environ. 2010, 408, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Klaper, R.D. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere 2013, 93, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and removal of PPCPs in municipal and hospital wastewater in Greece. J. Hazard Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef]
- Escher, B.I.; Baumgartner, R.; Koller, M.; Treyer, K.; Lienert, J.; McArdell, C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011, 45, 75–92. [Google Scholar] [CrossRef]
- Palmer, P.M.; Wilson, L.R.; O’Keefe, P.; Sheridan, R.; King, T.; Chen, C.-Y. Sources of pharmaceutical pollution in the New York City watershed. Sci. Total Environ. 2008, 394, 90–102. [Google Scholar] [CrossRef]
- Rodil, R.; Quintana, J.B.; Concha-Graña, E.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Arikan, O.A.; Rice, C.; Codling, E. Occurrence of antibiotics and hormones in a major agricultural watershed. Desalination 2008, 226, 121–133. [Google Scholar] [CrossRef]
- Miller, J.M.; Dahan, A. Predicting the solubility—Permeability interplay when using cyclodextrins in solubility-enabling formulations: Model validation. Int. J. Pharm. 2012, 430, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Jurecska, L.; Dobosy, P.; Barkács, K.; Fenyvesi, É.; Záray, G. Characterization of cyclodextrin containing nanofilters for removal of pharmaceutical residues. J. Pharm. Biomed. Anal. 2014, 98, 90–93. [Google Scholar] [CrossRef] [PubMed]

| Agitation (rpm) | 400 | 500 | 600 |
| Weight loss (%) | 3.0 | 6.7 | 6.9 |
| Membrane | PVC/poly(β-cyclodextrin) | PVC/DBP | PVC/DBP/poly(β-cyclodextrin) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Medium | HCl at 0.1 M | Phosphate buffer | NaOH at 0.1 M | HCl at 0.1 M | Phosphate buffer | NaOH at 0.1 M | HCl at 0.1 M | Phosphate buffer | NaOH at 0.1 M |
| Weight loss (%) | 3.9 | 4.5 | 17 | 0.5 | 0.5 | 0.5 | 4 | 4 | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulahcene, L.; Skiba, M.; Bounoure, F.; Benamor, M.; Milon, N.; Hallouard, F.; Lahiani-Skiba, M. New Polymer Inclusion Membrane Containing β-Cyclodextrin Polymer: Application for Pharmaceutical Pollutant Removal from Waste Water. Int. J. Environ. Res. Public Health 2019, 16, 414. https://doi.org/10.3390/ijerph16030414
Moulahcene L, Skiba M, Bounoure F, Benamor M, Milon N, Hallouard F, Lahiani-Skiba M. New Polymer Inclusion Membrane Containing β-Cyclodextrin Polymer: Application for Pharmaceutical Pollutant Removal from Waste Water. International Journal of Environmental Research and Public Health. 2019; 16(3):414. https://doi.org/10.3390/ijerph16030414
Chicago/Turabian StyleMoulahcene, Lamia, Mohamed Skiba, Frederic Bounoure, Mohamed Benamor, Nicolas Milon, Francois Hallouard, and Malika Lahiani-Skiba. 2019. "New Polymer Inclusion Membrane Containing β-Cyclodextrin Polymer: Application for Pharmaceutical Pollutant Removal from Waste Water" International Journal of Environmental Research and Public Health 16, no. 3: 414. https://doi.org/10.3390/ijerph16030414
APA StyleMoulahcene, L., Skiba, M., Bounoure, F., Benamor, M., Milon, N., Hallouard, F., & Lahiani-Skiba, M. (2019). New Polymer Inclusion Membrane Containing β-Cyclodextrin Polymer: Application for Pharmaceutical Pollutant Removal from Waste Water. International Journal of Environmental Research and Public Health, 16(3), 414. https://doi.org/10.3390/ijerph16030414





