The Waiting Time of Prostate Cancer Patients in Poland
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalbarczyk, W.P.; Gujski, M.; Brzozowski, S.; Tytko, Z. Walka z nowotworami i opieka onkologiczna w Polsce wobec wyzwań demograficznych i epidemiologicznych—Propozycje rozwiązań; Instytut Ochrony Zdrowia: Warszawa, Poland, 2015. [Google Scholar]
- Didkowska, J.W.U.; Olasek, P. Nowotwory złośliwe w Polsce w 2015 roku; Centrum Onkologii–Instytut im. M. Skłodowskiej–Curie: Warszawa, Poland, 2017. [Google Scholar]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE–5—A Population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef]
- EUROCARE Survival of Cancer Patients in Europe: Eurocare 5 Survival Analysis 2000–2007. Available online: https://w3.iss.it/site/EU5Results/forms/SA0007.aspx (accessed on 1 October 2018).
- Buchholz, T.A.; Austin-Seymour, M.M.; Moe, R.E.; Ellis, G.K.; Livingston, R.B.; Pelton, J.G.; Griffin, T.W. Effect of delay in radiation in the combined modality treatment of breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1993, 26, 23–35. [Google Scholar] [CrossRef]
- Chen, Z.; King, W.; Pearcey, R.; Kerba, M.; Mackillop, W.J. The relationship between waiting time for radiotherapy and clinical outcomes: A systematic review of the literature. Radiother. Oncol. 2008, 87, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Choan, E.; Dahrouge, S.; Samant, R.; Mirzaei, A.; Price, J. Radical radiotherapy for cervix cancer: The effect of waiting time on outcome. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1071–1077. [Google Scholar]
- Montella, M.; Crispo, A.; Botti, G.; De Marco, M.; de Bellis, G.; Fabbrocini, G.; Pizzorusso, M.; Tamburini, M.; D’Aiuto, G. An assessment of delays in obtaining definitive breast cancer treatment in Southern Italy. Breast Cancer Res Treat. 2001, 66, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.; Coy, P.; Pater, J.L.; Hodson, I.; Arnold, A.; Zee, B.C.; Payne, D.; Kostashuk, E.C.; Evans, W.K.; Dixon, P.; et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 1993, 11, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.A.; Westcombe, A.M.; Love, S.B.; Littlejohns, P.; Ramirez, A.J. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet 1999, 353, 1119–1126. [Google Scholar] [CrossRef]
- van Harten, M.C.; Hoebers, F.J.; Kross, K.W.; van Werkhoven, E.D.; van den Brekel, M.W.; van Dijk, B.A. Determinants of treatment waiting times for head and neck cancer in the Netherlands and their relation to survival. Oral Oncol. 2015, 51, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Osowiecka, K.; Rucinska, M.; Nowakowski, J.J.; Nawrocki, S. How Long Are Cancer Patients Waiting for Oncological Therapy in Poland? Int. J. Environ. Res. Public Health 2018, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Dale, W.; Bilir, P.; Han, M.; Meltzer, D. The role of anxiety in prostate carcinoma: A structured review of the literature. Cancer 2005, 104, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.P.; Vedsted, P.; Sokolowski, I.; Sondergaard, J.; Olesen, F. Time intervals from first symptom to treatment of cancer: A cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv. Res. 2011, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Helsper, C.C.W.; van Erp, N.N.F.; Peeters, P.; de Wit, N.N.J. Time to diagnosis and treatment for cancer patients in the Netherlands: Room for improvement? Eur. J. Cancer 2017, 87, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Bondy, S.J.; Loblaw, D.A. Wait times in prostate cancer diagnosis and radiation treatment. Can. Urol. Assoc. J. 2010, 4, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Subramonian, K.R.; Puranik, S.; Mufti, G.R. How will the two-weeks-wait rule affect delays in management of urological cancers? J. R. Soc. Med. 2003, 96, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Redaniel, M.T.; Martin, R.M.; Gillatt, D.; Wade, J.; Jeffreys, M. Time from diagnosis to surgery and prostate cancer survival: A retrospective cohort study. BMC Cancer 2013, 13, 559. [Google Scholar] [CrossRef] [PubMed]
- Baade, P.D.; Gardiner, R.A.; Ferguson, M.; Youlden, D.R.; Aitken, J.F.; Yaxley, J.; Chambers, S.K. Factors associated with diagnostic and treatment intervals for prostate cancer in Queensland, Australia: A large cohort study. Cancer Causes Control 2012, 23, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.; Kogel, A. Basic Clinical Radiobiology, 4th ed.; Hodder Arnold: London, UK, 2009. [Google Scholar]
- Hayes, J.H.; Barry, M.J. Screening for prostate cancer with the prostate-specific antigen test: A review of current evidence. JAMA 2014, 311, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Neuberger, M.M.; Djulbegovic, M.; Dahm, P. Screening for prostate cancer. Cochrane Database Syst. Rev. 2013, 319, 1946. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, P.C. Observational studies and the natural history of screen-detected prostate cancer. Curr. Opin. Urol. 2015, 25, 232–237. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Patients Included in | Patients Included in | ||
|---|---|---|---|---|
| Suspicion-Treatment Analysis | Suspicion-Diagnosis, Diagnosis-Treatment Analysis | |||
| n | % | n | % | |
| All patients | 123 | 100 | 101 | 100 |
| Age (years) | median 65; range 53–87 | |||
| Education | ||||
| Primary | 28 | 23 | 24 | 24 |
| Secondary | 65 | 53 | 52 | 51 |
| Higher | 30 | 24 | 25 | 25 |
| Place of residence | ||||
| City 101,000–500,000 | 40 | 33 | 32 | 32 |
| City 50,000–100,000 | 17 | 14 | 14 | 14 |
| City <50,0000 | 33 | 27 | 28 | 28 |
| Village | 33 | 27 | 27 | 27 |
| Professional activity | ||||
| Active | 26 | 21 | 24 | 24 |
| Unemployed | 3 | 2 | 2 | 2 |
| Pensioner | 94 | 76 | 75 | 74 |
| Marital status | ||||
| Married | 103 | 84 | 84 | 83 |
| Single | 10 | 8 | 9 | 9 |
| Widower | 10 | 8 | 8 | 8 |
| Type of “patient route” starting points | ||||
| Symptoms | 76 | 62 | 66 | 65 |
| Prevention | 38 | 31 | 32 | 32 |
| Follow-up | 9 | 7 | 3 | 3 |
| Method of treatment beginning | ||||
| Surgery | 26 | 21 | 14 | 14 |
| Radiotherapy | 44 | 36 | 35 | 35 |
| Chemotherapy | 3 | 2 | 3 | 3 |
| Hormonal therapy | 50 | 41 | 49 | 49 |
| Treatment intention | ||||
| Curative | 109 | 89 | 94 | 93 |
| Palliative | 14 | 11 | 7 | 7 |
| Private medical services | ||||
| Yes | 33 | 27 | 30 | 30 |
| No | 70 | 57 | 60 | 59 |
| No data | 20 | 16 | 11 | 11 |
| Variable Name | Suspicion-Treatment Time (weeks) n = 123 | Suspicion-Diagnosis Time (Weeks) n = 101 | Diagnosis-Treatment Time (Weeks) n = 101 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | (25–75% IQR) | p-Value | Median | (25–75% IQR) | p-Value | Median | (25–75% IQR) | p-Value | |
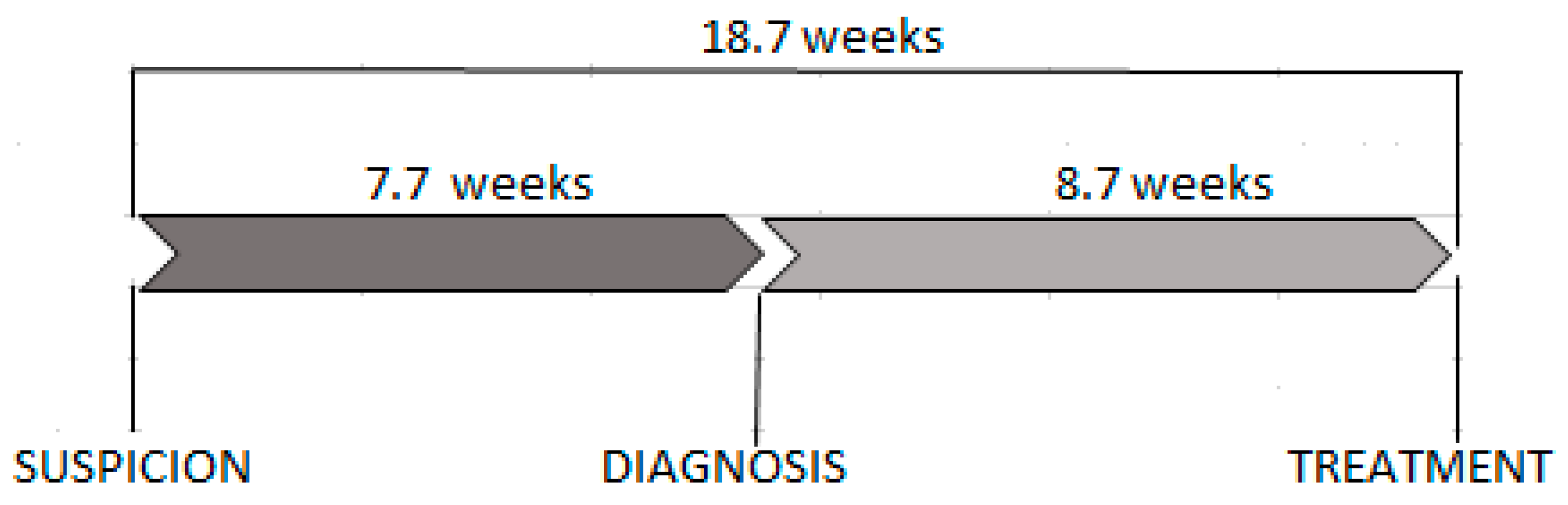
| All patients | 18.7 | (10.6–26.9) | 7.7 | (4–16.1) | 8.7 | (4.6–14.1) | |||
| Age | >0.05 | >0.05 | >0.05 | ||||||
| Education | |||||||||
| Primary | 18.1 | (11.8–26.0) | 0.86 | 8.4 | (4.7–14.4) | 0.70 | 8.0 | (3.9–12.7) | 0.55 |
| Secondary | 19.9 | (9.7–27.3) | 7.0 | (3.4–17.5) | 9.5 | (5.9–15.9) | |||
| Higher | 15.8 | (10.7–25.6) | 8.7 | (4.3–12.3) | 7.3 | (4.1–13.0) | |||
| Place of residence | |||||||||
| City 101,000–500,000 | 14.9 | (7.7–24.7) | 0.003 | 8.7 | (4.1–11.3) | 0.11 | 7.3 | (3.8–12.9) | 0.34 |
| City 50,000–100,000 | 25.0 | (20.1–47.4) | 11.5 | (5.3–42.9) | 13.4 | (6.3–16.7) | |||
| City <50,000 | 16.4 | (9.1–23.3) | 6.1 | (3.0–9.4) | 7.5 | (4.7–15.1) | |||
| Village | 19.9 | (13.1–26.9) | 9.6 | (4.0–21.0) | 9.3 | (4.6–12.9) | |||
| Professional activity | |||||||||
| Active | 17.3 | (10.4–23.9) | 0.09 | 4.4 | (2.9–10.6) | 0.20 | 9.9 | (6.0–14.5) | 0.63 |
| Unemployed | 36.9 | (25.6–58.9) | 17.9 | (6.4–29.4) | 13.3 | (7.4–19.1) | |||
| Pensioner | 18.1 | (10.6–26.9) | 8.7 | (4.1–17.4) | 8.4 | (4.0–14.1) | |||
| Marital status | |||||||||
| Married | 17.1 | (9.7–26.1) | 0.12 | 7.5 | (3.9–17.4) | 0.76 | 7.9 | (4.1–13.8) | 0.28 |
| Single | 22.9 | (21.4–40.3) | 9.0 | (4.4–12.7) | 12.3 | (10.6–15.1) | |||
| Widower | 19.6 | (15.4–24.3) | 7.9 | (6.3–12.6) | 13.0 | (5.9–15.0) | |||
| Type of “patient route” starting points | |||||||||
| Symptoms | 21.0 | (11.7–26.8) | 0.36 | 8.9 | (4.3–17.4) | 0.34 | 8.8 | (3.6–14.6) | 0.95 |
| Prevention | 14.8 | (9.7–26.9) | 5.9 | (3.3–12.9) | 8.3 | (6.0–13.8) | |||
| Follow-up | 14.0 | (13.6–20.1) | 4.0 | (4.0–32.3) | 10.0 | (2.0–16.1) | |||
| Method of treatment beginning | |||||||||
| Surgery | 13.6 | (7.1–24.0) | 8.3 | (4.1–15.7) | 8.6 | (6.6–12.9) | |||
| Radiotherapy | 21.3 | (14.6–27.7) | 0.06 | 8.7 | (4.7–16.1) | 0.78 | 12.7 | (8.7–17.7) | <0.001 |
| Chemotherapy | 34.7 | (9.7–47.4) | 32.0 | (1.3–44.7) | 2.7 | (2.7–8.4) | |||
| Hormonal therapy | 17.2 | (10.6–24.3) | 6.6 | (3.9–12.7) | 6.3 | (2.9–10.9) | |||
| Treatment intention | |||||||||
| Curative | 19.1 | (10.6–26.1) | 0.86 | 7.6 | (4.0–16.1) | 0.63 | 8.6 | (4.9–14.1) | 0.80 |
| Palliative | 14.5 | (13.6–27.3) | 9.7 | (4.0–21.0) | 10.0 | (3.0–32.7) | |||
| Private medical services | |||||||||
| Yes | 19.9 | (10.7–28.9) | 0.69 | 4.6 | (3.4–15.7) | 0.15 | 8.5 | (3.0–16.9) | 0.79 |
| No | 21.0 | (12.0–27.4) | 9.2 | (4.9–17.5) | 8.6 | (5.4–13.8) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osowiecka, K.; Nawrocki, S.; Kurowicki, M.; Rucinska, M. The Waiting Time of Prostate Cancer Patients in Poland. Int. J. Environ. Res. Public Health 2019, 16, 342. https://doi.org/10.3390/ijerph16030342
Osowiecka K, Nawrocki S, Kurowicki M, Rucinska M. The Waiting Time of Prostate Cancer Patients in Poland. International Journal of Environmental Research and Public Health. 2019; 16(3):342. https://doi.org/10.3390/ijerph16030342
Chicago/Turabian StyleOsowiecka, Karolina, Sergiusz Nawrocki, Marcin Kurowicki, and Monika Rucinska. 2019. "The Waiting Time of Prostate Cancer Patients in Poland" International Journal of Environmental Research and Public Health 16, no. 3: 342. https://doi.org/10.3390/ijerph16030342
APA StyleOsowiecka, K., Nawrocki, S., Kurowicki, M., & Rucinska, M. (2019). The Waiting Time of Prostate Cancer Patients in Poland. International Journal of Environmental Research and Public Health, 16(3), 342. https://doi.org/10.3390/ijerph16030342





