Leaching Characteristics of Heavy Metals and Plant Nutrients in the Sewage Sludge Immobilized by Composite Phosphorus-Bearing Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Materials
2.2. Experimental Methods
2.2.1. Leaching Column Experiment
2.2.2. Analytical Methods
3. Results and Discussion
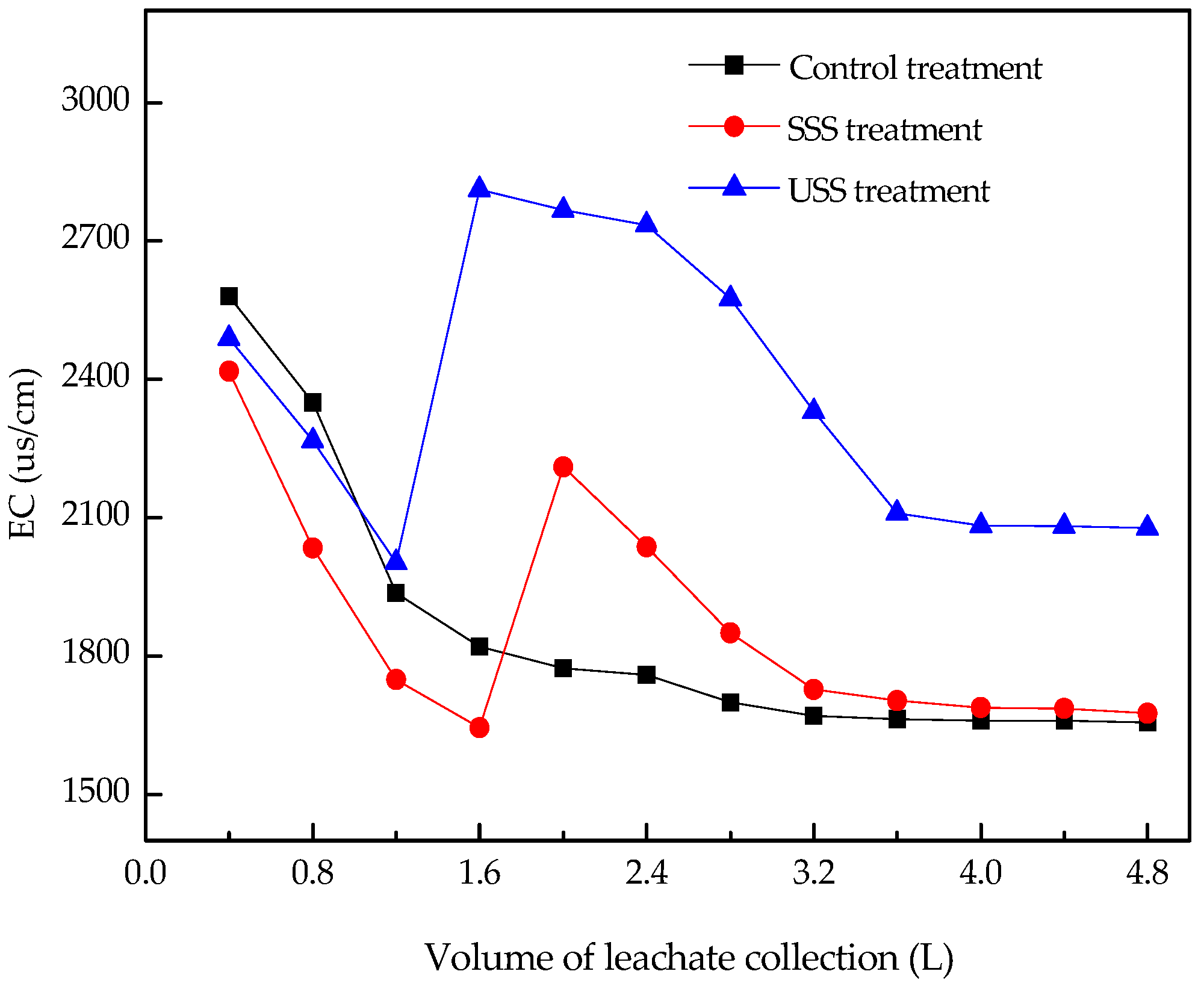
3.1. pH and EC in the Leachate
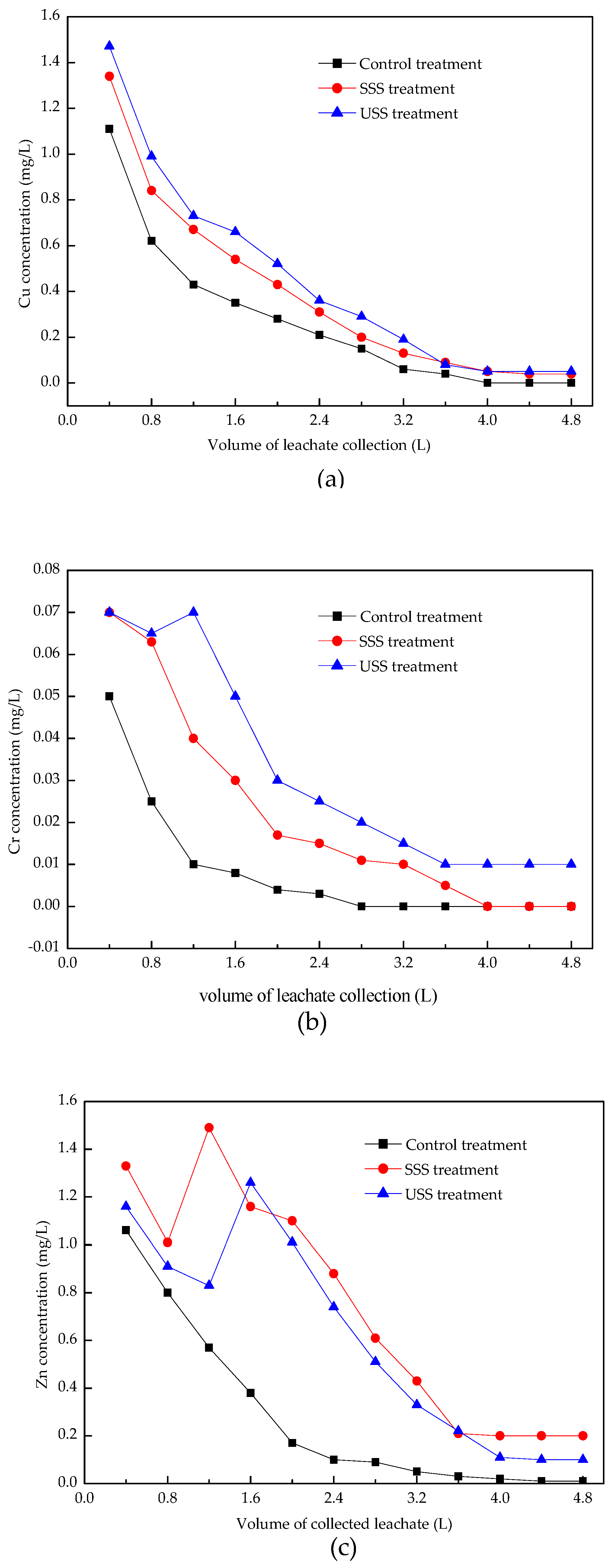
3.2. Leaching Characteristics of Heavy Metals
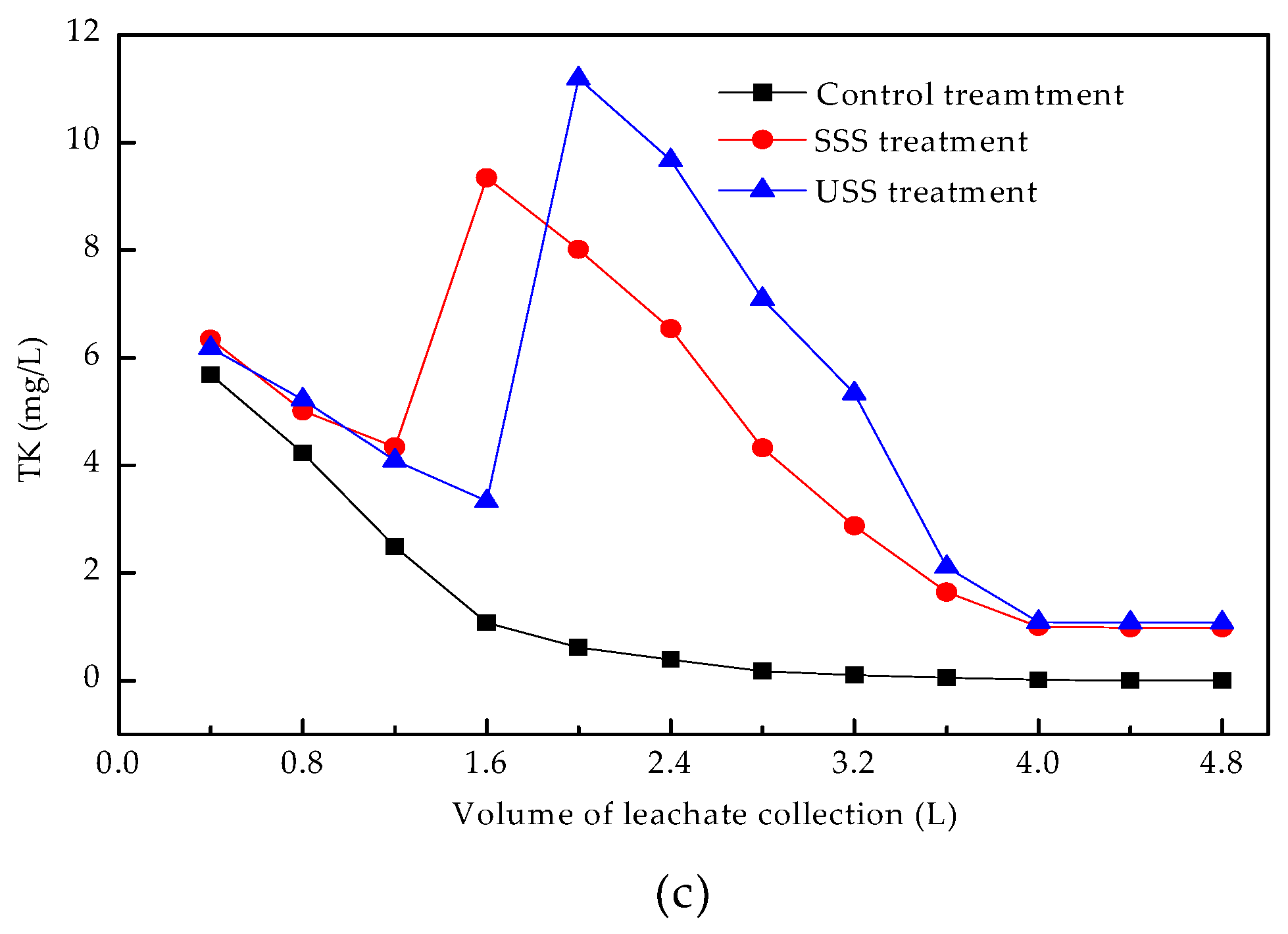
3.3. Release Kinetics of Heavy Metals
3.4. Leaching Characteristics of Plant Nutrients
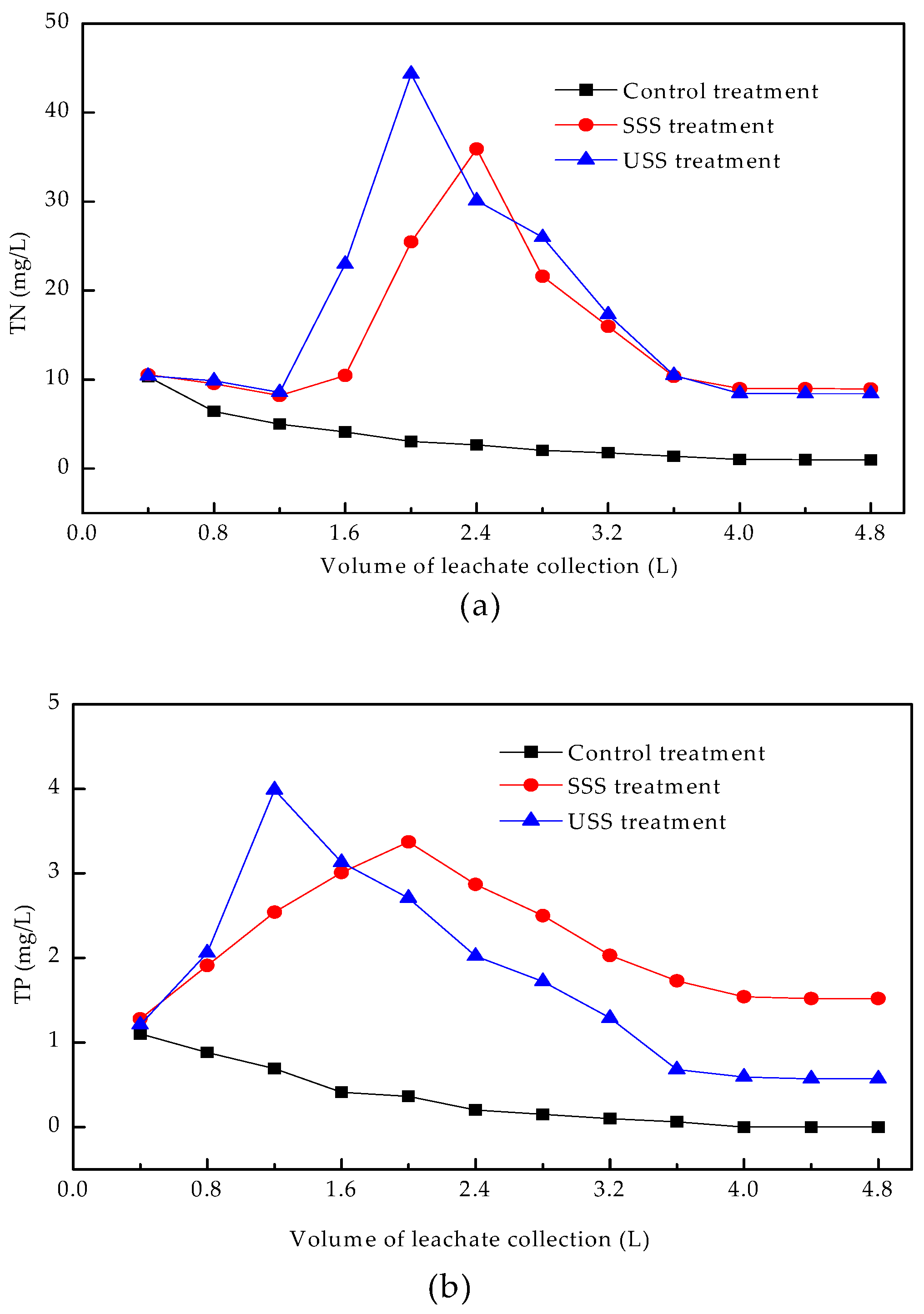
3.4.1. Concentrations of TN, TP, and TK in Leachate
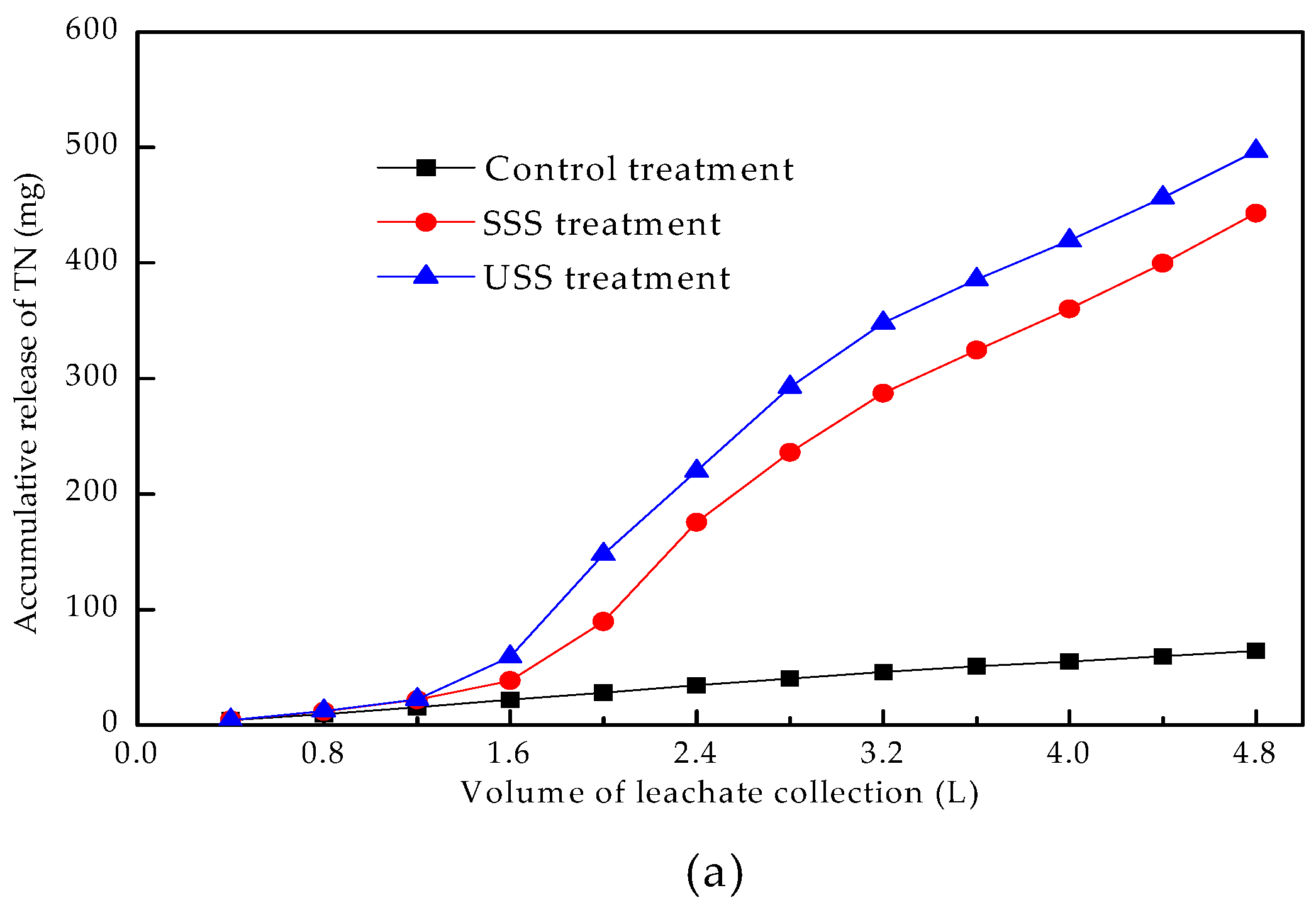
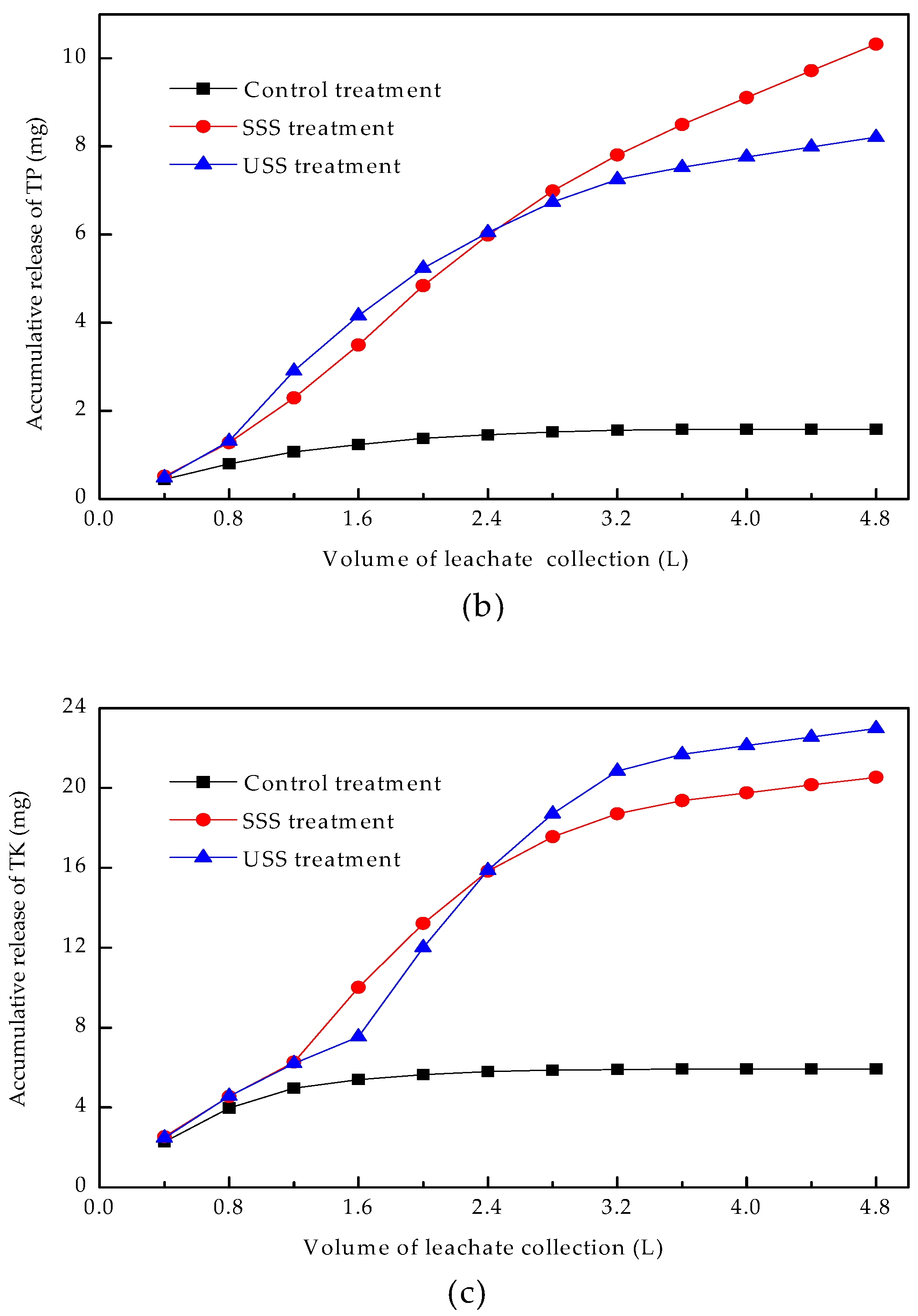
3.4.2. Accumulative Release Characteristics of Plant Nutrients
3.4.3. Accumulative Release Model of Plant Nutrients
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bai, Y.C.; Zang, C.Y.; Gu, M.J.; Gu, C.H.; Shao, H.B.; Guan, Y.X.; Wang, X.K.; Zhou, X.J.; Shan, Y.H.; Feng, K. Sewage sludge as an initial fertility driver for rapid improvement of mudflat salt-soils. Sci. Total Environ. 2017, 578, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Feizi, M.; Jalali, M.; Renella, G. Assessment of nutrient and heavy metal content and speciation in sewage sludge from different locations in Iran. Nat. Hazards 2019, 93, 657–675. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2018, 28, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hoeve, M.; Christensen, T.H.; Bruun, S.; Jensen, L.S.; Scheutz, C. Life cycle assessment of sewage sludge management options including long-term impacts after land application. J. Clean. Prod. 2017, 174, 538–547. [Google Scholar] [CrossRef]
- Alvarenga, P.; Mourinha, C.; Farto, M.; Santos, T.; Palma, P.; Sengo, J.; Morais, M.C.; Cunha-Queda, C. Sewage sludge, compost and other representative organic wastes as agricultural soil amendments: Benefits versus limiting factors. Waste Manag. 2015, 40, 44–52. [Google Scholar] [CrossRef]
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef]
- Hamdi, H.; Hechmi, S.; Khelil, M.N.; Zoghlami, I.R.; Benzarti, S.; Mokni-Tlili, S.; Hassen, A.; Jedidi, N. Repetitive land application of urban sewage sludge: Effect of amendment rates and soil texture on fertility and degradation parameters. Catena 2019, 172, 11–20. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Influence of organic amendments on soil structure and soil loss under simulated rain. Soil Till. Res. 2007, 93, 197–205. [Google Scholar] [CrossRef]
- Cheng, H.F.; Xu, W.P.; Liu, J.L.; Zhao, Q.J.; He, Y.Q.; Chen, G. Application of composted sewage sludge (CSS) as a soil amendment for turfgrass growth. Ecol. Eng. 2007, 29, 96–104. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; Mourinha, C.; Farto, M.; Dôres, J.; Patanita, M.; Cunha-Queda, C.; Natal-da-Luz, T.; Renaud, M.; Sous, J.P. Recycling organic wastes to agricultural land as a way to improve its quality: A field study to evaluate benefits and risks. Waste Manag. 2017, 61, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.H.; Bai, Y.C. Heavy metal leaching and plant uptake in mudflat soils amended with sewage sludge. Environ. Sci. Pollut. R. 2018, 25, 31031–31039. [Google Scholar] [CrossRef]
- Urbaniak, M.; Wyrwicka, A.; Tołoczko, W.; Serwecińska, L.; Zieliński, M. The effect of sewage sludge application on soil properties and willow (Salix sp.) cultivation. Sci. Total Environ. 2017, 586, 66–75. [Google Scholar] [CrossRef]
- Bai, Y.C.; Zuo, W.G.; Zhao, H.T.; Mei, L.J.; Gu, C.H.; Guan, Y.X.; Wang, X.K.; Gu, M.J.; Zang, C.Y.; Shan, Y.H. Distribution of heavy metals in maize and mudflat saline soil amended by sewage sludge. J. Soil. Sediment. 2017, 17, 1565–1578. [Google Scholar] [CrossRef]
- Ma, W.F.; Liu, F.; Cheng, X.; Jing, Y.; Nie, C.; Zhang, P.Y. Environmental evaluation of the application of compost sewage sludge to landscaping as soil amendments: A field experiment on the grassland soils in Beijing. Desalin. Water Treat. 2015, 54, 1118–1126. [Google Scholar] [CrossRef]
- Xu, C.Q.; Chen, W.; Hong, J.L. Life-cycle environment and economic assessment of sewage sludge treatment in China. J. Clean. Prod. 2014, 67, 79–87. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Gómez, I.; Navarro-Pedreño, J.; Jordán, M.M. The transfer of heavy metals to barley plants from soils amended with sewage sludge with different heavy metal burdens. J. Soil. Sediment. 2014, 14, 687–696. [Google Scholar] [CrossRef]
- Jalali, M.; Najafi, S. Effect of pH on potentially toxic trace elements (Cd, Cu, Ni, and Zn) solubility in two native and spiked calcareous soils: Experimental and modeling. Commun. Soil Sci. Plan. 2018, 49, 814–827. [Google Scholar] [CrossRef]
- Huanga, H.J.; Yuanb, X.Z. The migration and transformation behaviors of heavy metals during the hydrothermal treatment of sewage sludge. Bioresour. Technol. 2016, 200, 991–998. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Z.X.; Tang, L.; Zeng, G.M.; Yu, M.; Li, X.D.; Wu, H.P.; Qian, Y.Y.; Li, X.M.; Luo, Y. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 2017, 81, 281–288. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, S.Z.; Wu, W.Y.; Liu, H.L. Metal sorption on soils as affected by the dissolved organic matter in sewage sludge and the relative calculation of sewage sludge application. J. Hazard. Mater. 2007, 149, 399–407. [Google Scholar] [CrossRef]
- Jalali, M.; Latifi, Z. Measuring and simulating effect of organic residues on the transport of cadmium, nickel, and zinc in a calcareous soil. J. Geochem. Explor. 2018, 184, 372–380. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bulkowska, K.; Kierklo, K. Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.Q.; Wang, D.F. Immobilization of heavy metals in sewage sludge during land application process in China: A review. Sustainability 2017, 9, 2020. [Google Scholar] [CrossRef]
- Li, C.P.; Jiang, J.G.; Yin, M.; Gu, J. Influence of lime addition on sludge drying and metal passivation efficiency. China Water Wastewater 2010, 26, 28–31. [Google Scholar]
- Tica, D.; Udovic, M.; Lestan, D. Immobilization of potentially toxic metals using different soil amendments. Chemosphere 2011, 85, 577–583. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Jamil, T.S.; Hegazy, E.Z. Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals: II. Isotherm models. J. Hazard. Mater. 2010, 182, 842–847. [Google Scholar] [CrossRef]
- Wang, D.F.; Li, S.H.; Wang, X.Q.; Li, L.X.; Zhang, X. Synergistic passivation of fly ash and TMT on heavy metals in sewage sludge. Sustainability 2018, 10, 4731. [Google Scholar] [CrossRef]
- Huang, G.; Su, X.; Rizwan, M.S.; Zhu, Y.; Hu, H. Chemical immobilization of Pb, Cu, and Cd by phosphate materials and calcium carbonate in contaminated soils. Environ. Sci. Pollut. R. 2016, 23, 16845–16856. [Google Scholar] [CrossRef]
- Yuan, Y.; Chai, L.; Yang, Z.; Yang, W. Simultaneous immobilization of lead, cadmium, and arsenic in combined contaminated soil with iron hydroxyl phosphate. J. soil. Sediment. 2017, 19, 1–8. [Google Scholar] [CrossRef]
- Chen, S.L.; Tao, X.Y.; Liu, X.H.; Si, Y.B. Effect of the sulfide amendement on the speciation distributuion and bioavailability of heavy metals in municipal sludge. J. Saf. Environ. 2017, 17, 283–290. [Google Scholar] [CrossRef]
- Fang, W.; Delapp, R.C.; Kosson, D.S.; van der Sloot, H.A.; Liu, J. Release of heavy metals during long-term land application of sewage sludge compost: Percolation leaching tests with repeated additions of compost. Chemosphere 2017, 169, 271–280. [Google Scholar] [CrossRef]
- Mortula, M.M. Chemical treatment of sewage sludge to reduce leachability. In Proceedings of the 15th European Biosolids and Organic Resources Conference, Heraklion, Greece, 2012; Available online: https://www.researchgate.net/profile/Maruf_Mortula/publication/272021369_Chemical_Treatment_of_Sewage_Sludge_to_Reduce_Leachability/links/5ad1b6e8aca272fdaf77f447/Chemical-Treatment-of-Sewage-Sludge-to-Reduce-Leachability.pdf (accessed on 17 December 2019).
- Li, X.; Li, F.Y.; Rong, X.M. Risk and leaching characteristics of nitrogen and phosphorus in sandy soil amended with sewage sludge. J. Soil Water Conserv. 2013, 27, 93–97. [Google Scholar] [CrossRef]
- Oladeji, O.O.; Tian, G.L.; Cox, A.E.; Granato, T.C.; Connor, C.; Abedin, Z.; Pietz, R.I. Effect of long-term application of biosolids for mine land reclamation on groundwater chemistry: Nutrients and other selected qualities. J. Environ. Qual. 2013, 42, 94–102. [Google Scholar] [CrossRef]
- Mortula, M.M.; Ali, T.A.; Atabay, S.; Ghadban, A.A. Reduction of leachability of sewage sludge by alum treatment. J. Environ. Eng. Sci. 2014, 9, 105–112. [Google Scholar] [CrossRef]
- Hou, H.; Yao, N.; Li, J.N.; Wei, Y.; Zhao, L.; Zhang, J.; Li, F.S. Migration and leaching risk of extraneous antimony in three representative soils of China: Lysimeter and batch experiments. Chemosphere 2013, 93, 1980–1988. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agro-Chemistrical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2007; pp. 42–58, 76–80, 100–107. [Google Scholar]
- Veschetti, E.; Maresca, D.; Santarsiero, A.; Ottaviani, M. Sewage sludge microwave digestion procedure optimized by temperature and pressure analysis. Microchem. J. 1998, 59, 246–257. [Google Scholar] [CrossRef]
- Seo, B.H.; Kim, H.S.; Kwon, S.I.; Owens, G.; Kim, K.R. Heavy metal accumulation and mobility in a soil profile depend on the organic waste type applied. J. Soil Sediment. 2019, 19, 822–829. [Google Scholar] [CrossRef]
- Penido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; Guimaraes, I.D.; Guilherme, L.R.G. Combining biobar and sewage sludge for immobilization of heavy metals in mining soils. Ecotox. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef]
- Zheng, S.A.; Zheng, X.Q.; Zhang, T.L.; Liu, S.T. Study on leaching characteristics and release kinetics of heavy metals in polluted purple soil. J. Soil Water Conserv. 2011, 25, 253–256. [Google Scholar] [CrossRef]
- Cao, X.D.; Liang, Y.; Zhao, L. Mobility of Pb, Cu and Zn in the phosphorus-amended contaminated soils under simulated landfill and rainfall conditions. Environ. Sci. Pollut. R. 2013, 20, 5913–5921. [Google Scholar] [CrossRef]
- Mignardi, S.; Corami, A.; Ferrini, V. Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 2012, 86, 354–360. [Google Scholar] [CrossRef]
- Cao, X.D.; Ma, L.Q.; Rhue, D.R.; Appel, C.S. Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ. Pollut. 2004, 131, 435–444. [Google Scholar] [CrossRef]
- Cao, X.D.; Wahbi, A.; Ma, L.N.; Li, B.; Yang, Y.L. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2008, 164, 555–564. [Google Scholar] [CrossRef]
- Dai, S.G. Environmental Chemistry, 2nd ed.; Higher Education Press: Beijing, China, 2006; pp. 389–403. [Google Scholar]
- Inyang, H.I.; Onwawoma, A.; Bae, S.Y. The Elovich equation as a predictor of lead and cadmium sorption rates on contaminant barrier minerals. Soil Till. Res. 2016, 55, 124–132. [Google Scholar] [CrossRef]
- Zhang, S.Y.; He, X.W.; Li, Y.; Fang, Z.Q.; Wang, H. Leaching experimental study on heavy metals in soil lead-zinc mine. J. Min. Sci. Tech. 2018, 3, 406–416. [Google Scholar] [CrossRef]
- Lei, Q.R.; Qin, C.H.; Yang, W.J. A study on leaching characteristics of heavy metal in Chongqing life garbage. J. Chongqing Univ. 1993, 16, 64–69. [Google Scholar]


| Samples | Sewage Sludge | Background Soil | Rock Phosphate |
|---|---|---|---|
| pH | 6.84 ± 0.10 | 6.86 ± 0.06 | 7.17 ± 0.13 |
| EC (mS/cm) | 1.88 ± 0.11 | 0.13 ± 0.004 | 0.40 ± 0.01 |
| CEC (mmol/kg) | 179 ± 3 | 27.3 ± 2.8 | 114 ± 2 |
| OM (g/kg) | 367 ± 2 | 9.76 ± 0.78 | - |
| TN (g/kg) | 17.5 ± 1.1 | 0.19 ± 0.16 | - |
| TP (g/kg) | 15.6 ± 0.8 | 0.38 ± 0.04 | 35.0 ± 1.2 |
| TK (g/kg) | 3.00 ± 0.60 | 2.98 ± 0.46 | - |
| Cu (mg/kg) | 75.5 ± 7.2 | 11.4 ± 1.4 | 45.3 ± 4.4 |
| Cr (mg/kg) | 402 ± 8 | 12.6 ± 2.0 | 13.8 ± 2.6 |
| Zn (mg/kg) | 2161 ± 68 | 74.2 ± 4.4 | 13.5 ± 1.2 |
| Treatment | Background Soil | Sewage Sludge | Rock Phosphate | Superphosphate |
|---|---|---|---|---|
| Control | 100 | 0 | 0 | 0 |
| USS | 90 | 10 | 0 | 0 |
| SSS | 90 | 8.85 | 0.885 | 0.265 |
| Heavy Metals (mg/L) | Control Treatment | SSS Treatment | USS Treatment | Limit Value of the Fourth Level in GB/T14848-2017 |
|---|---|---|---|---|
| Cu | 1.11 | 1.34 | 1.47 | <1.5 |
| Cr | 0.05 | 0.07 | 0.06 | <0.1 |
| Zn | 1.06 | 1.49 | 1.26 | <5 |
| Treatment | First-Order Kinetic Model | Modified Elovich Model | Double-Constant Model | Hyperbolic Diffusion Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| lny = a + bx | R2 | y = alnx + b | R2 | lny = alnx + b | R2 | y = ax0.5 + b | R2 | ||
| Cu | Control treatment | lny = 0.2381x − 0.1348 | 0.6964 | y = 0.6879lnx − 1.2099 | 0.9811 | lny = 0.5206lnx − 0.0941 | 0.9458 | y = 1.0246x0.5 − 0.1459 | 0.9239 |
| SSS treatment | lny = 0.2263x − 0.2398 | 0.6889 | y = 0.5749lnx − 1.0682 | 0.9801 | lny = 0.4963lnx − 0.0233 | 0.9422 | y = 0.8538x0.5 − 0.1828 | 0.9170 | |
| USS treatment | lny = 0.1933x − 0.4609 | 0.6634 | y = 0.3703lnx − 0.8112 | 0.9649 | lny = 0.4282lnx − 0.2792 | 0.9289 | y = 0.5447x0.5 − 0.2491 | 0.8840 | |
| Cr | Control treatment | lny = 0.2996x − 3.0445 | 0.6832 | y = 0.5133lnx − 0.0740 | 0.9911 | lny = 0.6581lnx − 2.7587 | 0.9378 | y = 0.0796x0.5 − 0.0089 | 0.9470 |
| SSS treatment | lny = 0.2201x − 3.0824 | 0.6133 | y = 0.0319lnx − 0.0621 | 0.9686 | lny = 0.4969lnx − 2.8825 | 0.8986 | y = 0.0468x0.5 − 0.0139 | 0.8788 | |
| USS treatment | lny = 0.1032x − 3.5910 | 0.4747 | y = 0.0075lnx − 0.0310 | 0.8502 | lny = 0.2464lnx − 3.5074 | 0.7999 | y = 0.0105x0.5 − 0.0204 | 0.6946 | |
| Zn | Control treatment | lny = 0.3624x − 0.1496 | 0.7339 | y = 1.3790lnx − 1.4965 | 0.9746 | lny = 0.7795lnx − 0.2087 | 0.9595 | y = 2.0498x0.5 − 0.6526 | 0.9595 |
| SSS treatment | lny = 0.3593x − 0.3164 | 0.7420 | y = 1.1286lnx − 1.2558 | 0.9635 | lny = 0.7695lnx − 0.0413 | 0.9604 | y = 1.71570.5 − 0.5431 | 0.9489 | |
| USS treatment | lny = 0.1803x − 0.3950 | 0.5509 | y = 0.3573x − 0.8556 | 0.9281 | lny = 0.4173lnx − 0.2388 | 0.8572 | y = 0.5136x0.5 − 0.3316 | 0.8068 | |
| Treatment | First-Order Kinetic Model | Modified Elovich Model | Double-Constant Model | Hyperbolic Diffusion Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| lny = a + bx | R2 | y = alnx + b | R2 | lny = alnx + b | R2 | y = ax0.5 + b | R2 | ||
| N | Control treatment | lny = 0.5391x + 1.9338 | 0.8546 | y = 5.5703lnx − 0.5273 | 0.9956 | lny = 1.1104lnx + 2.5033 | 0.9959 | y = 40.492x0.5 − 26.741 | 0.9871 |
| SSS treatment | lny = 1.0225x + 1.9399 | 0.8810 | y = 50.733lnx − 90.988 | 0.975 | lny = 2.0541lnx + 3.0591 | 0.9766 | y = 317.14x0.5 − 289.54 | 0.9193 | |
| USS treatment | lny = 1.0319x + 2.0966 | 0.8422 | y = 44.664lnx − 90.971 | 0.9717 | lny = 2.1158lnx + 3.194 | 0.9713 | y = 363.69x0.5 − 321.87 | 0.9401 | |
| P | Control treatment | lny = 0.2157x−0.3438 | 0.641 | y = 0.4807lnx + 0.9528 | 0.9615 | lny = 0.4888lnx − 0.1494 | 0.9046 | y = 0.7013x0.5 + 0.2319 | 0.8739 |
| SSS treatment | lny = 0.5101x + 0.1428 | 0.8175 | y = 4.328lnx + 2.6617 | 0.9317 | lny = 1.2274lnx + 0.5786 | 0.9863 | y = 6.8223x0.5 − 4.6123 | 0.9885 | |
| USS treatment | lny = 0.5101x + 0.1428 | 0.7058 | y = 3.4993lnx + 2.8453 | 0.9745 | lny = 1.1232lnx + 0.6274 | 0.9399 | y = 5.3422x0.5 − 2.7681 | 0.9686 | |
| K | Control treatment | lny = 0.1398x + 1.2722 | 0.5123 | y = 1.3755lnx + 4.258 | 0.8732 | lny = 0.335lnx + 1.3848 | 0.8076 | y = 1.9309x0.5 + 2.3121 | 0.7347 |
| SSS treatment | lny = 0.4274x + 1.3633 | 0.7923 | y = 8.4972lnx + 7.6749 | 0.9494 | lny = 0.9014lnx + 1.7992 | 0.9677 | y = 13.067x0.5 + 6.1013 | 0.9586 | |
| USS treatment | lny = 0.4801x + 1.2437 | 0.8493 | y = 9.8643x + 7.4019 | 0.9093 | lny = 0.9809lnx + 1.757 | 0.9735 | y = 15.46x0.5 − 9.0399 | 0.9537 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Fang, B.; Wang, D.; Wang, X.; Man, X.; Zhang, X. Leaching Characteristics of Heavy Metals and Plant Nutrients in the Sewage Sludge Immobilized by Composite Phosphorus-Bearing Materials. Int. J. Environ. Res. Public Health 2019, 16, 5159. https://doi.org/10.3390/ijerph16245159
Li S, Fang B, Wang D, Wang X, Man X, Zhang X. Leaching Characteristics of Heavy Metals and Plant Nutrients in the Sewage Sludge Immobilized by Composite Phosphorus-Bearing Materials. International Journal of Environmental Research and Public Health. 2019; 16(24):5159. https://doi.org/10.3390/ijerph16245159
Chicago/Turabian StyleLi, Shihe, Baihui Fang, Dongfang Wang, Xianqing Wang, Xiaobing Man, and Xuan Zhang. 2019. "Leaching Characteristics of Heavy Metals and Plant Nutrients in the Sewage Sludge Immobilized by Composite Phosphorus-Bearing Materials" International Journal of Environmental Research and Public Health 16, no. 24: 5159. https://doi.org/10.3390/ijerph16245159
APA StyleLi, S., Fang, B., Wang, D., Wang, X., Man, X., & Zhang, X. (2019). Leaching Characteristics of Heavy Metals and Plant Nutrients in the Sewage Sludge Immobilized by Composite Phosphorus-Bearing Materials. International Journal of Environmental Research and Public Health, 16(24), 5159. https://doi.org/10.3390/ijerph16245159




