Human Health Risk Assessment and Potentially Harmful Element Contents in the Fruits Cultivated in the Southern Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Sampling and Preparation
2.2. Sample Analyses
2.3. Quality Control
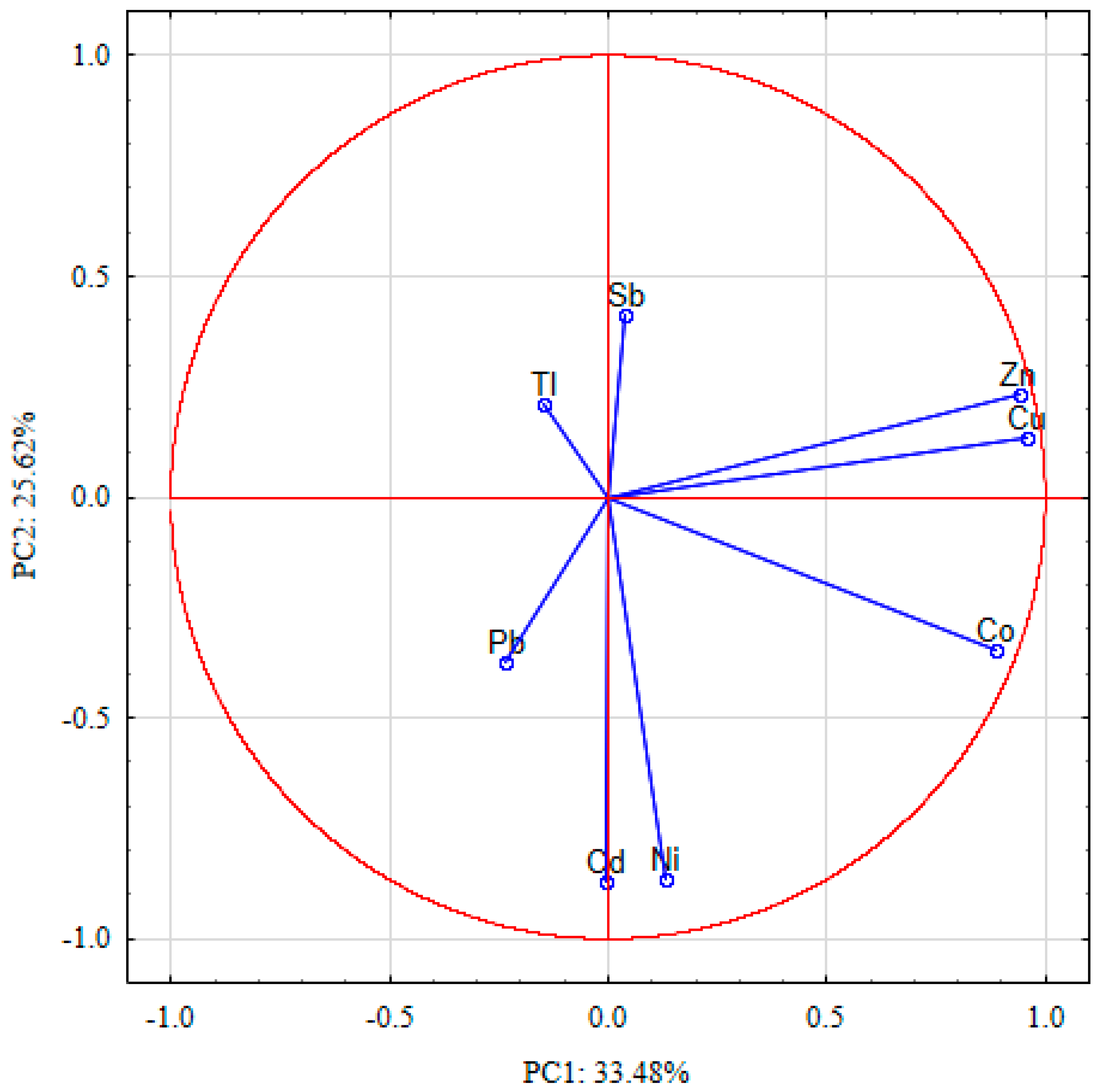
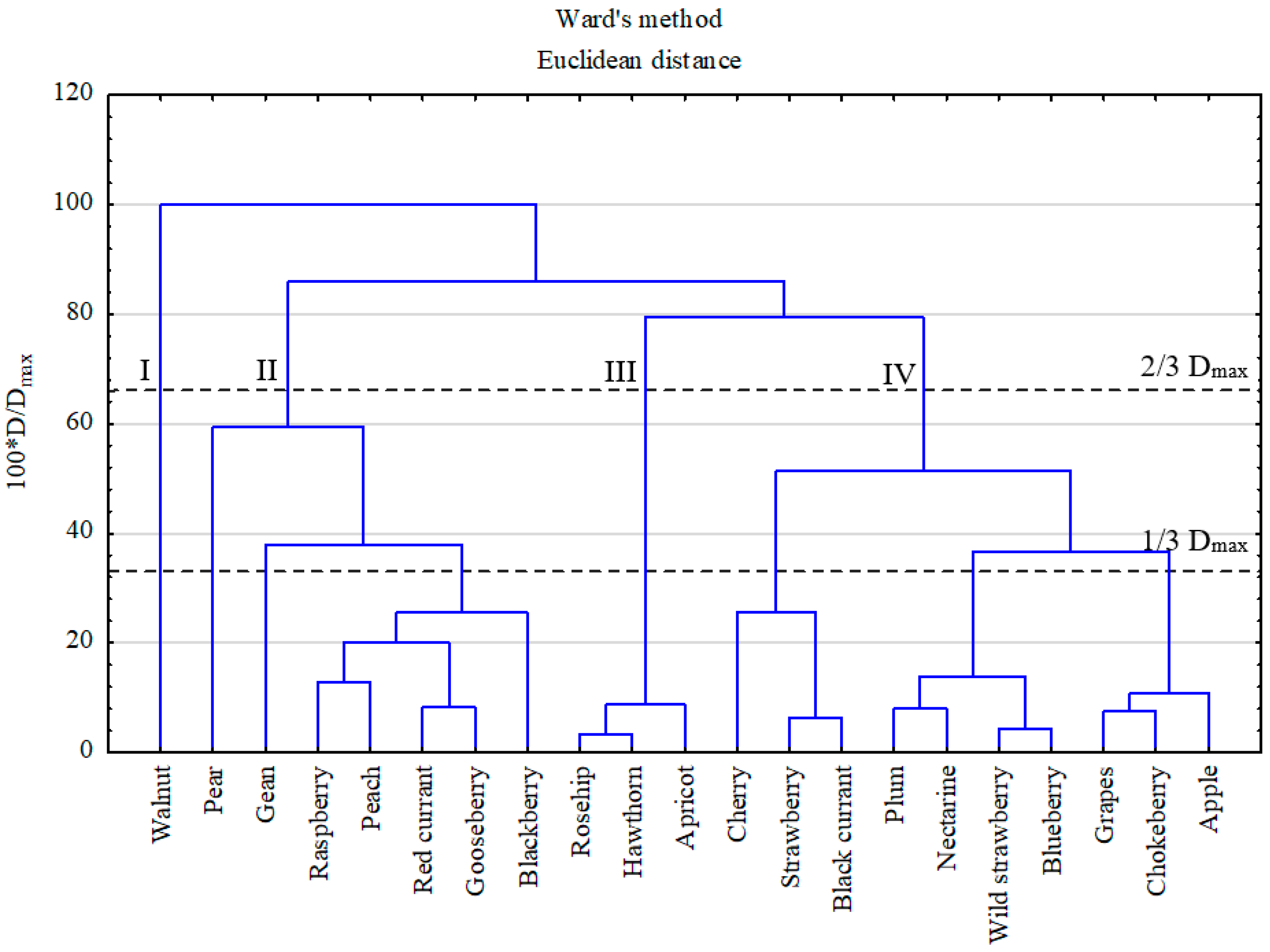
2.4. Statistical Analysis
2.5. Soil-to-Plant Transfer Indices
2.6. Human Health Risk Assessment
3. Results and Discussion
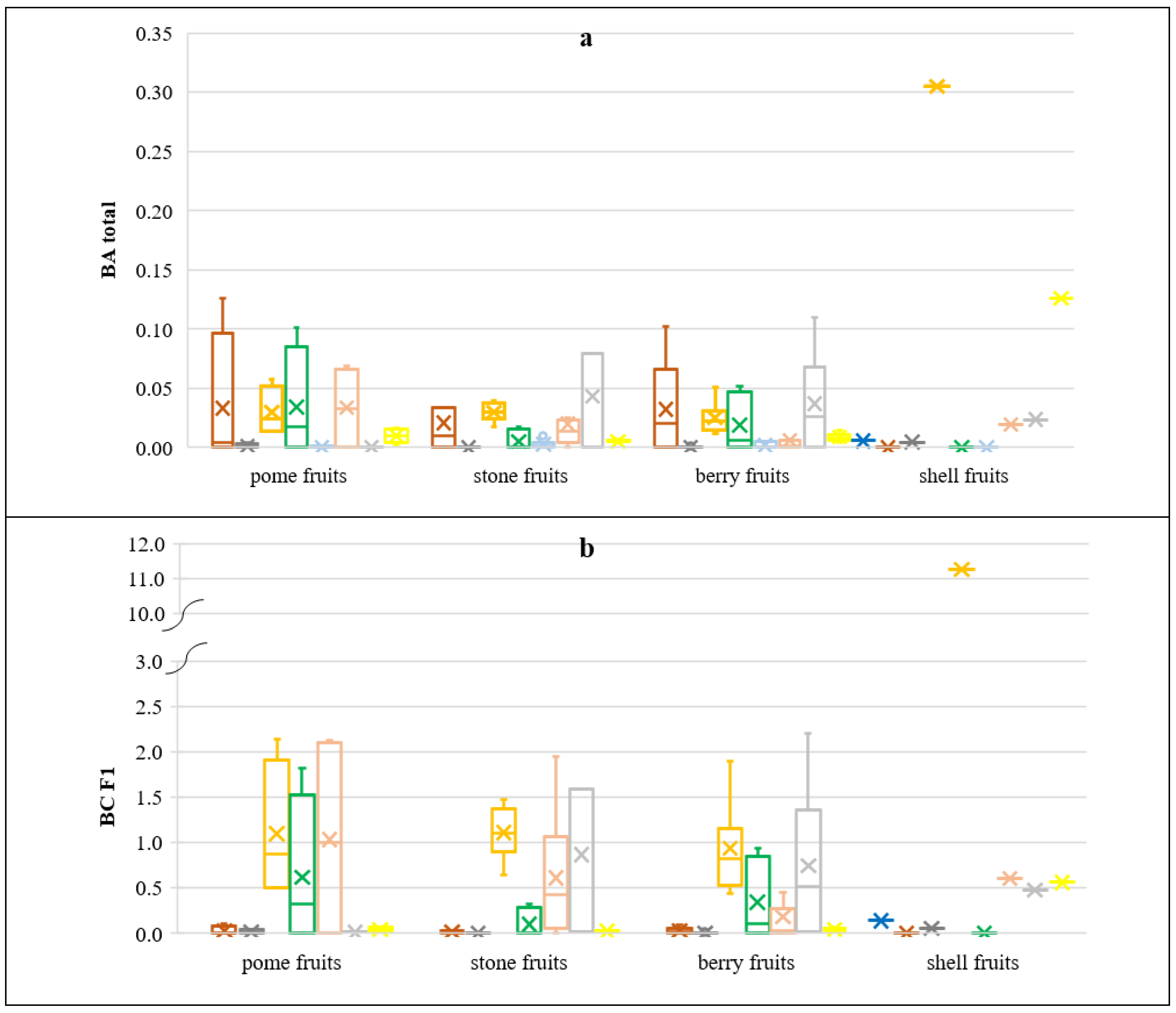
3.1. PHE Levels in Groups of Fruits
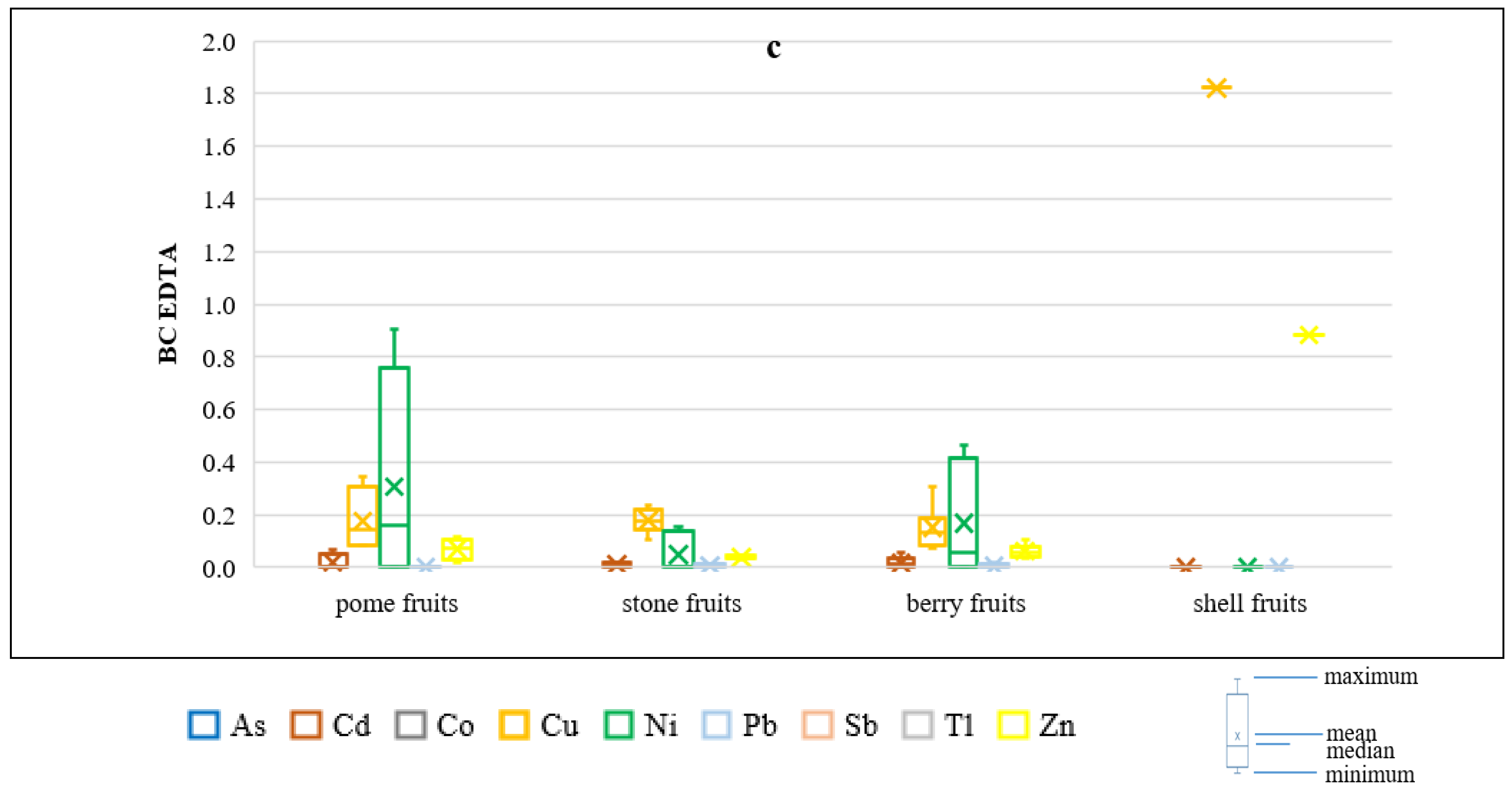
3.2. Soil-to-Plant Transfer Indices
3.3. Human Health Risk Assessment
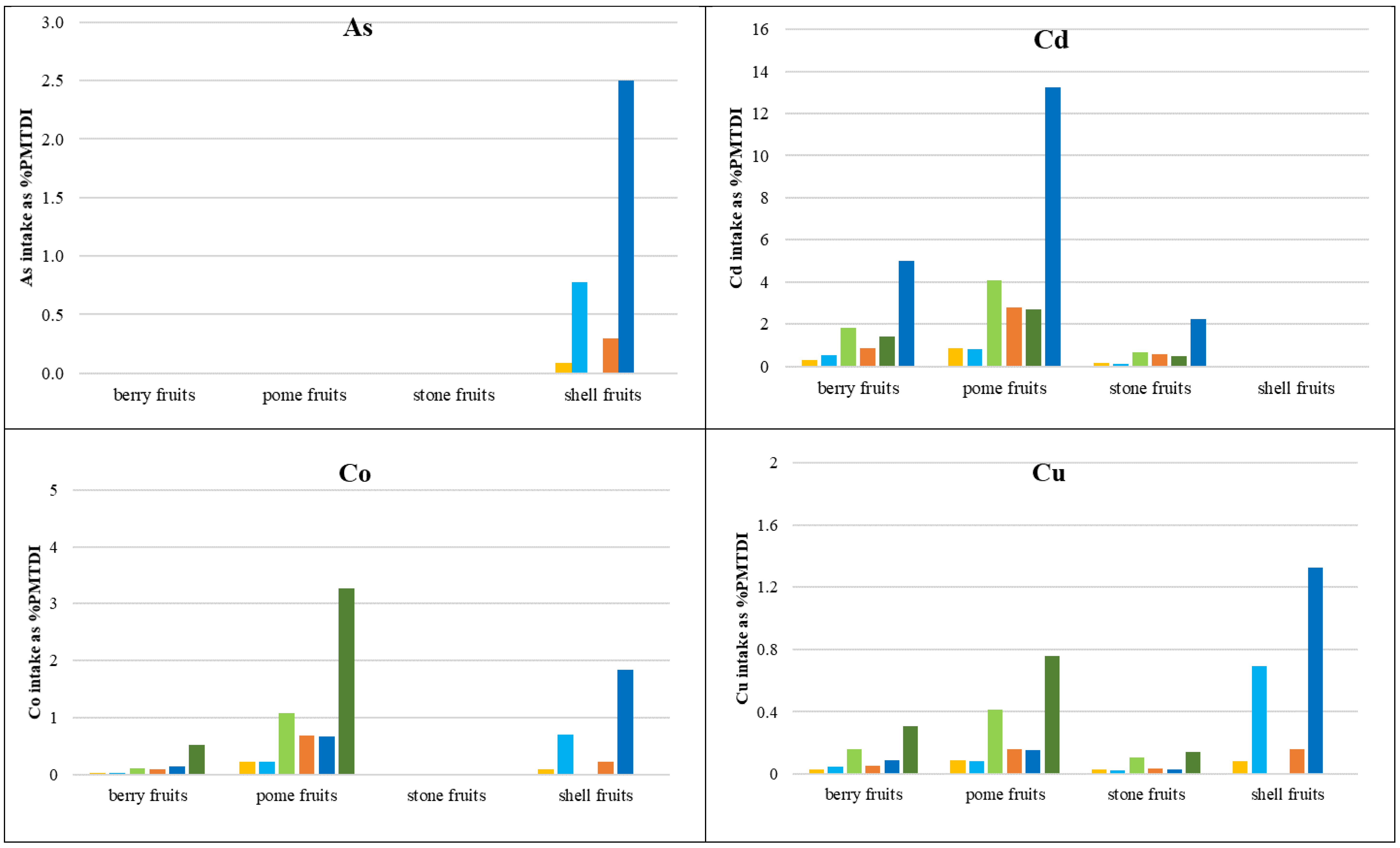
3.3.1. Daily Intake Rates
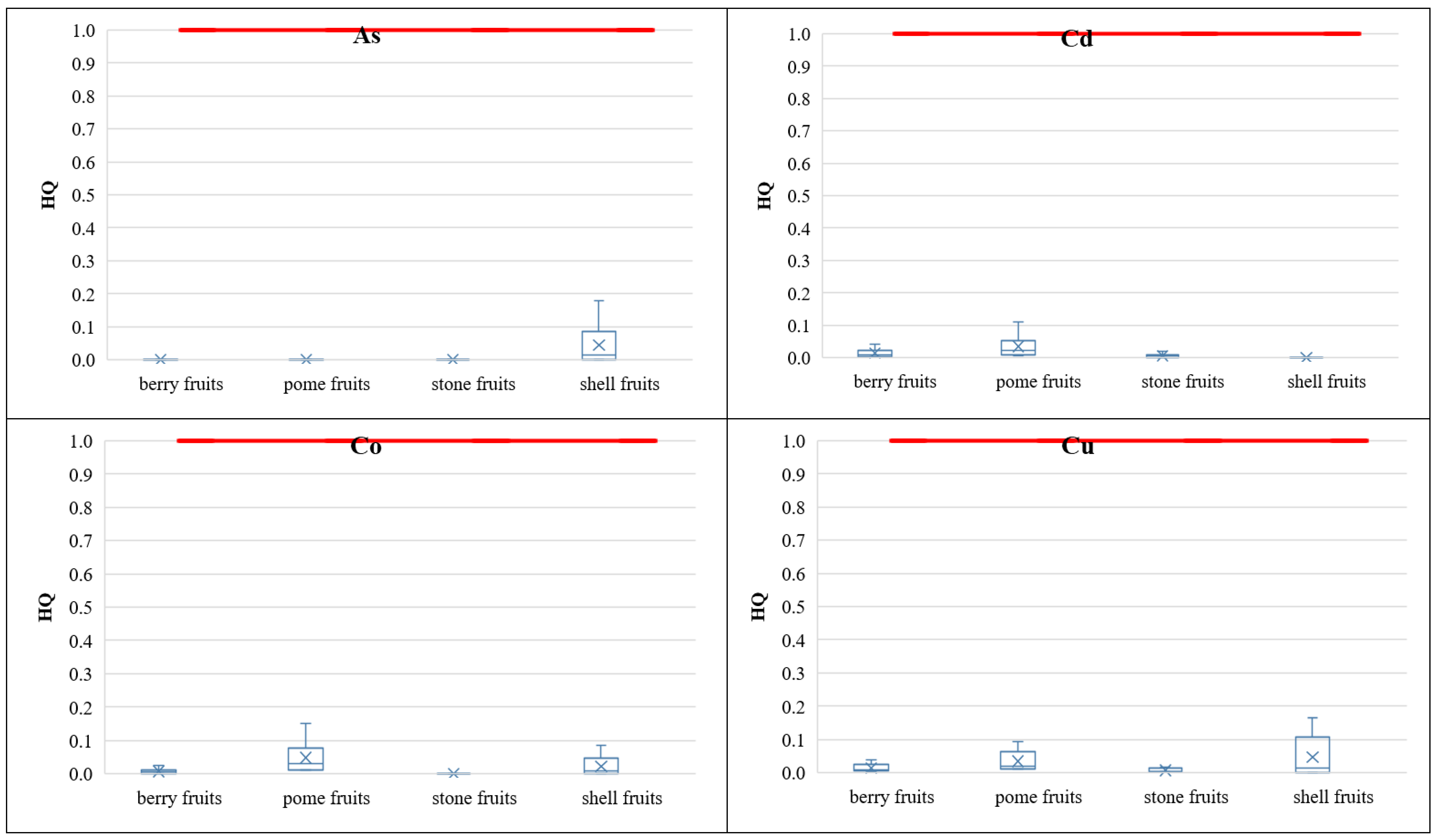
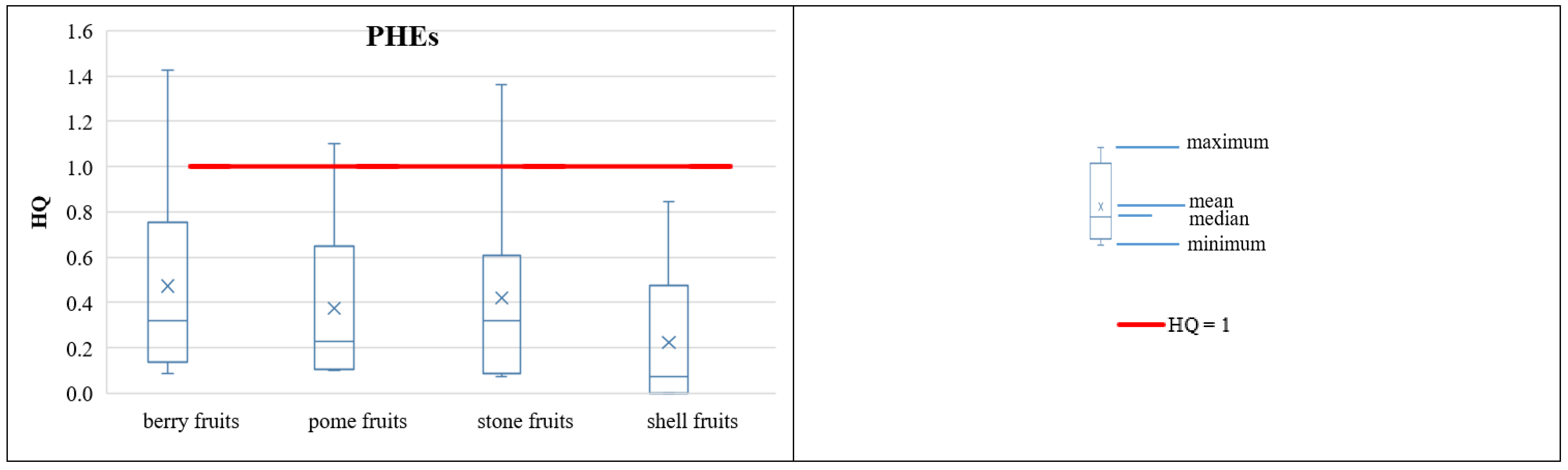
3.3.2. Non-Carcinogenic Risk of PHEs
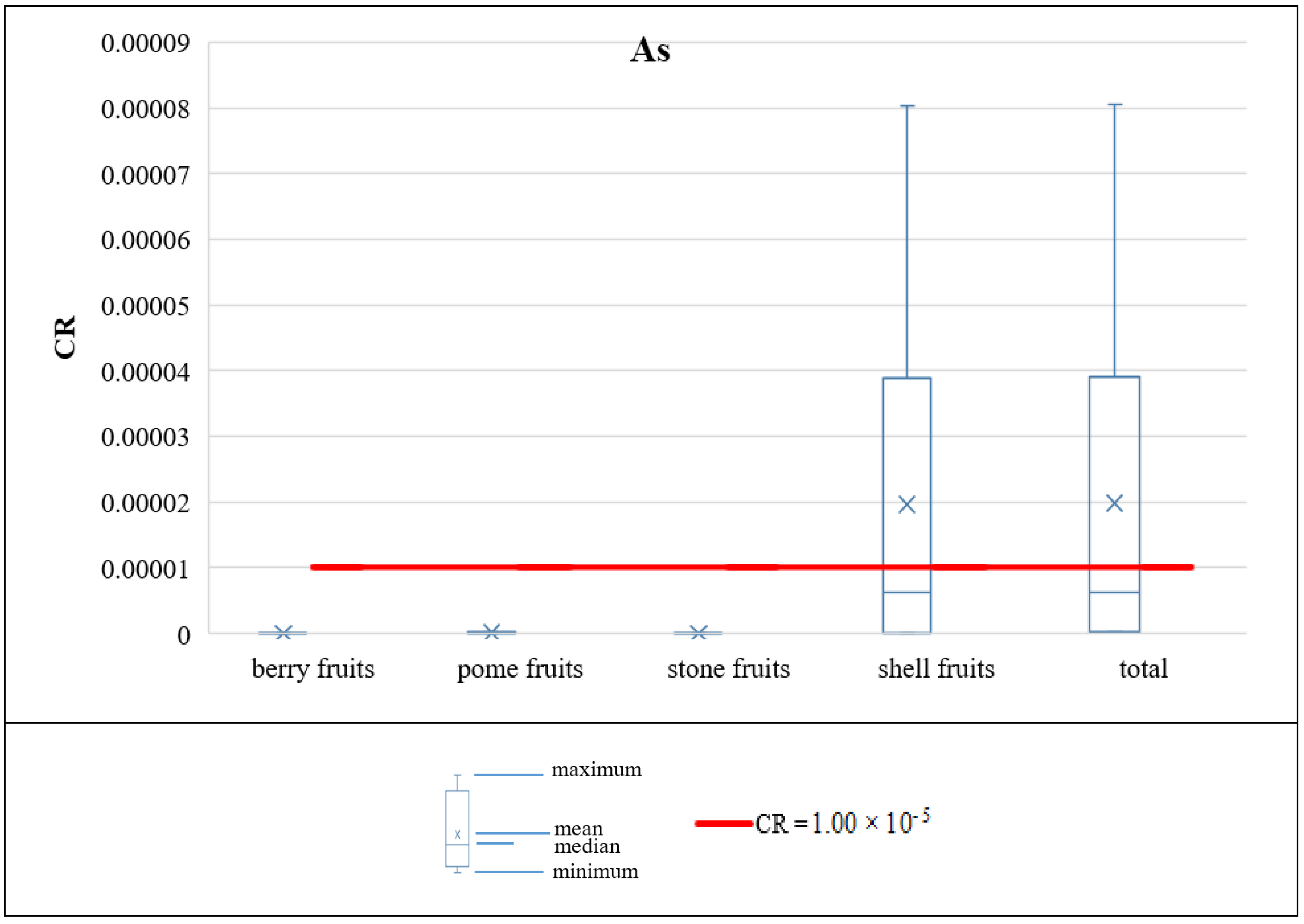
3.3.3. Carcinogenic Risk of PHEs
3.3.4. Margin of Exposure to Pb
3.3.5. Uncertainties in HHRA
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Vicente, A.R.; Manganaris, G.A.; Sozzi, G.O.; Crisosto, C.H. Nutritional quality of fruits and vegetables. In Postharvest Handling: A Systems Approach, 2nd ed.; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Elsevier—Academic Press: Waltham, MA, USA, 2009; ISBN 978-0-12-374112-7. [Google Scholar]
- Raj, R.D.A. Nutritional composition of fruits of Alangium salviifolium, ssp. Sundanum (Miq.) Bloemp.: An underutilized edible fruit plant. J. Pharmacogn. Phytochem. 2018, 7, 3145–3148. [Google Scholar]
- Zhong, T.; Xue, D.; Zhao, L.; Zhang, X. Concentration of heavy metals in vegetables and potential health risk assessment in China. Environ. Geochem. Health 2018, 40, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food and crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Karalliedde, L.; Murray, V.; Maynard, R.; Parkinson, N.H.T. Essentials of Toxicology for Health Protection: A Handbook for Field Professionals, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Rodríguez-Eugenio, N.; McLaughlin, M.; Pennock, D. Soil Pollution: A Hidden Reality; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- USEPA. Risk Assessment Guidance for Superfund. Volume 1. Human Health Evaluation Manual. Part A. Interim Report (Final); Technical Report No. PB-90-155581/XAB; US Environmental Protection Agency: Washington, DC, USA, 1989.
- Chefri, A.; Abdoun, S.; Gaci, O. Food survey: Levels and potential health risks of chromium, lead, zinc, and copper content in fruits and vegetables consumed in Algeria. Food Chem. Toxicol. 2014, 70, 48–53. [Google Scholar]
- Gupta, S.K.; Ansari, F.A.; Nasr, M.; Chabukdhara, M.; Bux, F. Multivariate analysis and health risk assessment of heavy metal contents in foodstuffs of Durban, South Africa. Environ. Monit. Assess. 2018, 190, 151. [Google Scholar] [CrossRef]
- Nie, J.; Kuang, L.; Li, Z.; Xu, W.; Wang, C.; Chen, Q.; Li, A.; Zhao, X.; Xie, H.; Zhao, D.; et al. Assessing the concentration and potential health risk of heavy metals in China’s main deciduous fruits. J. Integr. Agric. 2016, 15, 1645–1655. [Google Scholar] [CrossRef]
- Roba, C.; Roşu, C.; Piştea, I.; Ozunu, A.; Baciu, C. Heavy metal content in vegetables and fruits cultivated in Baia Mare mining area (Romania) and health risk assessment. Environ. Sci. Pollut. Res. Int. 2016, 23, 6062–6073. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A. Potentially harmful element concentrations in the vegetables cultivated on arable soils, with human health risk implications. Int. J. Environ. Res. Public Health 2019, 16, 4053. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A.; Baran, A.; Wdowin, M.; Mazur-Kajta, K.; Czech, T. The contents of the potentially harmful elements in the arable soils of southern Poland, with the assessment of ecological and health risks: A case study. Environ. Geochem. Health 2019. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A.; Baran, A.; Mazur-Kajta, K.; Czech, T. Geochemical fractions of the agricultural soils of southern Poland and the assessment of the potentially harmful element mobility. Minerals 2019, 9, 674. [Google Scholar] [CrossRef]
- USEPA. Method 6020B: Inductively Coupled Plasma—Mass Spectrometry. Revision 2; US Environmental Protection Agency: Washington, DC, USA, 2014.
- ISO. Water Quality—Use of Inductively Induced Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of 62 Elements; ISO 17294-2:2003; International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- USEPA. Exposure Factors Handbook: 2011 Edition; Technical Report No. EPA/600/R-09/052F; US Environmental Protection Agency: Washington, DC, USA, 2011.
- ISO. Water Quality—Use of Inductively Induced Plasma Mass Spectrometry (ICP-MS)—Part 1: General Guidelines; ISO 17294-1:2007; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- ISO. Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); ISO 11885:2009; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- WHO. GEMS/Food-EURO Second Workshop on Reliable Evaluation of Low-Level Contamination of Food: Report on a Workshop in the Frame of GEMS Food-EURO; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Inacio, M.; Neves, O.; Pereira, V.; da Silva, E.F. Levels of selected potential harmful elements (PHEs) in soils and vegetables used in diet of the population living in the surroundings of the Estarreja Chemical Complex (Portugal). Appl. Geochem. 2014, 44, 38–44. [Google Scholar] [CrossRef]
- Wang, G.; Su, M.Y.; Chen, Y.H.; Lin, F.F.; Luo, D.; Gao, S.F. Transfer characteristic of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ. Pollut. 2006, 144, 127–135. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Dietary Exposure Assessment of Chemicals in Food. Report of Joint FAO/WHO Consultation; WHO Library: Annapolis, MD, USA, 2005. [Google Scholar]
- Nosecka, B. No. 54/2019 Fruits and Vegetables Market: State and Perspectives; Institute of Agricultural and Food Economics—National Research Institute: Warsaw, Poland, 2019; ISSN 1231-2584. [Google Scholar]
- USEPA. Update for Chapter 9 of the Exposure Factors Handbook. Intake of Fruits and Vegetables; Technical Report No. EPA/600/R-18/098F; US Environmental Protection Agency: Washington, DC, USA, 2018.
- Neale, E.P.; Tapsell, L.C.; Martin, A.; Batterham, M.J.; Wibisono, C.; Probst, Y.C. Impact of providing walnut samples in a lifestyle intervention for weight loss: A secondary analysis of the HealthTrack trial. Food Nutr. Res. 2017, 61, 1344522. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.G.M.; Schincaglia, R.M.; Pimentel, G.D.; Mota, J.F. Nuts and human health outcomes: A systematic review. Nutrients 2017, 9, 1311. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Risk Assessment Guidance for Superfund: Volume III—Part A, Process for Conducting Probabilistic Risk Assessment; US Environmental Protection Agency: Washington, DC, USA, 2001.
- USEPA. Regional Screening Level (RSL) Summary Table (TR = 1E-06, HQ = 1), April 2019; US Environmental Protection Agency: Washington, DC, USA, 2019.
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on lead in food. EFSA J. 2010, 8. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005, 282. [Google Scholar] [CrossRef]
- Wang, M.; Liang, B.; Zhang, W.; Chen, K.; Zhang, Y.; Zhou, H.; Cheng, Y.; Liu, H.; Zhong, X.; Li, Y.; et al. Dietary lead exposure and associated health risks in Guangzhou, China. Int. J. Environ. Res. Public Health 2019, 16, 1417. [Google Scholar] [CrossRef]
- Dziubanek, G.; Piekut, A.; Rusin, M.; Baranowska, R.; Hajok, I. Contamination of food crops grown on soils with elevated heavy metals content. Ecotoxicol. Environ. Saf. 2015, 118, 183–189. [Google Scholar] [CrossRef]
- Dziubanek, G.; Baranowska, R.; Ćwieląg-Drabek, M.; Spychała, A.; Piekut, A.; Rusin, M.; Hajok, I. Cadmium in edible plants from Silesia, Poland, and its implications for health risk in populations. Ecotoxicol. Environ. Saf. 2017, 142, 8–13. [Google Scholar] [CrossRef]
- Patil, H.; Tank, R.V.; Bennurmath, P.; Doni, S. Role of zinc, copper and boron in fruit crops: A review. Int. J. Chem. Stud. 2018, 6, 1040–1045. [Google Scholar]
- Gad, N.; Hassan, N.M.K. Response of growth and yield of sweet pepper (Capsicum annuum L.) to cobalt nutrition. World Appl. Sci. J. 2013, 21, 760–765. [Google Scholar]
- Smolders, E. Cadmium uptake by plants. Int. J. Occup. Med. Environ. Health 2001, 14, 177–183. [Google Scholar]
- Kashem, M.A.; Singh, B.R. Metal availability in contaminated soils: II. Uptake of Cd, Ni and Zn in rice plants grown under flooded culture with organic matter addition. Nutr. Cycl. Agroecosyst. 2001, 61, 257–266. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil-plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Hayat, S.; Ahmad, A. Nickel: An overview of uptake, essentiality and toxicity in plants. Bull. Environ. Contam. Toxicol. 2011, 86, 1–17. [Google Scholar] [CrossRef]
- He, S.; He, Z.; Yang, X.; Baligar, V.C. Mechanisms of nickel uptake and hyperaccumulation by plants and implications for soil remediation. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 117, pp. 117–179. [Google Scholar]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremed. 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Cabala, J.; Smieja-Król, B.; Jablonska, M.; Chrost, L. Mineral components in a peat deposit: Looking for signs of early mining and smelting activities in Silesia-Cracow region (southern Poland). Environ. Earth Sci. 2013, 69, 2559–2568. [Google Scholar] [CrossRef]
- Tschan, M.; Robinson, B.H.; Nodari, M.; Schulin, R. Antimony uptake by different plant species from nutrient solution, agar and soil. Environ. Chem. 2009, 6, 144–152. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Kazantzis, G. Thallium in the environment and health effects. Environ. Geochem. Health 2000, 22, 275–280. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Woch, M.W.; Kapusta, P. Inconspicuous waste heaps left by historical Zn–Pb mining are hot spots of soil contamination. Geoderma 2014, 235–236, 1–8. [Google Scholar] [CrossRef]
- Potysz, A.; Kierczak, J.; Pietranik, A.; Kądziołka, K. Mineralogical, geochemical, and leaching study of historical Cu-slags issued from processing of the Zechstein formation (Old Copper Basin, south-western Poland). Appl. Geochem. 2018, 98, 22–35. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead uptake, toxicity, and detoxification in plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [PubMed]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Cindrić, I.J.; Zeiner, M.; Hlebec, D. Mineral composition of elements in walnuts and walnut oils. Int. J. Environ. Res. Public Health 2018, 15, 2674. [Google Scholar] [CrossRef]
- Grembecka, M.; Szefer, P. Comparative assessment of essential and heavy metals in fruits from different geographical regions. Environ. Monit. Assess. 2013, 185, 9139–9160. [Google Scholar] [CrossRef]
- Figurska-Ciura, D.; Łoźna, K.; Styczyńska, M. Cadmium, lead, zinc and copper contents in selected vegetables and fruit from garden allotments of the south-western Poland. Pol. J. Food Nutr. Sci. 2007, 57, 137–143. [Google Scholar]
- Zupka, S.; Vollmannová, A.; Harangozo, L.; Slávik, M.; Medvecký, M. Risk of contamination of wild berries from upper Orava region by cadmium. Potravinarstvo 2016, 10, 126–131. [Google Scholar] [CrossRef][Green Version]
- Bednarek, W.; Tkaczyk, P.; Dresler, S. Contents of heavy metals as a criterion for apple quality assessment and soil properties. Pol. J. Soil Sci. 2007, 40, 47–56. [Google Scholar]
- Bednarek, W.; Tkaczyk, P.; Dresler, S. Contents of heavy metals as a criterion of the quality of strawberry fruit and soil properties. Pol. J. Soil Sci. 2006, 39, 165–174. [Google Scholar]
- European Union. Commission regulation (EC) No 1881/2006 of 19 December 2006: Setting maximum levels for certain contaminants in foodstuff. Off. J. Eur. Union 2006, 49, 364. [Google Scholar]
- Gambuś, F.; Wieczorek, J. Pollution of fertilizers with heavy metals. Ecol. Chem. Eng. A 2012, 19, 353–360. [Google Scholar]
- Irshad, M.; Malik, A.H.; Shaukat, S.; Mushtaq, S.; Ashraf, M. Characterization of heavy metals in livestock manures. Pol. J. Environ. Stud. 2013, 22, 1257–1262. [Google Scholar]
- FAO. Fertilizers Use by Crop in Poland; Food and Agriculture Organization of the UN: Rome, Italy, 2003. [Google Scholar]
- Motesharezadeh, B.; Etesami, H.; Bagheri-Novair, S.; Amirmokri, H. Fertilizer consumption trend in developing countries vs developed countries. Environ. Monit. Assess. 2017, 189, 103. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plant nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants. Thirty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series No. 776; WHO: Geneva, Switzerland, 1989. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. 73rd Joint FAO/WHO Expert Committee on Food Additives (JECFA) Meeting—Food Additives and Contaminants. Summary and Conclusions; Technical Report No. JECFA/73/SC; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Baars, A.J.; Theelen, R.M.C.; Janssen, P.J.C.M.; Hesse, J.M.; van Apeldoorn, M.E.; Meijerink, M.C.M.; Verdam, L.; Zeilmaker, M.J. Re-Evaluation of Human-Toxicological Maximum Permissible Risk Levels; RIVM Report 711701 025; National Institute of Public Health and the Environment: Bilthoven, The Netherlands, 2001; Available online: https://www.rivm.nl/bibliotheek/rapporten/711701025.pdf (accessed on 18 June 2019).
- Joint FAO/WHO Expert Committee on Food Additives. Joint FAO/WHO Food Standards Programme: Codex Alimentarius Commission; Technical Report No. ALI-NORM01/12A; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001; pp. 1–289. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives. Mercury (addendum). In Safety Evaluation of Certain Contaminants in Food; WHO Food Additives Series 63; FAO JECFA Monographs 8; WHO: Geneva, Switzerland; Food and Agriculture Organization of the UN: Rome, Italy, 2011. [Google Scholar]
- WHO. Guidelines for Drinking Water Quality, 3rd Edition: Volume 1—Recommendations. Incorporating First and Second Addenda; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Sander, S.; Kappenstein, O.; Ebner, I.; Fritsch, K.A.; Schmidt, R.; Pfaff, K.; Luch, A. Release of aluminum and thallium ions from uncoated food contact materials made of aluminum alloys into food and food simulant. PLoS ONE 2018. [Google Scholar] [CrossRef]
- Ministry of the Environment. Regulation of the Minister of the Environment of 1 September 2016 on the Conduct of the Assessment of Contamination of the Surface of the Earth; OJ 2016, Item 1395; Ministry of the Environment: Warsaw, Poland, 2016.
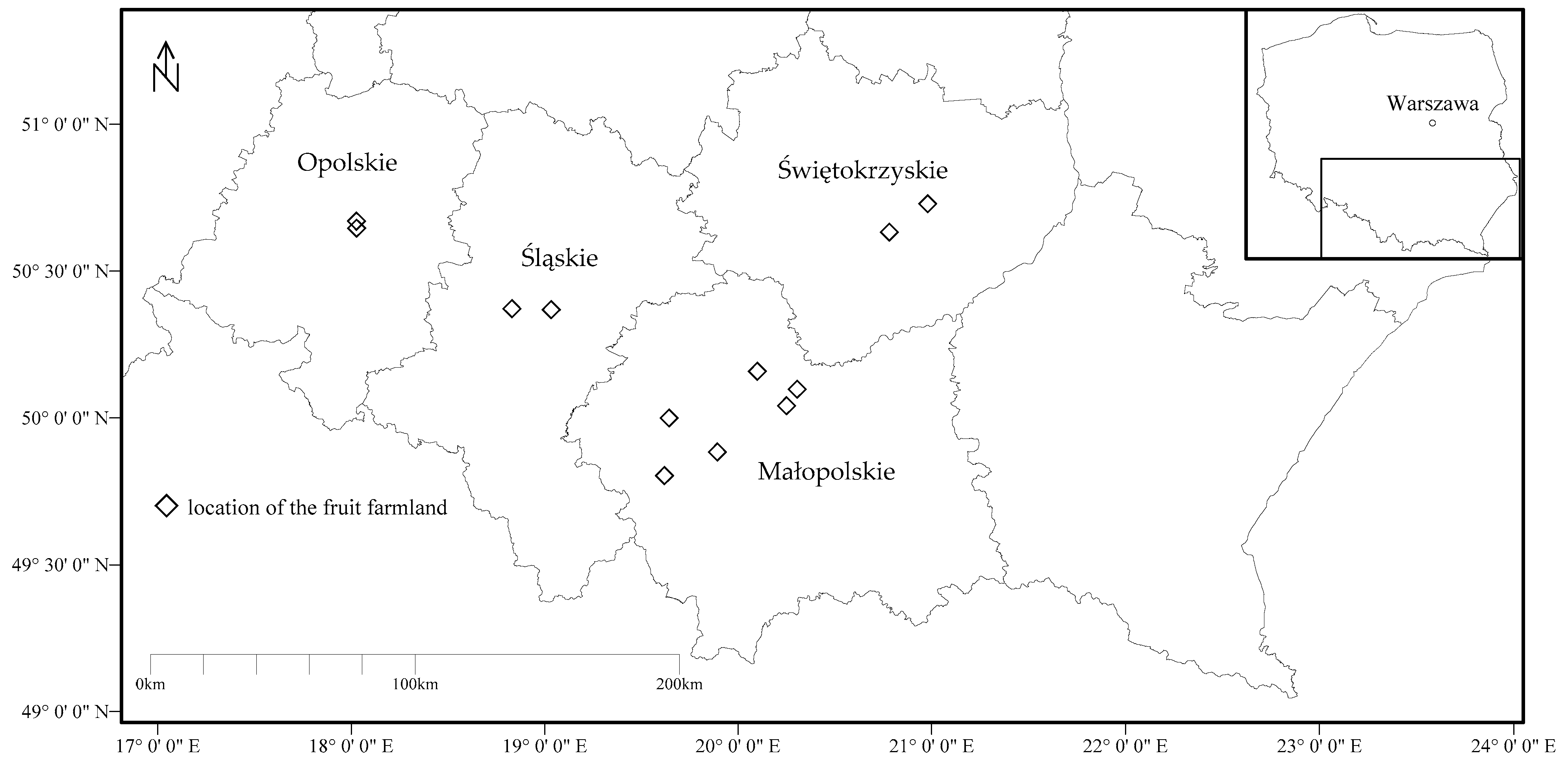



| No. | Common Name | Botanical Name | Number of Samples (n) | Group of Fruit |
|---|---|---|---|---|
| 1. | Black currant | Ribes nigrum | 3 | berry fruit |
| 2. | Blackberry | Rubus fruticosus | 2 | berry fruit |
| 3. | Blueberry | Vaccinium myrtillus | 2 | berry fruit |
| 4. | Gooseberry | Ribes uva-crispa | 4 | berry fruit |
| 5. | Grapes | Vitis vinifera | 5 | berry fruit |
| 6. | Raspberry | Rubus idaeus | 5 | berry fruit |
| 7. | Red currant | Ribes rubrum | 6 | berry fruit |
| 8. | Strawberry | Fragaria × ananassa Duchesne | 6 | berry fruit |
| 9. | Wild strawberry | Fragaria vesca | 2 | berry fruit |
| 10. | Apple | Malus domestica | 7 | pome fruit |
| 11. | Chokeberry | Aronia melanocarpa | 3 | pome fruit |
| 12. | Hawthorn | Crataegus oxyacantha | 1 | pome fruit |
| 13. | Pear | Pyrus communis | 4 | pome fruit |
| 14. | Rosehip | Rosa canina | 3 | pome fruit |
| 15. | Walnut | Juglans regia | 6 | shell fruit |
| 16. | Apricot | Prunus armeniaca | 4 | stone fruit |
| 17. | Cherry | Prunus cerasus | 5 | stone fruit |
| 18. | Gean | Prunus avium | 6 | stone fruit |
| 19. | Nectarine | Prunus persica var. nucipersica | 2 | stone fruit |
| 20. | Peach | Prunus persica | 2 | stone fruit |
| 21. | Plum | Prunus domestica | 9 | stone fruit |
| IR (g ww./Person-Day) | |||
|---|---|---|---|
| Type of Fruit | Adult PL | Adult USEPA | Child USEPA |
| Berry fruits | 14.0 | 23.8 | 18.0 |
| Pome fruits | 38.4 * | 37.1 | 39.3 |
| Stone fruits | 12.3 | 9.80 | 10.1 |
| Shell fruits | 3.56 ** | 30.0 *** | nd |
| Sum | 68.3 | 100.7 | 67.4 |
| PHEs | Statistical Parameters | Berry Fruits (n = 35) | Pome Fruits (n = 18) | Shell Fruits (Walnut) (n = 6) | Stone Fruits (n = 28) |
|---|---|---|---|---|---|
| As | min | ND | ND | nd | ND |
| mean | 0.039 | ||||
| SD | 0.013 | ||||
| max | 0.143 | ||||
| P95 | 0.125 | ||||
| Cd | min | nd | nd | ND | nd |
| mean | 0.023 | 0.021 | 0.012 | ||
| SD | 0.006 | 0.005 | 0.004 | ||
| max | 0.081 | 0.116 | 0.069 | ||
| P95 | 0.058 | 0.044 | 0.027 | ||
| MAC | 0.05 | ||||
| Co | min | nd | nd | nd | ND |
| mean | 0.006 | 0.010 | 0.023 | ||
| SD | 0.002 | 0.006 | 0.006 | ||
| max | 0.014 | 0.058 | 0.062 | ||
| P95 | 0.013 | 0.023 | 0.060 | ||
| Cu | min | 0.003 | 0.006 | 3.082 | nd |
| mean | 0.706 | 0.710 | 8.105 | 0.797 | |
| SD | 0.142 | 0.092 | 0.202 | 0.120 | |
| max | 2.300 | 2.292 | 15.478 | 2.754 | |
| P95 | 1.356 | 1.254 | 14.603 | 1.576 | |
| Ni | min | nd | nd | ND | nd |
| mean | 0.414 | 0.701 | 0.186 | ||
| SD | 0.323 | 0.390 | 0.117 | ||
| max | 1.624 | 2.230 | 0.790 | ||
| P95 | 0.967 | 1.300 | 0.581 | ||
| Pb | min | nd | nd | ND | nd |
| mean | 0.166 | 0.095 | 0.203 | ||
| SD | 0.021 | 0.008 | 0.020 | ||
| max | 0.713 | 0.390 | 2.069 | ||
| P95 | 0.322 | 0.200 | 0.520 | ||
| MAC | 0.20/0.10 | ||||
| Sb | min | nd | nd | nd | nd |
| mean | 0.016 | 0.058 | 0.024 | 0.029 | |
| SD | 0.008 | 0.016 | 0.008 | 0.009 | |
| max | 0.077 | 0.240 | 0.121 | 0.197 | |
| P95 | 0.034 | 0.328 | 0.097 | 0.081 | |
| Tl | min | nd | nd | nd | nd |
| mean | 0.006 | 0.003 | 0.002 | 0.013 | |
| SD | 0.001 | 0.001 | 0.001 | 0.001 | |
| max | 0.050 | 0.009 | 0.007 | 0.064 | |
| P95 | 0.020 | 0.008 | 0.006 | 0.051 | |
| Zn | min | 0.78 | 0.37 | 31.9 | 0.49 |
| mean | 2.31 | 2.55 | 35.6 | 1.54 | |
| SD | 0.96 | 1.07 | 1.99 | 0.76 | |
| max | 4.76 | 5.34 | 37.7 | 3.75 | |
| P95 | 3.24 | 3.14 | 37.7 | 2.78 | |
| PHEs | Varimax Rotated | |||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |
| Cd | −0.008 | −0.871 | −0.119 | −0.115 |
| Co | 0.886 | −0.347 | 0.116 | 0.149 |
| Cu | 0.958 | 0.132 | −0.006 | −0.221 |
| Ni | 0.131 | −0.866 | 0.015 | 0.397 |
| Pb | −0.232 | −0.372 | −0.219 | −0.837 |
| Sb | 0.039 | 0.411 | −0.744 | 0.192 |
| Tl | −0.148 | 0.208 | 0.835 | −0.094 |
| Zn | 0.939 | 0.231 | 0.002 | −0.201 |
| Eigenvalues | 2.68 | 2.05 | 1.33 | 1.03 |
| Explained variance % | 33.5 | 25.6 | 16.6 | 12.9 |
| Cumulative variance % | 33.5 | 59.1 | 75.7 | 88.5 |
| Fruit Intake Scenario | MOE (Mean Exposure) | MOE (P95 Exposure) |
|---|---|---|
| Adult PL | 15.7 | 5.52 |
| Adult USEPA | 14.0 | 4.89 |
| Child USEPA | 1.64 | 0.58 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszecka-Kosowska, A. Human Health Risk Assessment and Potentially Harmful Element Contents in the Fruits Cultivated in the Southern Poland. Int. J. Environ. Res. Public Health 2019, 16, 5096. https://doi.org/10.3390/ijerph16245096
Gruszecka-Kosowska A. Human Health Risk Assessment and Potentially Harmful Element Contents in the Fruits Cultivated in the Southern Poland. International Journal of Environmental Research and Public Health. 2019; 16(24):5096. https://doi.org/10.3390/ijerph16245096
Chicago/Turabian StyleGruszecka-Kosowska, Agnieszka. 2019. "Human Health Risk Assessment and Potentially Harmful Element Contents in the Fruits Cultivated in the Southern Poland" International Journal of Environmental Research and Public Health 16, no. 24: 5096. https://doi.org/10.3390/ijerph16245096
APA StyleGruszecka-Kosowska, A. (2019). Human Health Risk Assessment and Potentially Harmful Element Contents in the Fruits Cultivated in the Southern Poland. International Journal of Environmental Research and Public Health, 16(24), 5096. https://doi.org/10.3390/ijerph16245096





