Evaluating Biochar Impact on Topramezone Adsorption Behavior on Soil under No-Tillage and Rotary Tillage Treatments: Isotherms and Kinetics
Abstract
:1. Introduction
- Topramezone adsorption behavior under no-tillage and rotary tillage treatments,
- Biochar addition impact on topramezone adsorption under no-tillage and rotary tillage treatments. It is planned to carry out both a laboratory and field-based study because there may be some differences between the lab analysis results and what really happens in the natural cropping system, which should be further examined in detail.
2. Materials and Methods
2.1. Chemicals
2.1.1. Maize Straw and Soil Sampling
2.1.2. Biochar Preparation
2.2. Biochar Characterization
2.3. Batch Adsorption Experiments
2.3.1. Adsorption Kinetics
2.3.2. Adsorption Thermodynamics
2.3.3. Thermodynamic and Kinetic Modeling
Kinetics
2.4. Statistical Analysis
3. Results and Discussion
3.1. Biochar Characterization
3.2. Batch Experiments
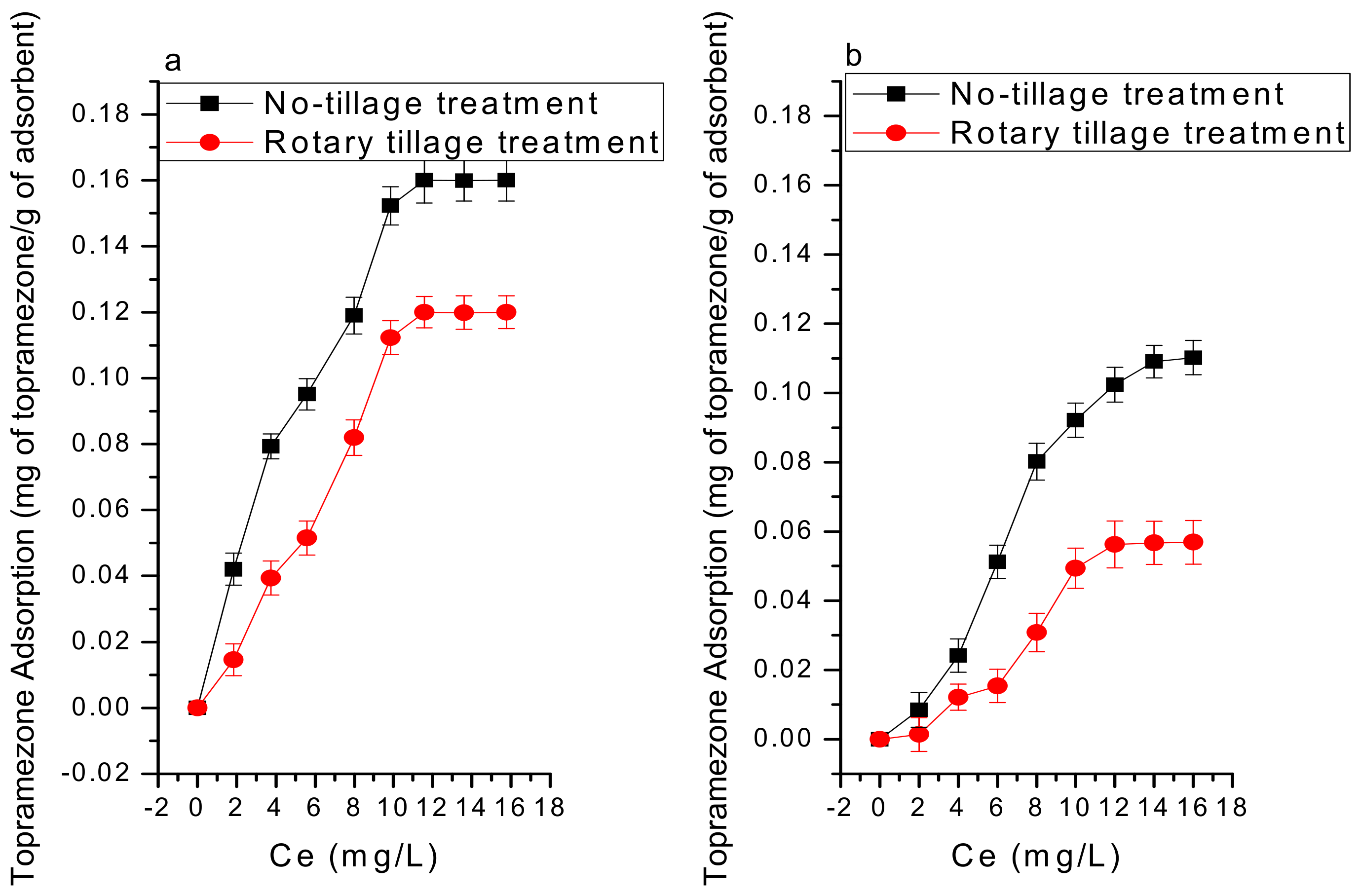
3.2.1. Adsorption Thermodynamics
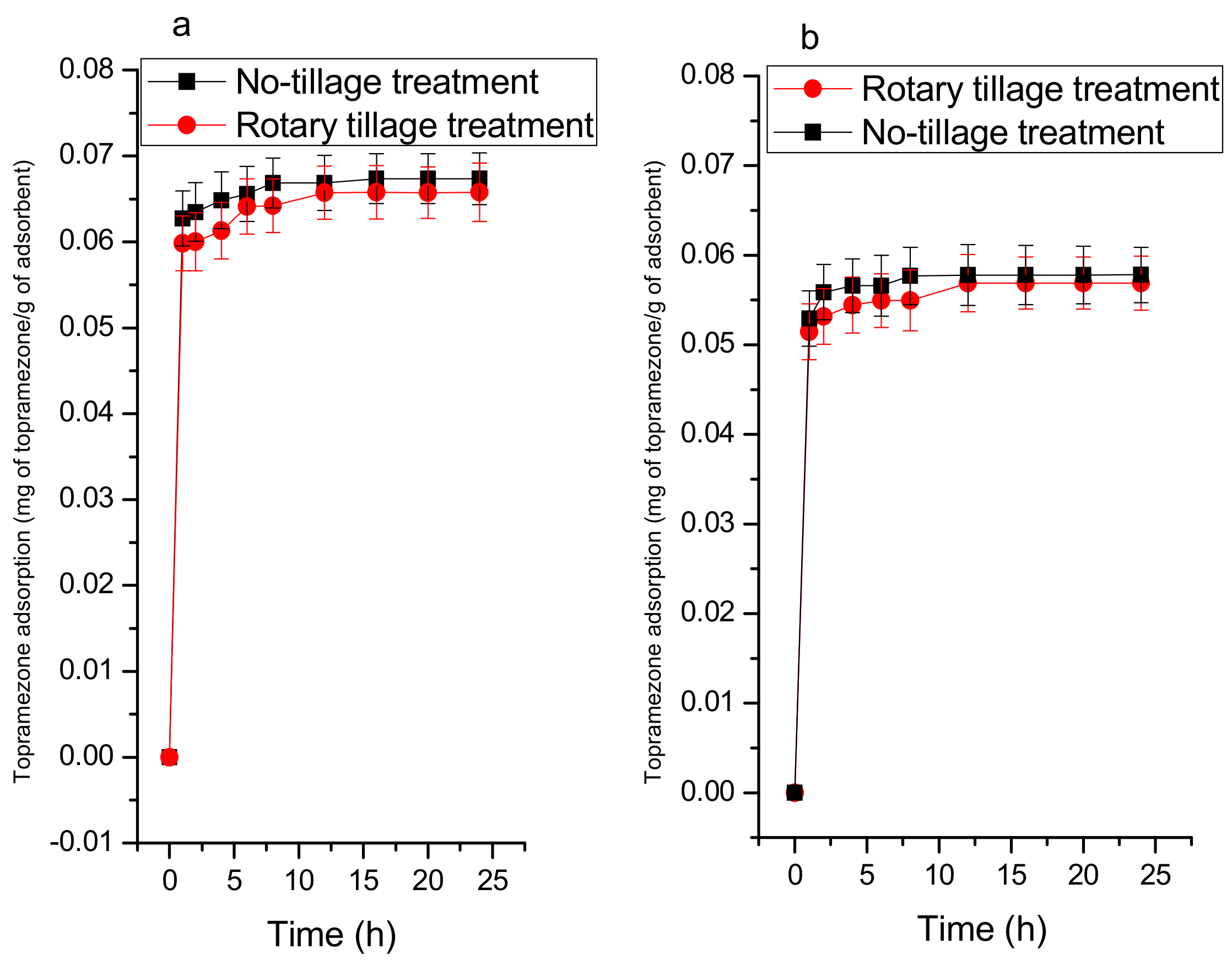
3.2.2. Adsorption Kinetics
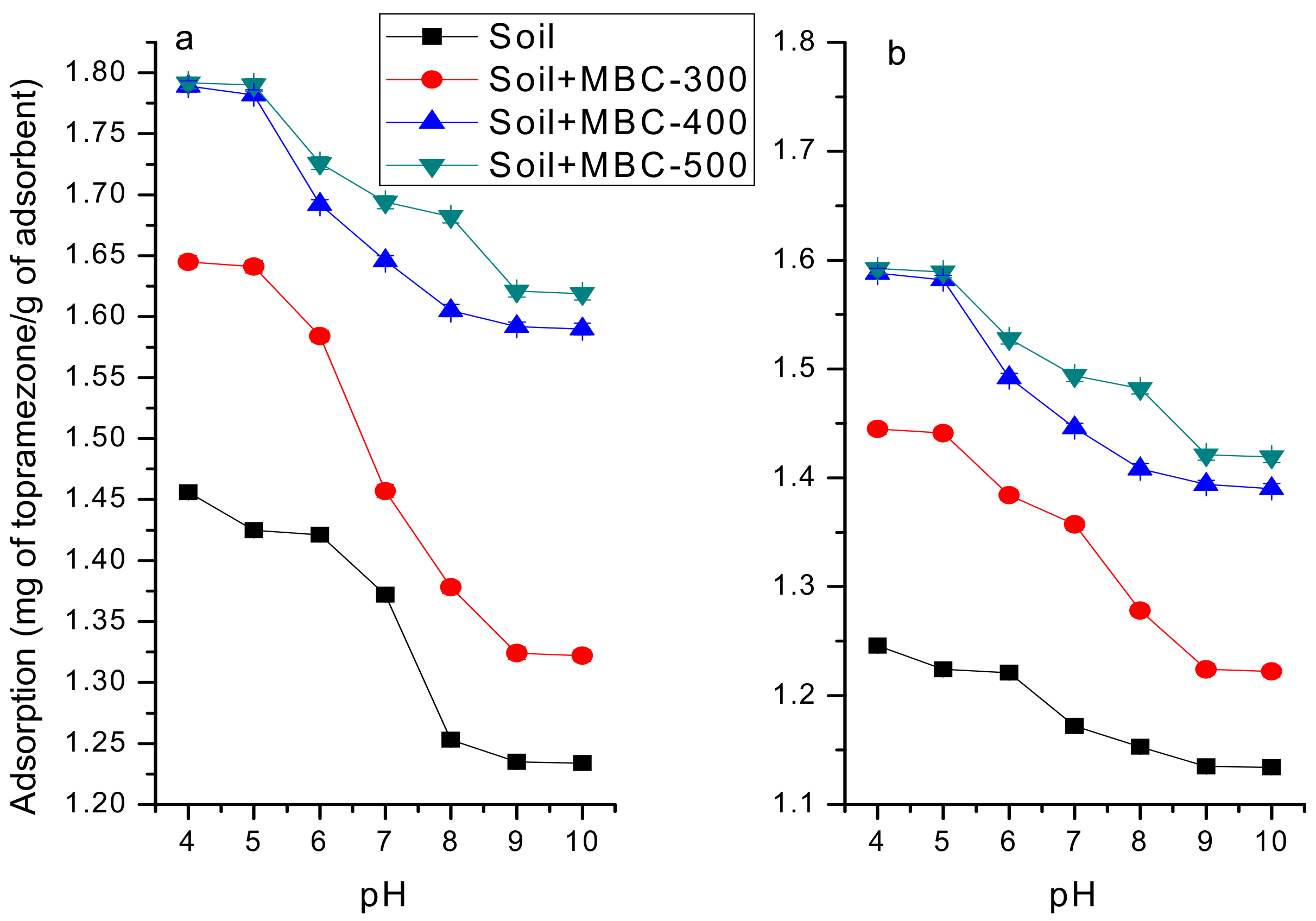
Effect of pH on Topramezone Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gereslassie, T.; Workineh, A.; Atieno, O.J.; Jun Wang, J. Determination of Occurrences, Distribution, Health Impacts of Organochlorine Pesticides in Soils of Central China. Int. J. Environ. Res. Public Health 2019, 16, 146. [Google Scholar]
- Pan, L.; Sun, J.; Li, Z.; Zhan, Y.; Xu, S.; Zhu, L. Organophosphate pesticide in agricultural soils from the Yangtze River Delta of China: concentration, distribution and risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 4–11. [Google Scholar]
- EPA (U.S. Environmental Protection Agency). 2005 c. Guidelines for Carcinogen Risk Assessment; EPA/630/P-03/001F; Risk Assessment Forum, U.S. Environmental Protection Agency: Washington, DC, USA, March 2005. Available online: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=116283 (accessed on 2 September 2019).
- Arias-Estevez, M.; Lopez-Periago, E.; Martinez-Carballo, E.; Simal-Gandara, J.; Mejuto, J.C.; Garcia-Rio, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar]
- Cook, P.G.; Favreau, G.; Dighton, J.C.; Tickell, S. Determining natural groundwater influx to a tropical river using radon, chlorofluorocarbons and ionic environmental tracers. J. Hydrol. 2003, 277, 74–88. [Google Scholar]
- Ren, X.; Yuan, X.; Sun, H. Dynamic changes in atrazine and phenanthrene sorption behaviors during the aging of biochar in soils. Environ. Sci. Pollut. Res. 2018, 25, 81–90. [Google Scholar]
- Jiang, Y.F.; Sun, H.; Yves, U.J.; Li, H.; Hu, X.F. Impact of biochar produced from post-harvest residue on the adsorption behavior of diesel oil on loess soil. Environ. Geochem. Health 2016, 38, 243–253. [Google Scholar]
- Jiang, Y.F.; Uwamungu, J.Y.; Sun, H.; Hu, X.F.; Mu, Z.F.; Zhan, H.Y. Effect of wheat-waste biochar on the adsorption behavior of benzonitrile onto loess soil. China Environ. Sci. 2016, 36, 1506–1513. (In Chinese) [Google Scholar]
- Jiang, Y.F.; Mu, Z.F.; Uwamungu, J.Y.; Sun, H.; Hu, X.F.; Zhan, H.Y. Study on the Adsorption Behavior of Atrazine onto Loess Soil in Northwest China. Res. Environ. Sci. 2016, 29, 547–552. (In Chinese) [Google Scholar]
- Du, T.; Kang, S.; Sun, J.; Zhang, X.; Zhang, J. An improved water use efficiency of cereals under temporal and spatial deficit irrigation in north China. Agric. Water Manag. 2010, 97, 66–74. [Google Scholar]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar]
- Alothman, Z.A.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar]
- Elliott, J.A.; Cessna, A.J.; Hilliard, C.R. Influence of tillage systems on water quality and quantity in Prairie Pothole wetlands. Can. Water Res. J. 2011, 26, 165–181. [Google Scholar]
- Isensee, A.R.; Sadeghi, A.M. Interactions of tillage and rainfall on atrazine leaching under field and laboratory conditions. Chemosphere 1997, 34, 2715–2723. [Google Scholar]
- Cullum, R.F. Macropore flow estimations under no-till and till systems. Catena 2009, 78, 87–91. [Google Scholar]
- Siczek, A.; Horn, R.; Lipiec, J.; Usowicz, B.; Lukowsk, M. Effects of soil deformation and surface mulching on soil physical properties and soybean response related to weather conditions. Soil Tillage Res. 2015, 153, 175–184. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size Analysis. In Methods of Soil Analysis, Part 1; Klute, A., Ed.; American Society of Agronomy Madison: Madison, WI, USA, 1986; pp. 91–100. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon and Organic Matter. In Methods of Soil Analysis, Part 2; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; America Society of Agronomy: Madison, WI, USA, 1982; Volume 2. [Google Scholar]
- Studzińska, S.; Sprynskyy, M.; Buszewski, B. Study of sorption kinetics of some ionic liquids on different soil types. Chemosphere 2008, 71, 2121–2128. [Google Scholar]
- Chun, Y.; Sheng, G.; Chiou, G.T.; Xing, X. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar]
- OECD/OCDE. Lignes directrices de l’OCDE pour les essais de produits chimiques, Section 1 Essai n° 106: Adsorption/désorption selon une méthode d’équilibres successifs; OECD Publishing: Paris, France, 2000. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar]
- Freundlich, H. Uber die adsorption in losungen (Adsorption in solution). Phys. Chem. Period. 1906, 57, 384–470. [Google Scholar]
- Dubinin, M.M.; Zaverina, E.D.; Radushkevich, L.V. Sorption and structure of active carbons. I. Adsorption of organic vapors. Rus. J. Bioorg. Chem. 1947, 21, 1351–1362. [Google Scholar]
- Liu, Y. Is the free energy change of adsorption correctly calculated? J. Chem. Eng. Data 2009, 54, 1981–1985. [Google Scholar]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar]
- Trigo, C.; Cox, L.; Spokas, K. Influence of pyrolysis temperature and hardwood species on resulting biochar properties and their effect on azimsulfuron sorption as compared to other sorbents. Sci. Total. Environ. 2016, 566, 1454–1464. [Google Scholar]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of cadmium by biochar derived from municipal sewage sludge: Impact factors and adsorption mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar]
- Yang, F.; Zhao, L.; Gao, B.; Xu, X.; Cao, X. The interfacial behavior between biochar and soil minerals and its effect on biochar stability. Environ. Sci. Technol. 2016, 50, 2264–2271. [Google Scholar]
- Jeong, C.Y.; Dodla, K.; Wang, J.J. Fundamental and molecular composition characteristics of biochars produced from sugarcane and rice crop residues and by-products. Chemosphere 2016, 142, 4–13. [Google Scholar]
- Zama, E.F.; Brian, J.; Sun, G.; Yuan, H.; Li, X.; Zhu, Y. Silicon (Si) biochar for the mitigation of arsenic (As) bioaccumulation in spinach (Spinacia oleracean) and improvement in the plant growth. J. Clean. Prod. 2018, 189, 386–395. [Google Scholar]
- Kumar, A.; Gaurav Sharma, S.; Naushad, M.; Kumar, A.; Kalia, S.; Guo, C.S.; Mola, G.T. Facile hetero-assembly of superparamagnetic Fe3O4/BiVO4 stacked on biochar for solar photo-degradation of methyl paraben and pesticide removal from soil. J. Photochem. Photobiol. A. Chem. 2017, 337, 118–131. [Google Scholar]
- Pignatello, J.J.; Oliveros, E.; Mackay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Env. Sci. Tec. 2007, 36, 1–84. [Google Scholar]
- Lonappan, L.; Rouissi, T.; Das, R.K.; Brar, S.K.; Ramirez, A.A.; Verma, M.; Surampalli, R.Y.; Valero, J.R. Adsorption of methylene blue on biochar microparticles derived from different waste materials. Waste Manag. 2016, 49, 537–544. [Google Scholar]
- Naushad, M. Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem. Eng. J. 2014, 235, 100–108. [Google Scholar]
- Tsai, W.T.; Chang, Y.M.; Lai, C.W.; Lo, C.C. Adsorption of ethyl violet dye in aqueous solution by regenerated spent bleaching earth. J. Colloid Interf. Sci. 2005, 289, 333–338. [Google Scholar]
- Chen, S.; Chen, S.Y.; Sun, H.Y.; Zhang, X.; Pei, D. Effects of Tillage Methods on Soil Evaporation and Water Use Efficiency of Winter Wheat. Chin. J. Soil Sci. 2006, 37, 34–0817. [Google Scholar]
- Zhao, D.; Zhang, J.; Duan, E.; Wang, J. Adsorption equilibrium and kinetics of dibenzothiophene from n-octane on bamboo charcoal. Appl. Surf. Sci. 2008, 254, 3242–3247. [Google Scholar]
- Chauhan, B.S.; Gill, G.S.; Preston, C. Tillage system effects on weed ecology, herbicide activity and persistence: A review. Aust. J. Exp. Agric. 2006, 46(12), 1557–1570. [Google Scholar]
- Bai, W.; Kong, L.; Guo, A. Effects of physical properties on electrical conductivity of compacted lateritic soil. J. Rock Mech. Geotech. Eng. 2013, 5, 406–411. [Google Scholar]
- Fasfous, I.I.; Radwan, E.S.; Dawoud, J.N. Kinetics, equilibrium and thermodynamics of the sorption of tetrabromobisphenol A on multiwalled carbon nanotubes. Appl. Surf. Sci. 2010, 256, 7246–7252. [Google Scholar]
- Tsai, W.T.; Lai, C.W.; Hsien, K.J. Effect of particle size of activated clay on the adsorption of paraquat from aqueous solution. J. Colloid Interface Sci. 2003, 263, 29–34. [Google Scholar]
- Al-Meshragi, M.; Hesham, G.; Aboabboud, M.M. Equilibrium and Kinetics of Chromium Adsorption on Cement Kiln Dust; WCECS: San Francisco, CA, USA, 2008. [Google Scholar]
- Rodrigues, A.E. What’s wrong with Lagergreen pseudo first order model for adsorption kinetics? Chem. Eng. J. 2016, 306, 1138–1142. [Google Scholar]
- Xu, R.K.; Xiao, S.C.; Yuan, J.H.; Zhao, A.Z. Adsorption of methyl violet from aqueous solutions by the biochars. Bioresour.Technol. 2011, 102, 10293–10298. [Google Scholar]
- Naushad, M.; Gaurav Sharma, G.; Zeid, A.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinkedpoly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241, 118263. [Google Scholar]
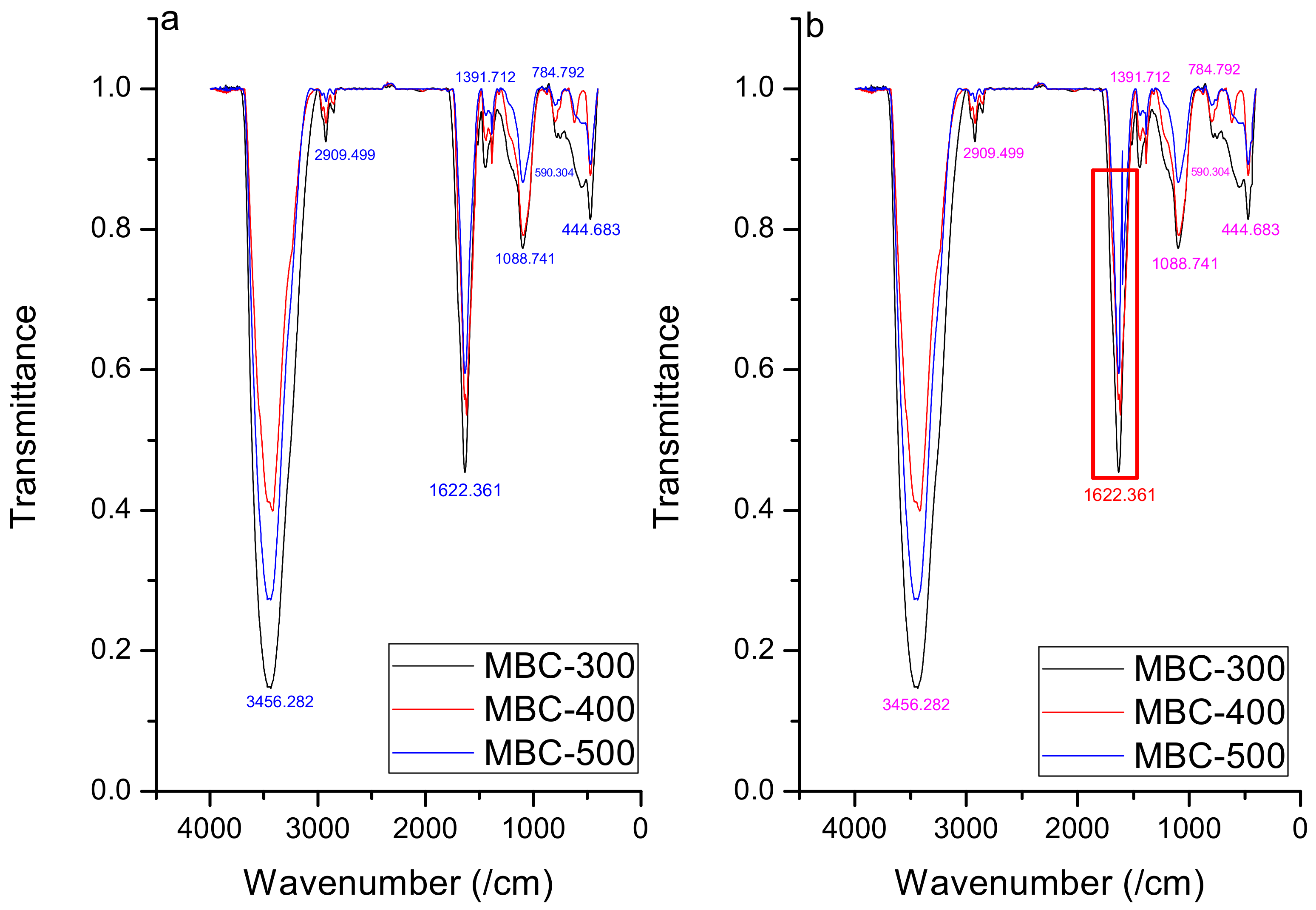

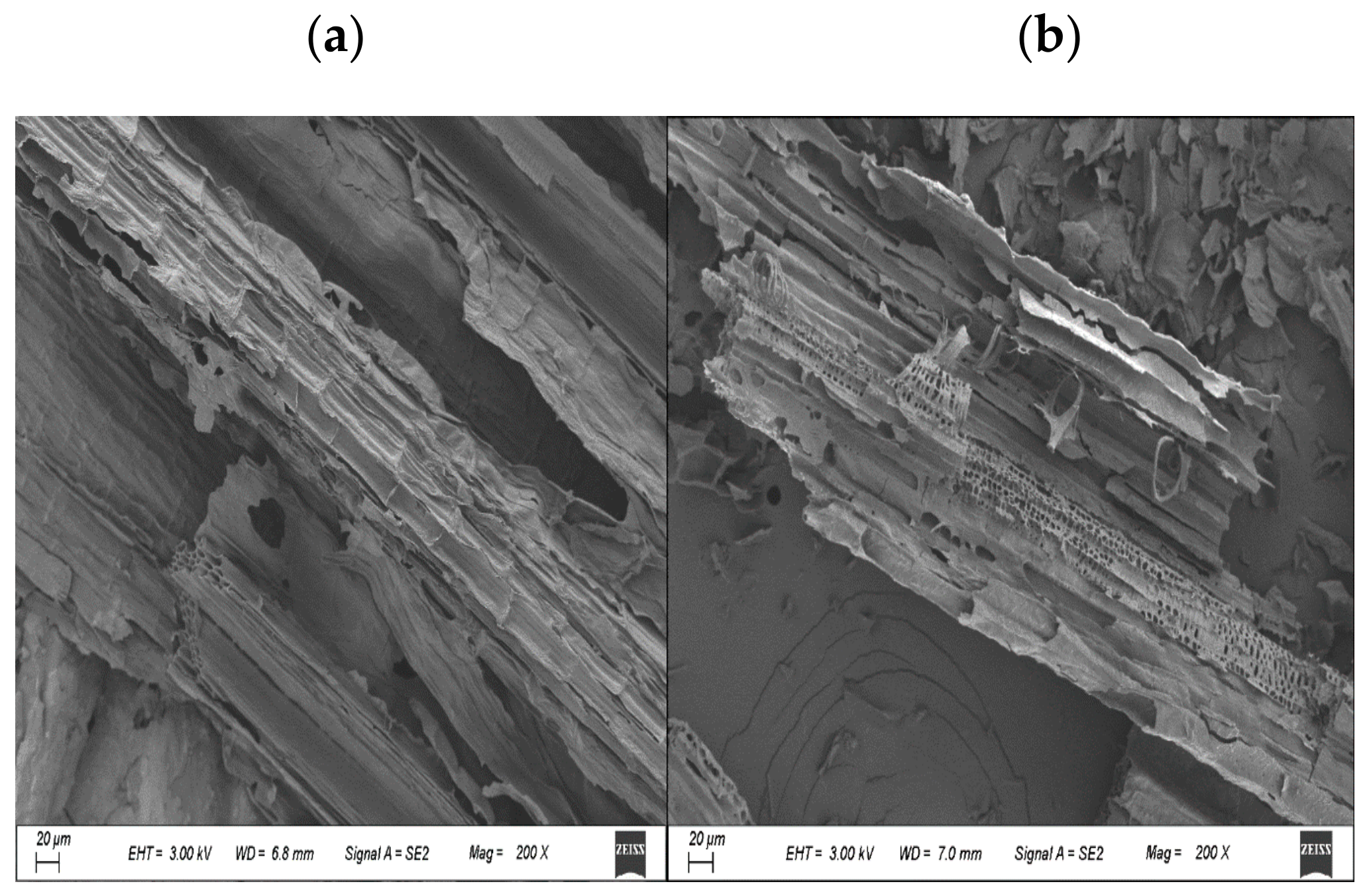

| Biochar Sample | BET Surface Area (m2/g) | BJH Adsorption Cumulative Surface Area (m2/g) | Langmuir Surface Area (m2/g) | Pore Diameter (nm) | BJH Adsorption Average Pore Diameter (nm) | SF Micro Pore Volume cm3/g |
|---|---|---|---|---|---|---|
| MBC-300 | 2.063 | 2.191 | 2.978 | 17.749 | 17.089 | 6.16 × 10−4 |
| MBC-400 | 20.286 | 21.780 | 30.339 | 14.822 | 18.820 | 7.86 × 10−3 |
| MBC-500 | 20.897 | 30.295 | 40.556 | 19.208 | 17.424 | 8.99 × 10−3 |
| Parameter | Rotary Tillage Treatment | No-Tillage Treatment |
|---|---|---|
| Value | Value | |
| Sand (%) | 24.07 ± 0.04 | 21.64 ± 0.02 |
| Silt (%) | 60.02 ± 0.02 | 60.12 ± 0.02 |
| Clay (%) | 15.91 ± 0.02 | 18.24 ± 0.01 |
| pH | 8.37 ± 0.04 | 8.36 ± 0.04 |
| Electrical Conductivity (µS/cm) | 162.20 ± 0.01 | 115.10 ± 0.01 |
| Cation Exchange Capacity (meq/100 g) | 14.03 ±0.02 | 15.11 ± 0.00 |
| Total Organic Carbon (%) | 1.583 ± 0.00 | 1.734 ± 0.00 |
| Total Organic Nitrogen (%) | 0.148 ± 0.00 | 0.158 ± 0.00 |
| Bulk Density | 1.40 ± 0.02 | 1.43 ± 0.02 |
| Langmuir Equation | Freundlich Equation | D-R Equation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KL(L/mol) | Qm (mg/g) | r2 | KF((mol/g) (L/mol)1/n) | n | r2 | Β (mol2/J−2) | lnQm (mol/g) | E (J/mol) | r2 | ||
| 15 °C | Soil NT | 0.039 | 0.129 | 0.926 | 0.005 | 0.759 | 0.807 | −5.4×10−8 | −0.142 | 9512.02 | 0.739 |
| RT | 0.021 | 0.065 | 0.914 | 0.0039 | 0.762 | 0.801 | −5.6 ×10−8 | −0.124 | 9482.02 | 0.714 | |
| S+MBC300 NT | 0.163 | 0.187 | 0.972 | 0.015 | 1.092 | 0.865 | −2.3 ×10−8 | −0.225 | 14,821.14 | 0.554 | |
| RT | 0.125 | 0.098 | 0.968 | 0.015 | 1.094 | 0.845 | −2.4 ×10−8 | −0.208 | 14,515.12 | 0.558 | |
| S+MBC400 NT | 0.225 | 0.21 | 0.989 | 0.047 | 1.927 | 0.927 | −1.7 ×10−9 | −0.252 | 60,271.07 | 0.625 | |
| RT | 0.218 | 0.143 | 0.978 | 0.042 | 2.001 | 0.889 | −1.9 ×10−9 | −0.247 | 60,249.52 | 0.611 | |
| S+MBC500 NT | 0.217 | 0.217 | 0.984 | 0.046 | 1.873 | 0.974 | −2.2 ×10−9 | −0.250 | 49,020.00 | 0.646 | |
| RT | 0.202 | 0.146 | 0.972 | 0.042 | 1.881 | 0.965 | −2.2 ×10−9 | −0.241 | 49,020.00 | 0.651 | |
| Soil NT | 0.04 | 0.085 | 0.914 | 0.0006 | 0.559 | 0.83 | −5.2 ×10−8 | −0.215 | 9456 | 0.762 | |
| RT | 0.028 | 0.059 | 0.902 | 0.0006 | 0.584 | 0.825 | −5.3 ×10−8 | −0.210 | 9446.019 | 0.658 | |
| 25 °C | S+MBC300 NT | 0.125 | 0.15 | 0.94 | 0.0007 | 0.536 | 0.903 | −2.0 ×10−8 | −0.279 | 13,074.14 | 0.656 |
| RT | 0.012 | 0.121 | 0.923 | 0.0005 | 0.561 | 0.901 | −2.1 ×10−8 | −0.244 | 49,520.00 | 0.627 | |
| S+MBC400 NT | 0.158 | 0.178 | 0.962 | 0.0081 | 0.944 | 0.927 | −1.3 ×10−9 | −0.282 | 57,071.02 | 0.62 | |
| RT | 0.032 | 0.123 | 0.928 | 0.0062 | 1.021 | 0.901 | −1.3 ×10−9 | −0.277 | 57,071.02 | 0.614 | |
| S+MBC500 NT | 0.165 | 0.175 | 0.961 | 0.0157 | 1.247 | 0.926 | −2.1 ×10−8 | −0.272 | 49,520.00 | 0.642 | |
| RT | 0.033 | 0.122 | 0.954 | 0.0095 | 1.243 | 0.913 | −2.3 ×10−8 | −0.213 | 14,821.14 | 0.645 | |
| Soil NT | 0.04 | 0.085 | 0.903 | 0.00005 | 0.556 | 0.86 | −5.4 ×10−8 | −0.174 | 9512.02 | 0.79 | |
| RT | 0.023 | 0.048 | 0.892 | 0.00005 | 0.562 | 0.885 | −5.4 ×10−8 | −0.145 | 9512.02 | 0.741 | |
| 40 °C | S+MBC300 NT | 0.036 | 0.088 | 0.938 | 0.00017 | 0.178 | 0.88 | −2.3 ×10−8 | −0.225 | 14,821.14 | 0.589 |
| RT | 0.011 | 0.073 | 0.92 | 0.00011 | 0.183 | 0.819 | −2.6 ×10−8 | −0.158 | 13,846.02 | 0.582 | |
| S+MBC400 NT | 0.08 | 0.129 | 0.938 | 0.0026 | 0.478 | 0.927 | −2.1 ×10−8 | −0.225 | 49,520.00 | 0.589 | |
| RT | 0.03 | 0.094 | 0.933 | 0.0027 | 0.478 | 0.921 | −2.4 ×10−8 | −0.166 | 14,515.12 | 0.603 | |
| S+MBC500 NT | 0.079 | 0.134 | 0.935 | 0.0048 | 0.402 | 0.804 | −2.3 ×10−8 | −0.164 | 14,821.14 | 0.578 | |
| RT | 0.031 | 0.098 | 0.933 | 0.0041 | 0.411 | 0.905 | −2.3 ×10−8 | −0.152 | 14,821.14 | 0.57 |
| SOIL | T | ΔG | ΔH | ΔS |
| (K) | (J/mol) | (J/mol) | (J/MOL/K) | |
| 288 | −1848.07 −366.531 | |||
| 298 | −1974.93 −1091.67 | −1313.73 −23,274.6 | −21.92 −78.296 | |
| 303 | −2008.07 1242.541 | |||
| MBC300 | 288 | −5270.91 −4635.64 | ||
| 298 | −4796.6 1006.563 | −64,670.1 −95,149 | −134.89 −419.655 | |
| 303 | −1742.78 1242.541 | |||
| MBC400 | 288 | −6042.38 −5966.74 | ||
| 298 | −5376.77 −1422.34 | −46,169.5 −10,2196 | −193.24 −335.126 | |
| 303 | −3753.36 −1283.7 | |||
| MBC500 | 288 | −5955.74 −5784.31 | ||
| 298 | −5484.12 −1498.54 | −44,328.9 −96,562.2 | −53.15076 −316.137 | |
| 303 | −3721.69 −1366.27 |
| Pseudo-First Order Equation | Pseudo-Second Order Equation | Intraparticle Diffusion Equation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| q1 (mg g−1) | k1(min−1) | r12 | q2 mg g−1) | k2(gmg−1min−1) | r22 | kp (gmg−1min−1/2) | cp | rp2 | |
| Soil NT RT | 0.089 0.084 | 0.075 0.070 | 0.211 0.188 | 0.021 0.020 | 22.956 23.574 | 0.950 0.942 | 0.003 0.001 | 0.032 0.0512 | 0.335 0.328 |
| Soil+MBC300NT RT | 0.092 0.090 | 0.076 0.075 | 0.208 0.204 | 0.022 0.021 | 22.006 22.537 | 0.950 0.943 | 0.008 0.005 | 0.056 0.047 | 0.735 0.562 |
| Soil+MBC400NT RT | 0.103 0.100 | 0.074 0.074 | 0.507 0.206 | 0.025 0.024 | 19.842 20.294 | 0.952 0.951 | 0.012 0.008 | 0.062 0.060 | 0.919 0.732 |
| Soil+MBC500NT RT |
0.104 0.061 |
0.076 0.069 |
0.504 0.304 |
0.025 0.024 |
19.648 19.947 |
0.949 0.937 |
0.011 0.007 |
0.063 0.060 |
0.696 0.654 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwamungu, J.Y.; Nartey, O.D.; Uwimpaye, F.; Dong, W.; Hu, C. Evaluating Biochar Impact on Topramezone Adsorption Behavior on Soil under No-Tillage and Rotary Tillage Treatments: Isotherms and Kinetics. Int. J. Environ. Res. Public Health 2019, 16, 5034. https://doi.org/10.3390/ijerph16245034
Uwamungu JY, Nartey OD, Uwimpaye F, Dong W, Hu C. Evaluating Biochar Impact on Topramezone Adsorption Behavior on Soil under No-Tillage and Rotary Tillage Treatments: Isotherms and Kinetics. International Journal of Environmental Research and Public Health. 2019; 16(24):5034. https://doi.org/10.3390/ijerph16245034
Chicago/Turabian StyleUwamungu, Jean Yves, Obemah David Nartey, Fasilate Uwimpaye, Wenxu Dong, and Chunsheng Hu. 2019. "Evaluating Biochar Impact on Topramezone Adsorption Behavior on Soil under No-Tillage and Rotary Tillage Treatments: Isotherms and Kinetics" International Journal of Environmental Research and Public Health 16, no. 24: 5034. https://doi.org/10.3390/ijerph16245034
APA StyleUwamungu, J. Y., Nartey, O. D., Uwimpaye, F., Dong, W., & Hu, C. (2019). Evaluating Biochar Impact on Topramezone Adsorption Behavior on Soil under No-Tillage and Rotary Tillage Treatments: Isotherms and Kinetics. International Journal of Environmental Research and Public Health, 16(24), 5034. https://doi.org/10.3390/ijerph16245034







