Effects of Nigella Sativa on Type-2 Diabetes Mellitus: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Selection of Research Articles
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Management
3. Results
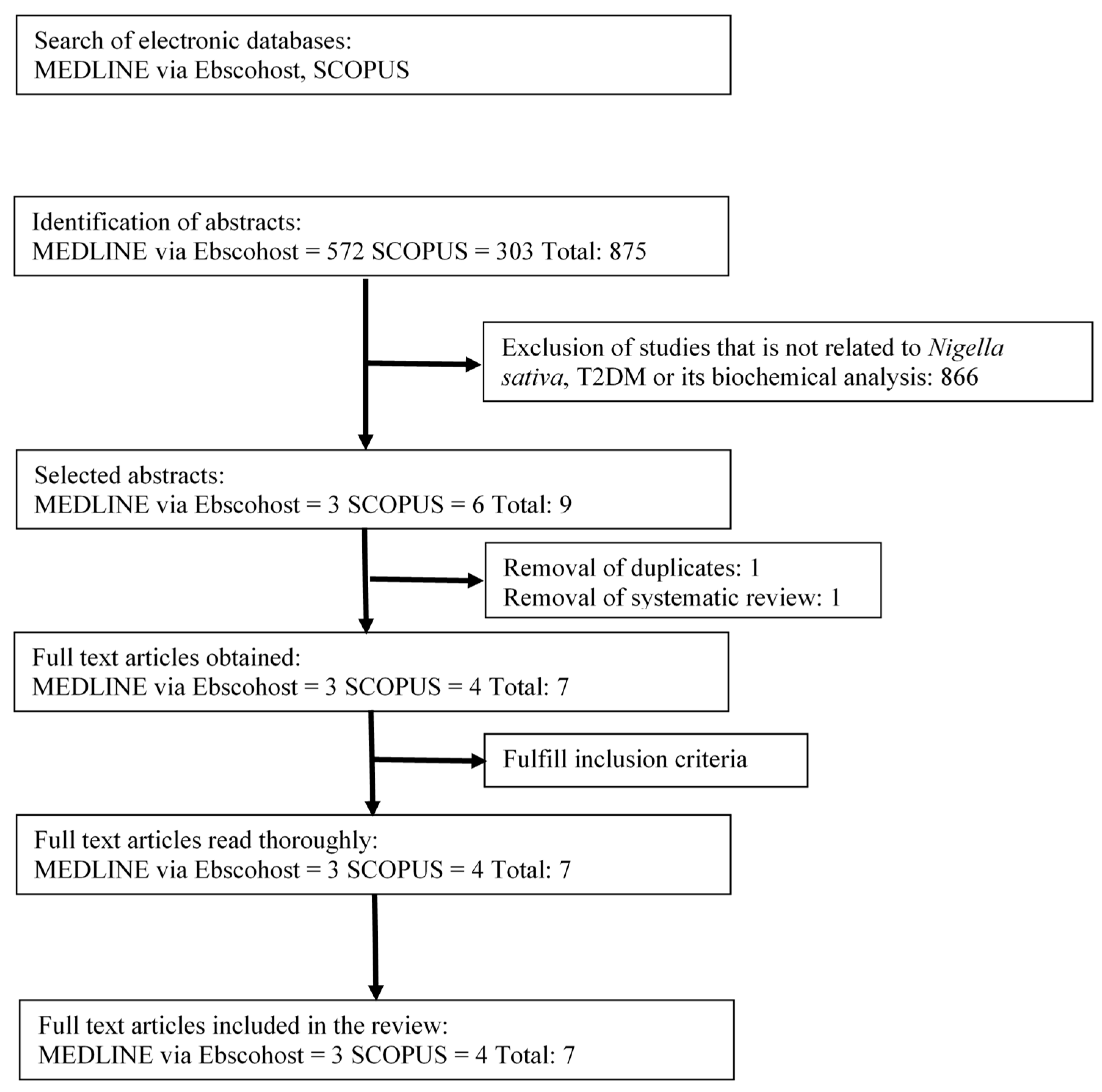
3.1. Search Results
3.2. Study Characteristics
3.3. Fasting Blood Glucose (FBG)
3.4. Blood Glucose Level 2 h Postprandial (2hPG)
3.5. Glycated Hemoglobin (HbA1c)
3.6. Insulin Level and Insulin Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reimann, M.; Bonifacio, E.; Solimena, M.; Schwarz, P.E.; Ludwig, B.; Hanefeld, M.; Bornstein, S.R. An update on preventive and regenerative therapies in diabetes mellitus. Pharmacol. Ther. 2009, 121, 317–331. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D. Hyperglycemia and the pathobiology of diabetic complications. Adv. Cardiol. 2008, 45, 1–16. [Google Scholar] [PubMed]
- Monnier, L.; Colette, C.; Dunseath, G.J.; Owens, D.R. The loss of postprandial glycemic control precedes stepwise deterioration of fasting with worsening diabetes. Diabetes Care 2007, 30, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Putignano, P.; Bossi, A.C.; Bonaventura, A.; Querci, F.; Franzetti, I.G.; Guazzini, B.; Testori, G.; Fogari, E.; Maffioli, P. Exenatide or glimepiride added to metformin on metabolic control and on insulin resistance in type 2 diabetic patients. Eur. J. Pharmacol. 2011, 666, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid. Based Complement. Altern. Med. 2013, 2013, 25. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Borji, A. An overview on cardioprotective and anti-diabetic effects of thymoquinone. Asian Pac. J. Trop. Med. 2017, 10, 849–854. [Google Scholar] [CrossRef]
- Khader, M.; Eckl, P.M. Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran. J. Basic Med Sci. 2014, 17, 950–957. [Google Scholar] [PubMed]
- Abdelrazek, H.M.A.; Kilany, O.E.; Muhammad, M.A.A.; Tag, H.M.; Abdelazim, A.M. Black Seed Thymoquinone Improved Insulin Secretion, Hepatic Glycogen Storage, and Oxidative Stress in Streptozotocin-Induced Diabetic Male Wistar Rats. Oxidative Med. Cell. Longev. 2018, 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Daryabeygi-Khotbehsara, R.; Golzarand, M.; Ghaffari, M.P.; Djafarian, K. Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: A systematic review and meta-analysis. Complement. Ther. Med. 2017, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. Based Complement. Altern. Med. 2019, 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Namazi, N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: A systematic review. Complement. Ther. Med. 2015, 23, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Mirkarimi, S.A.; Amini, M.; Mohtashami, R.; Kianbakht, S.; Fallah Huseini, H. Effects of Nigella sativa L. Seed Oil in Type II Diabetic Patients: A Randomized, Double-Blind, Placebo Controlled Clinical Trial. JMPIR 2013, 3, 93–99. [Google Scholar]
- Ahmad, B.; Tariq, M.; Uppal, A.M.; Naveed, A.K. Effects of Nigella sativa oil on some blood parameters in type 2 diabetes mellitus patients. Asian J. Chem. 2009, 21, 5373–5381. [Google Scholar]
- Kaatabi, H.; Bamosa, A.O.; Badar, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Al-Khadra, A.; Al-Almaie, S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant blinded clinical trial. PLoS ONE 2015, 10, e0113486. [Google Scholar] [CrossRef]
- Bamosa, A.O.; Kaatabi, H.; Lebdaa, F.M.; Elq, A.M.; Al-Sultanb, A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J. Physiol. Pharmacol. 2010, 54, 344–354. [Google Scholar]
- Bamosa, A.; Kaatabi, H.; Badar, A.; Al-Khadra, A.; Al Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Al-Almaie, S. Nigella sativa: A potential natural protective agent against cardiac dysfunction in patients with type 2 diabetes mellitus. J. Fam. Community Med. 2015, 22, 88–95. [Google Scholar] [CrossRef]
- El-Shamy, K.A.; Mosa, M.M.A.; El-Nabarawy, S.K.; El-Qattan, M. Effect of Nigella sativa tea in type 2-diabetic patients as regards glucose homeostasis, liver and kidney functions. J. Appl. Sci. Res. 2011, 7, 1982–1991. [Google Scholar]
- Javed, S. Nutritional, phytochemical potential and pharmacological evaluation of Nigella Sativa (Kalonji) and Trachyspermum Ammi (Ajwain). J. Med. Plants Res. 2012, 6, 768–775. [Google Scholar]
- De Luca, V.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the Biodiversity of Plants: A Revolution in the Making. Science 2012, 336, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Aisa, H.A.; Xin, X.; Tang, D. Chapter 40-Nigella sativa: A Medicinal and Edible Plant That Ameliorates Diabetes. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 629–640. [Google Scholar]
- El Rabey, H.A.; Al-Seeni, M.N.; Bakhashwain, A.S. The Antidiabetic Activity of Nigella sativa and Propolis on Streptozotocin-Induced Diabetes and Diabetic Nephropathy in Male Rats. Evid. Based Complement. Altern. Med. 2017, 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Akiyama, T.; Shimizu, S.; Kamiya, A.; Uemura, K.; Li, M.; Shirai, M.; Sugimachi, M. Detection of endogenous acetylcholine release during brief ischemia in the rabbit ventricle: A possible trigger for ischemic preconditioning. Life Sci. 2009, 85, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Nasiruddin, M.; Khan, R.A.; Haque, S.F. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int. J. Diabetes Dev. Ctries. 2008, 28, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Rachman, P.N.R.; Darmawan, E.A. The efficacy of black cumin seed (Nigella sativa) oil and hypoglycemic drug combination to reduce HbA1c level in patients with metabolic syndrome risk. IOP Conf. Ser. Mater. Sci. Eng. 2017, 259, 012018. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef]
- Pitocco, D.; Tesauro, M.; Alessandro, R.; Ghirlanda, G.; Cardillo, C. Oxidative stress in diabetes: Implications for vascular and other complications. Int. J. Mol. Sci. 2013, 14, 21525–21550. [Google Scholar] [CrossRef]
- Soskic, S.S.; Dobutovic, B.D.; Sudar, E.M.; Obradovic, M.M.; Nikolic, D.M.; Djordjevic, J.D.; Radak, D.J.; Mikhailidis, D.P.; Isenovic, E.R. Regulation of Inducible Nitric Oxide Synthase (iNOS) and its Potential Role in Insulin Resistance, Diabetes and Heart Failure. Open Cardiovasc. Med. J. 2011, 5, 153–163. [Google Scholar] [CrossRef]
- Tiganis, T. Reactive oxygen species and insulin resistance: The good, the bad and the ugly. Trends Pharmacol. Sci. 2011, 32, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Nehar, S.; Kumari, M. Ameliorating Effect of Nigella sativa Oil in Thioacetamide-induced Liver Cirrhosis in Albino Rats. Indian J. Pharm. Educ. Res. 2013, 47, 135–139. [Google Scholar]
- Fararh, K.M.; Atoji, Y.; Shimizu, Y.; Shiina, T.; Nikami, H.; Takewaki, T. Mechanisms of the hypoglycaemic and immunopotentiating effects of Nigella sativa L. oil in streptozotocin-induced diabetic hamsters. Res. Vet. Sci. 2004, 77, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Louis, M.C.; Diane, V.; Yara, H.; Pierre, H.S. Antidiabetic effects of Nigella sativa are mediated by activation of insulin and AMPK pathways, and by mitochondrial uncoupling. Can. J. Diabetes 2008, 32, 333. [Google Scholar] [CrossRef]
- Badary, O.A.; Taha, R.A.; Gamal el-Din, A.M.; Abdel-Wahab, M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem. Toxicol. 2003, 26, 87–98. [Google Scholar] [CrossRef]
- Al Wafai, R.J. Nigella sativa and thymoquinone suppress cyclooxygenase-2 and oxidative stress in pancreatic tissue of streptozotocin-induced diabetic rats. Pancreas 2013, 42, 841–849. [Google Scholar] [CrossRef]
- Mohmoud Saleh Mansi, K. Effects of Oral Administration of Water Extract of Nigella sativa on Serum Concentrations of Insulin and Testosterone in Alloxan-induced Diabetic Rats. Pak. J. Biol. Sci. 2005, 8, 1152–1156. [Google Scholar]
- Meddah, B.; Ducroc, R.; El Abbes Faouzi, M.; Eto, B.; Mahraoui, L.; Benhaddou-Andaloussi, A.; Martineau, L.C.; Cherrah, Y.; Haddad, P.S. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J. Ethnopharmacol. 2009, 121, 419–424. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 241–253. [Google Scholar]
- Geng, D.; Zhang, S.; Lan, J. Analysis on chemical components of volatile oil and determination of thymoquinone from seed of Nigella glandulifera. Zhongguo Zhong Yao Za Zhi = China J. Chin. Mater. Med. 2009, 34, 2887–2890. [Google Scholar]
| REF | Study Design | Type of Nigella sativa | Methodology | T2DM Related Biochemical Outcome | Conclusions | |
|---|---|---|---|---|---|---|
| Treatment Group | Biochemical Analysis | |||||
| [17] | Interventional study (pre-post study). | Nigella sativa oil (from 0.7 g Nigella sativa seeds purchased from Rawalpindi, Pakistan) to be consumed orally for 40 d. | Forty-one T2DM patients consumed Nigella sativa oil for the 1st 40 d (NS treatment) followed by wheat bran for the 2nd 40 d (placebo), combined with their usual oral antidiabetic drug at constant dose. | Analysis of blood samples: Fasting blood glucose (FBG), insulin level blood urea, platelet count, total leucocytes count (TLC), serum alanine aminotransferase (ALT), and serum aspartate aminotransferase (AST). | FBG significantly decreased from 190.780 ± 8.042 mg/dL to 168.317 ± 7.150 mg/dL following the 1st treatment before significantly increasing back to 186.487 ± 7.491 mg/dL after the 2nd treatment. Although not significant, insulin levels increased from 8.013 ± 0.758 lU/mL to 13.194 ± 1.404 ulU/mL after the 1st treatment before decreasing back to 8.850 ± 0.694 ulU/mL after the 2nd treatment. | Nigella sativa oil decreased FBG and increased insulin levels when combined with oral antidiabetic drug. |
| [19] | Interventional study (pre-post study). | Nigella sativa seed (Bioextract (Pvt) Ltd, Sri Lanka) in capsules contain 500 mg of grounded Nigella sativa to be consumed orally in three different doses 1, 2, and 3 g/day for 12 weeks. | Ninety-four T2DM patients divided randomly into 3 groups. Thirty patients receiving 1 g/day dose, 32 patients receiving 2 g/day dose, and 32 patients receiving 3 g/day dose together with their oral antidiabetic drug for 4, 8, and 12 weeks. | Analysis of blood samples: FBG, blood glucose level 2 h postprandial (2hPG), glycated hemoglobin (HbA1c), insulin resistance index, β-cell function, serum C-peptide, and body mass index (BMI). | (a) FBG at 0, 4, 8, and 12 weeks showed no significant changes with the 1 g/day capsule with 189 ± 14.3, 186 ± 38, 171 ± 10.1, and 171 ± 7.8 mg/dL, respectively. With 2 g/day capsule, FBG at 0, 4, 8, and 12 weeks showed a significant reduction from 219 ± 12.3, 174 ± 10.1, 157 ± 10.8, to 162 ± 9.2 mg/dL accordingly. With 3 g/day capsule, FBG at 0, 4, 8, and 12 weeks showed a significant reduction from 204 ± 18.2, 176 ± 15.2, 157 ± 9.9, to 169 ± 16.4 mg/dL, respectively. (b) 2hPG levels at 0, 4, 8, and 12 weeks did not change with 1 g/day capsule with readings of 286 ± 23.3, 244 ± 22.5, 241 ± 19.2, and 218 ± 15.6 mg/dL, respectively. With 2 g/day capsule, 2hPG levels at 0, 4, 8, and 12 weeks decreased significantly with readings of 289 ± 24.2, 213 ± 27.8, 231 ± 26.58, and 256 ± 28.1 mg/dL, respectively. Although not significant, 2hPG levels increased with 3 g/day capsule with readings at 0, 4, 8, and 12 weeks of 277 ± 54.3, 301 ± 54.3, 229 ± 9.9, and 234 ± 80.3 mg/dL, respectively. (c) HbA1c at 12 weeks did not significantly decrease from baseline with 1 g/day capsule, with readings from 8.36 ± 0.31 to 8.01 ± 0.27 %. With 2 g/day capsule, HbA1c decreased significantly from 9.09 ± 0.24 to 7.57 ± 0.30 %. With 3 g/day capsule, HbA1c decreased significantly from 9.35 ± 0.41 to 7.31 ± 0.37 %. (d) Insulin resistance index did not significantly change from 2.75 ± 0.34 to 2.82 ± 0.26 with 1 g/day capsule. With 2 g/day capsule, insulin resistance index significantly decreased at 12 weeks from 3.20 ± 0.36 to 2.37 ± 0.20. Although not significant, with 3 g/day capsule, insulin resistance index increased at 12 weeks from 4.11 ± 0.55 to 2.98 ± 0.49. (e) β-cell function decreased with 1 g/day capsule from 61.75 ± 7.79 to 59.12 ± 8.19, while it increased with 2 g/day and 3 g/day capsule, going from 45.03 ± 6.28 to 63.63 ± 9.59 and from 41.89 ± 9.83 to 88.90 ± 36.05, respectively. All changes in β-cell function were not statistically significant. | Nigella sativa at dose 2 g/day significantly improves diabetic control when combined with oral antidiabetic drug. |
| [21] | Interventional study (pre-post study). | Nigella sativa tea. Extract by hot water. The tea was 5 g/day for 6 months. | Sixty-six T2DM patients divided into 2 groups. Forty-one T2DM patients (diabetic group) and 25 healthy peoples (normal group). T2DM patients combined their oral antidiabetic drug with Nigella sativa tea. | Analysis of blood samples: FBG; 2hPG; HbA1c, kidney function test (serum creatinine), kidney function test (blood urea), liver function test (AST), liver function test (ALT), serum total bilirubin, direct bilirubin, and indirect bilirubin. | (a) FBG for the normal group: FBG significantly decreased from 80.22 ± 10.8 to 78.14 ± 10.3 after 1 month, highly significant decrease to 76.79 ± 8.66 after 2 months, very highly significant decrease to 75.30 ± 8.97 after 3 months and after 6 months to 73.34 ± 8.71. FBG for the diabetic group: Very highly significant decrease in FBG for all months from 148.7 ± 26.59 to 137.93 ± 28.36 after 1 month, to 131.64 ± 26.33 after 2 months, to 126.46 ± 23.14 after 3 months, and to 127.67 ± 22.01 after 6 months. Nigella sativa tea significantly decreased FBG for the diabetic group after 3 months, and also 6 months for normal group. (b) 2hPG for the normal group: Very highly significant decrease in 2hPG for all months from 101.13 ± 15.25 to 96.01 ± 14.12 after 1 month, to 93.16 ± 12.93 after 2 months, to 92.20 ± 13.58 after 3 months, and 89.49 ± 12.38 after 6 months. 2hPG for the diabetic group: Very highly significant decrease in 2hPG for all months from 251.42 ± 76.88 to 216.39±61.09 after 1 months, to 192.86 ± 46.11 after 2 months, to 174.27 ± 36.60 after 3 months, and to 164.12 ± 28.72 after 6 months. Nigella sativa tea very highly significant decrease in 2hPG for normal and diabetic groups. (c) HbA1c for the normal group: No significant decrease in HbA1c from 4.43±0.36 to 4.26 ± 0.51 after 3 months, highly significant decrease to 4.14 ± 0.47 after 6 months. HbA1c for the diabetic group: Very highly significant decrease in HbA1c from 7.18 ± 0.83 to 6.59 ± 0.62 after 3 months and to 6.02 ± 0.58 after 6 months. Nigella sativa tea very highly significant decrease in HbA1c after 3 and 6 months treatment. | Nigella sativa tea extract 5 g/day showed improvement in blood glucose levels and when combined with oral antidiabetic drug. |
| [16] | Randomized, double-blind, and controlled trial. | Nigella sativa oil purchased from local market in Tehran city. Cold press procedure is used to produce the Nigella sativa oil. 5 mL Nigella sativa per day for 3 months. | Seventy T2DM patients divided into 2 groups of 35 each. Group 1 (Nigella sativa group - Nigella sativa oil), group 2 (placebo group-mineral oil). All subjects taken oral antidiabetic drug combined with Nigella sativa oil. | Analysis of blood samples: FBG, 2hPG, HbA1C, creatinine, cholesterol, triglyceride, high-density lipoproteins (HDL); low-density lipoproteins (LDL), body mass index (BMI), serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and alkaline phosphatase (ALP). | (a) FBG for group 1: Significant decrease in FBG from 180.2 ± 31.8 to 161.9 ± 45.3. FBG for group 2: Increased FBG from 179.8 ± 32.3 to 186.3 ± 42.1. Nigella sativa oil significantly decreased FBG after treatment combined with oral antidiabetic drug. (b) 2hPG for group 1: Significant decrease in 2hPG from 183.0 ± 38.7 to 167.9 ± 37.5. 2hPG for group 2: Increase in 2hPG from 189.7 ± 42.8 to 192.2 ± 41.7. Nigella sativa oil significantly decreased 2hPG for Group 1. (c) HbA1c for group 1: Significant decrease in HbA1c from 8.82 ± 0.73 to 8.52 ± 0.68. HbA1c for group 2: Decrease in HbA1c from 8.79 ± 0.55 to 8.70 ± 0.67. Nigella sativa oil significantly decreased HbA1c for Group 1. | Nigella sativa oil at dose 5 mL significantly decreased FBG levels, 2hPG, and HbA1c when combined with oral antidiabetic drug. |
| [18] | Randomized, single-blind, and controlled trial. | Nigella sativa seeds as powder in capsules of 500 mg (Sri Lanka). Dose used in this study was 2 g/day. | One-hundred-and-fourteen T2DM patients divided into 2 groups. Fifty-seven patients in control group (Charcoal capsule) and 57 patients in Nigella sativa group. Results were taken every 3 months until 1 year. All patients continued their own oral antidiabetic drug. | Analysis of blood samples: FBG, HbA1c, insulin resistance (IR), β-cell activity, C-peptide, total antioxidant capacity (TAC), superoxide dismutase (SOD), catalase (CAT), and glutathione and thiobarbituric acid reactive substances (TBARS). | (a) FBG for the Nigella sativa group: Significant decrease in FBG from 195 ± 6.57 to 163 ± 6.31 after 3 months, to 164 ± 5.97 after 6 months, to 176 ± 6.59 after 9 months, and to 172 ± 5.83 after 12 months. FBG for the control group: Increase in FBG from 180 ± 5.75 to 184 ± 5.81 after 3 months, to 185 ± 5.59 after 6 months, to 183 ± 5.41 after 9 months, and to 180 ± 5.59 after 12 months. Nigella sativa capsule significant decrease FBG after 3, 6, 9, and 12 months for the Nigella sativa group. (b) HbA1c for the Nigella sativa group: Significant decrease in HbA1c from 8.6 ± 0.13 to 7.9 ± 0.18 after 3 months, to 7.8 ± 0.22 after 6 months, to 7.9 ± 0.19 after 9 months, and to 8.2 ± 0.14 after 12 months. HbA1c for the control group: Increase in HbA1c from 8.2 ± 0.12 to 8.3 ± 0.12 after 3 months, to 8.3 ± 0.13 after 6 months, to 8.5 ± 0.15 after 9 months, and to 8.5 ± 0.14 after 12 months. Nigella sativa capsule significantly decreased HbA1c after 3, 6, 9, and 12 months for the Nigella sativa group. (c) Insulin resistance (IR) for the Nigella sativa group: Significant decrease in insulin resistance from 3.0±0.24 to 2.5±0.16 after 3 months, to 2.4 ± 0.17 after 6 months, to 2.5 ± 0.19 after 9 months, and to 2.5 ± 0.18 after 12 months. IR for the control group: Increase in insulin resistance from 2.5 ± 0.17 to 2.6 ± 0.16 after 3 months, to 2.7 ± 0.19 after 6 months, to 2.7 ± 0.16 after 9 months, and to 2.5 after 12 months. Nigella sativa capsule significantly decreased insulin resistance after 3, 6, 9, and 12 months for the Nigella sativa group. | Nigella sativa capsule 2 g/day significantly decreased FBG, HbA1c, insulin resistance (IR) when combined with oral antidiabetic drug. |
| [20] | Randomized, single-blind, and controlled trial. | Nigella sativa powdered 2 g/day for 1 year. | Sixty T2DM patients divided into a control group that received charcoal and test group that received Nigella sativa powdered. Combined with oral antidiabetic drug. | Analysis of blood samples: HbA1c, BMI, pulse rate, and mean arterial pressure (MAP). | (a) HbA1c for the test group: Significant decrease in HbA1c from 8.84 ± 0.96 to 8.40 ± 1.07 after 12 months. Decrease in HbA1c from 8.78 ± 0.95 to 8.14 ± 1.69 after 6 months. HbA1c for the control group: Increase in HbA1c from 8.14 ± 0.79 to 8.28 ± 0.80 after 6 months. Increase in HbA1c from 8.18 ± 0.77 to 8.26 ± 0.90 after 12 months. | Nigella sativa powdered 2 g/day for 1 year significantly decreased HbA1c when combined with oral antidiabetic drug. |
| [15] | Randomized, double-blind, and controlled trial. | Nigella sativa oil in soft gel capsule 3 g/day for 12 weeks. | Seventy-two T2DM patients. Thirty-six participants in the intervention group received Nigella sativa oil, and 36 participants in control group received sunflower soft gel capsules. Combined with oral antidiabetic drug. | Analysis of blood samples: FBG, HbA1c, insulin, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), triglyceride, total cholesterol, HDL-cholesterol, and LDL-cholesterol. | (a) FBG for the intervention group: Significant decrease in FBG from 183.4 ± 42.1 to 166.3 ± 38.5. FBG for the control group: Increase in FBG from 201.8 ± 63.9 to 204.9 ± 63.2. Nigella sativa soft gel capsule significantly decreased FBG. (b) HbA1c for the intervention group: Significant decrease in HbA1c from 8.3 ± 0.9 to 7.8 ± 0.8. HbA1c for the control group: Increase in HbA1c from 8.3 ± 1.0 to 8.6 ± 1.0 Nigella sativa soft gel capsule significantly decreased HbA1c. | Nigella sativa oil in soft gel capsule 3 g/day significantly decreased FBG and HbA1c when combined with oral antidiabetic drug. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdan, A.; Haji Idrus, R.; Mokhtar, M.H. Effects of Nigella Sativa on Type-2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 4911. https://doi.org/10.3390/ijerph16244911
Hamdan A, Haji Idrus R, Mokhtar MH. Effects of Nigella Sativa on Type-2 Diabetes Mellitus: A Systematic Review. International Journal of Environmental Research and Public Health. 2019; 16(24):4911. https://doi.org/10.3390/ijerph16244911
Chicago/Turabian StyleHamdan, Amiza, Ruszymah Haji Idrus, and Mohd Helmy Mokhtar. 2019. "Effects of Nigella Sativa on Type-2 Diabetes Mellitus: A Systematic Review" International Journal of Environmental Research and Public Health 16, no. 24: 4911. https://doi.org/10.3390/ijerph16244911
APA StyleHamdan, A., Haji Idrus, R., & Mokhtar, M. H. (2019). Effects of Nigella Sativa on Type-2 Diabetes Mellitus: A Systematic Review. International Journal of Environmental Research and Public Health, 16(24), 4911. https://doi.org/10.3390/ijerph16244911





