The Effects of Anterior Cruciate Ligament Reconstruction on Individual Quadriceps Muscle Thickness and Circulating Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
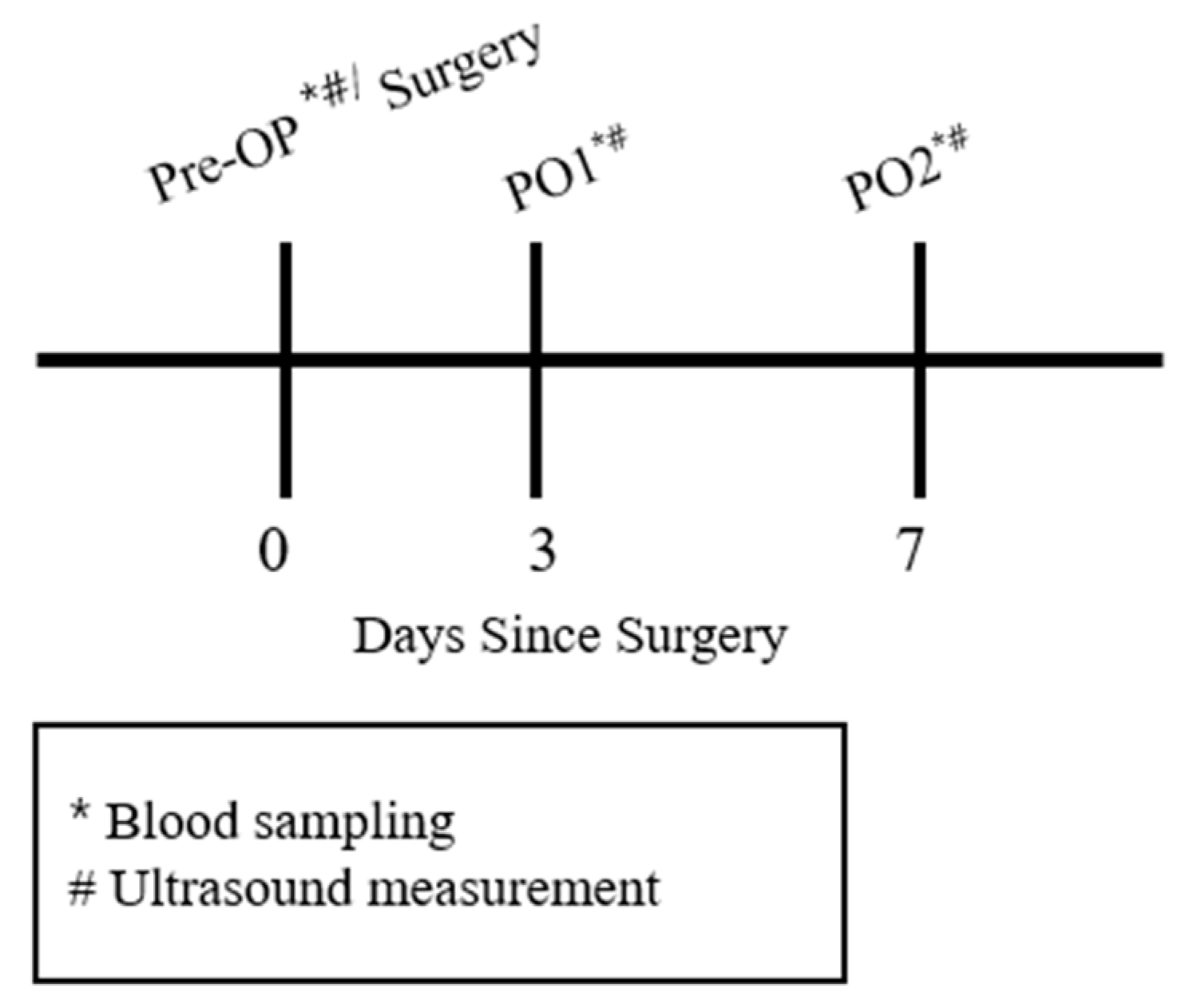
2.3. Experimental Protocol
2.3.1. Blood Sampling
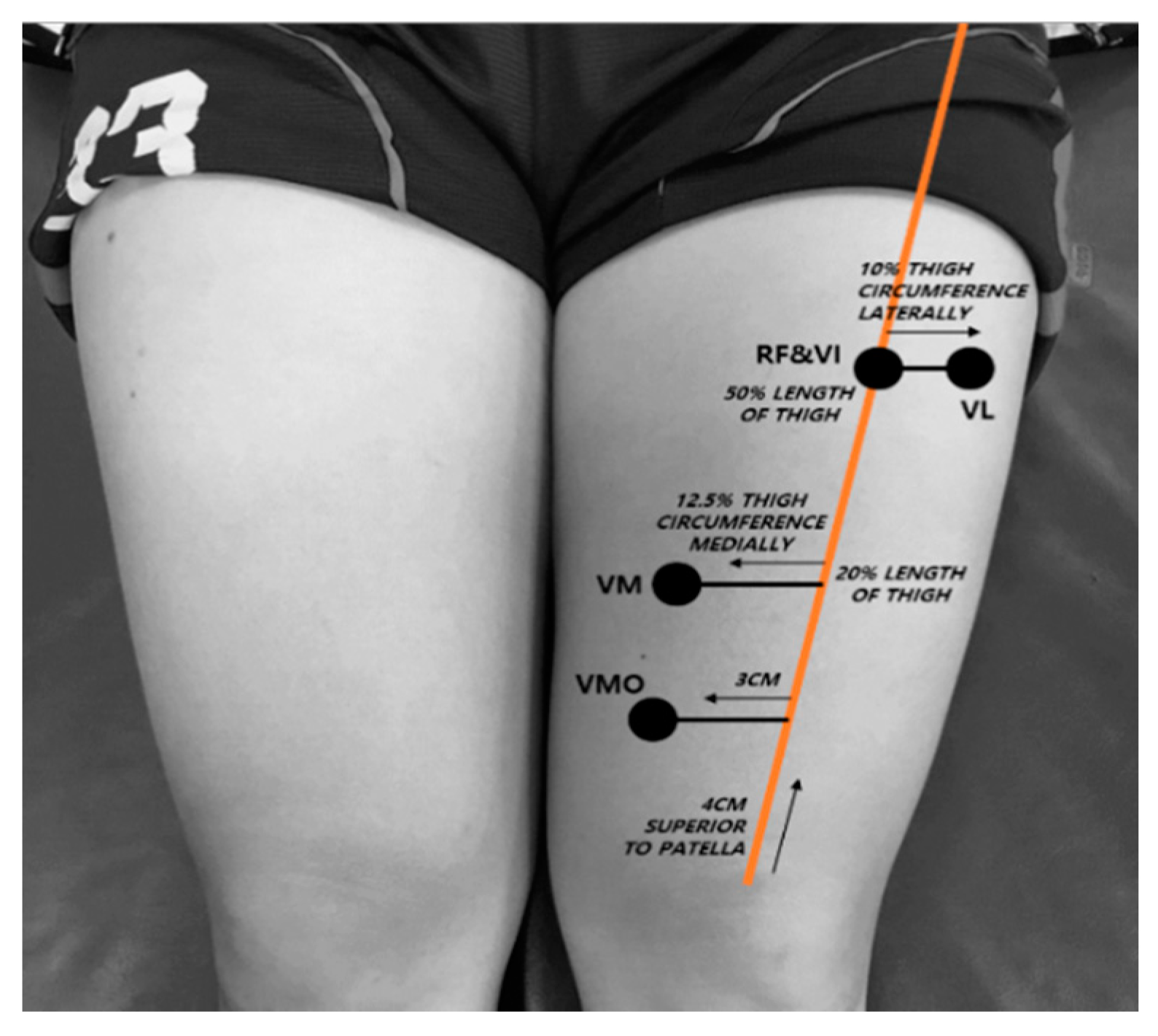
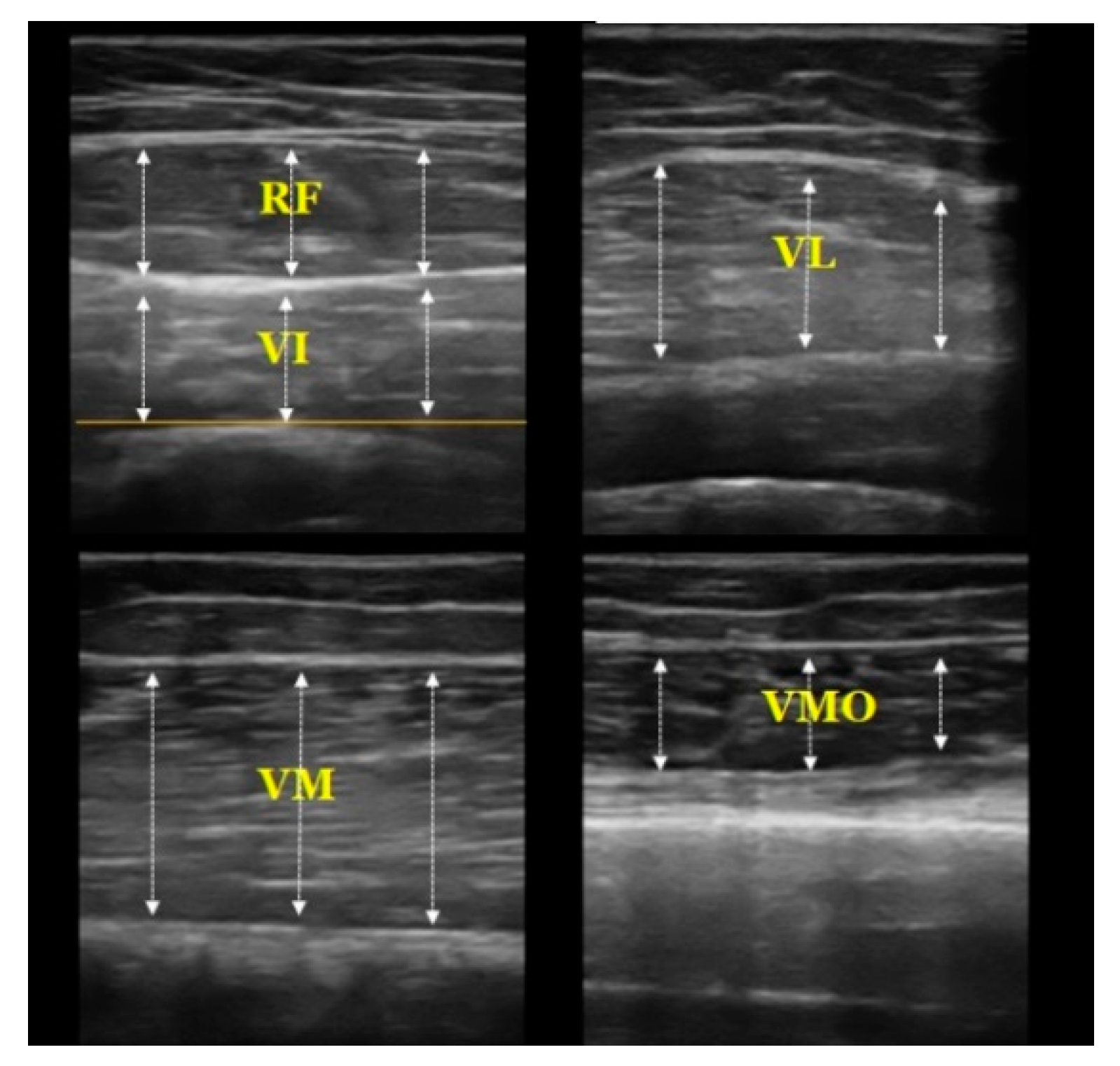
2.3.2. Muscle Thickness Measurement
2.4. Measurement of Biomarkers
2.5. Statistical Analysis
3. Results
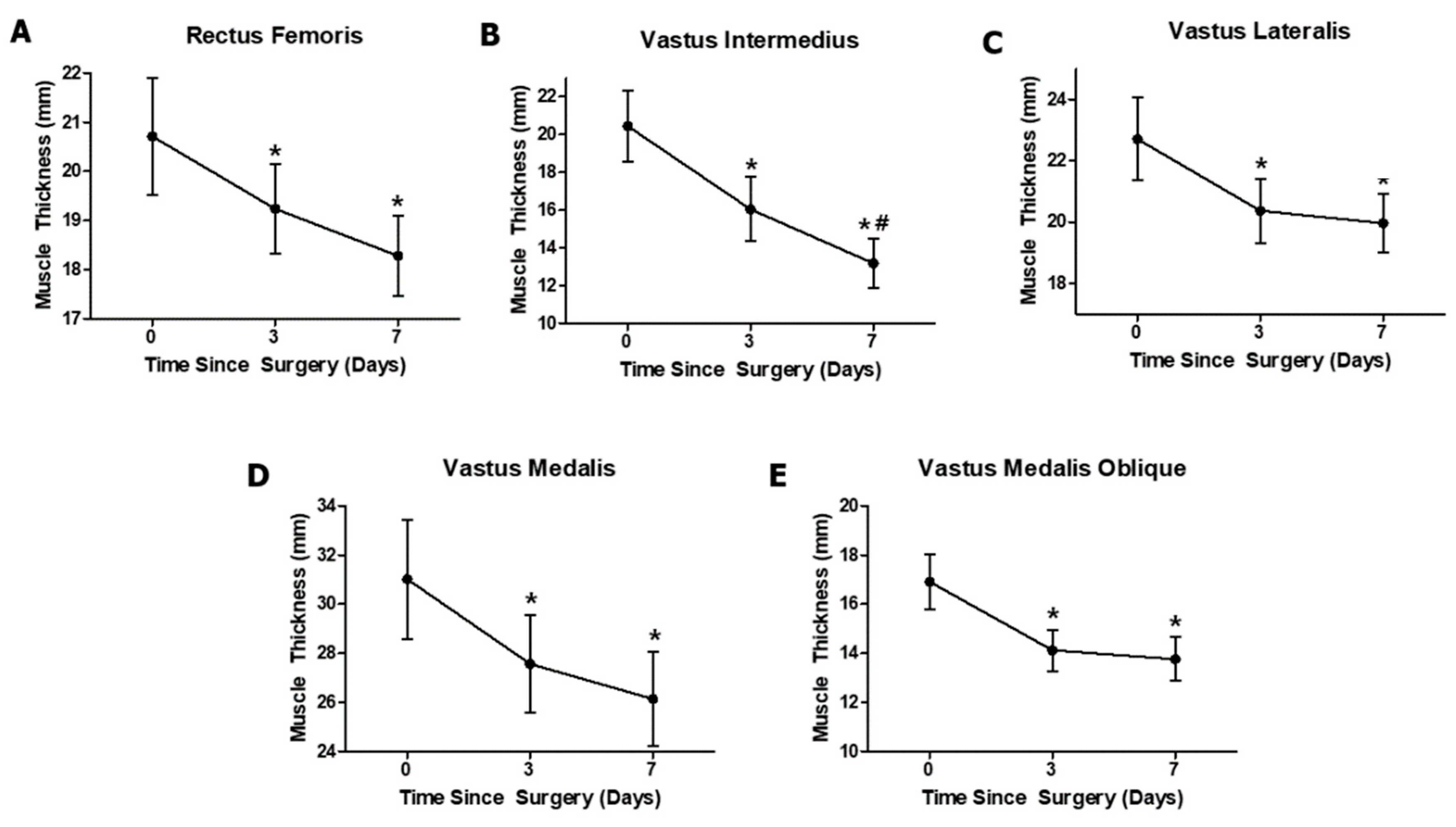
3.1. Effects of Anterior Cruciate Ligament Reconstruction on Individual Quadriceps Muscle Thickness
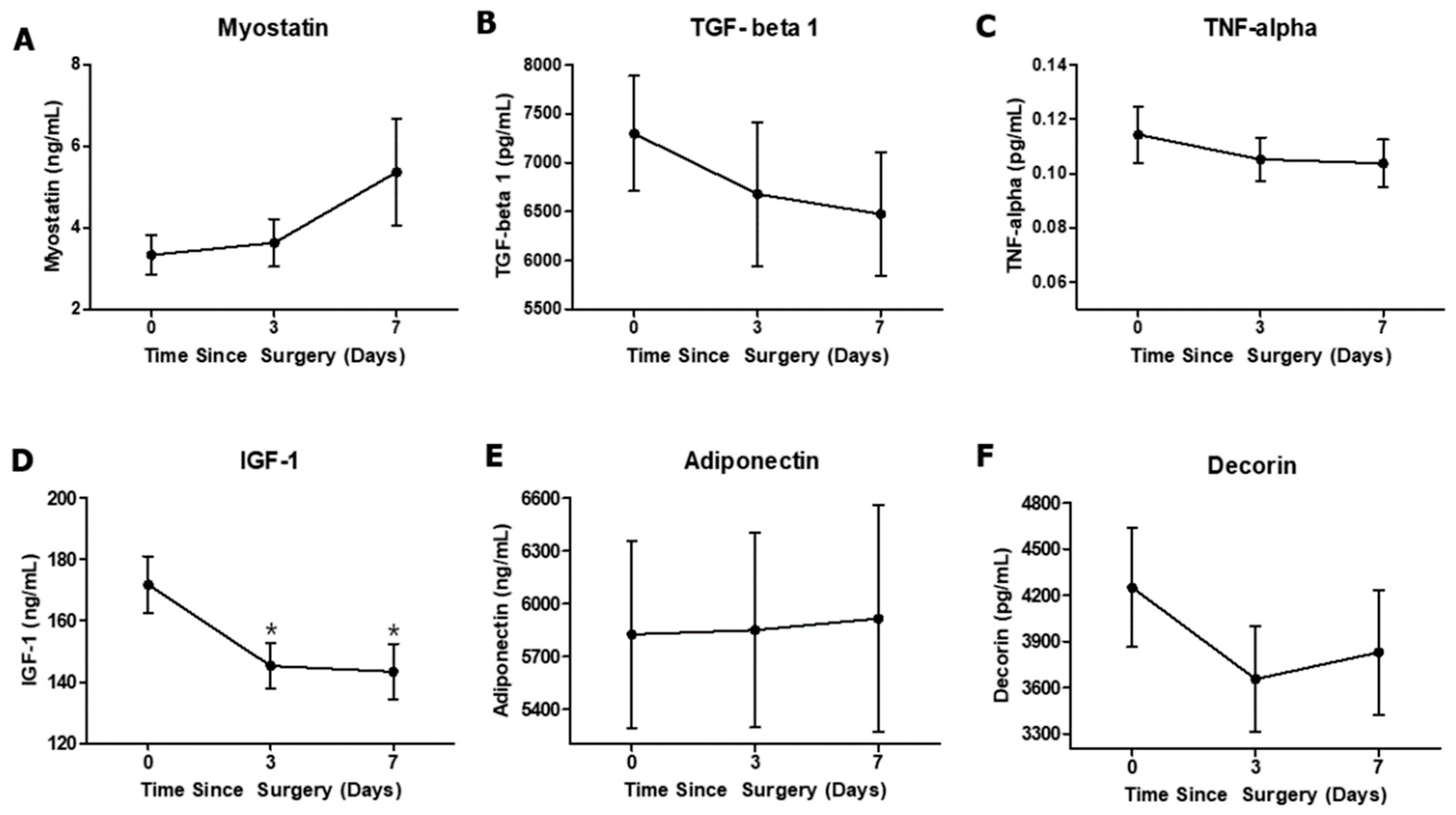
3.2. Effect of Anterior Cruciate Ligament Reconstruction on Circulating Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RF | Rectus Femoris | VI | Vastus Intermedius |
| VL | Vastus Lateralis | VM | Vastus Medialis |
| VMO | Vastus Medialis Obliques | ||
References
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Larson, D.R.; Dahm, D.L.; Levy, B.A.; Stuart, M.J.; Krych, A.J. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am. J. Sports Med. 2016, 44, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.C.; Wojtys, E.M.; Brandon, C.; Palmieri-Smith, R.M. Muscle atrophy contributes to quadriceps weakness after anterior cruciate ligament reconstruction. J. Sci. Med. Sport 2016, 19, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Ikeda, K.; Nishino, A.; Sunaga, M.; Aihara, Y.; Fukubayashi, T. Relationship between quadriceps femoris muscle volume and muscle torque after anterior cruciate ligament repair. Scand. J. Med. Sci. Sports 2007, 17, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Oda, T.; Tsukazaki, S.; Kinugasa, R.; Fukubayashi, T. Relationship between quadriceps femoris muscle volume and muscle torque at least 18 months after anterior cruciate ligament reconstruction. Scand. J. Med. Sci. Sports 2012, 22, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.L.; Kelly, J.; Hohmann, E. Neuromuscular adaptations and correlates of knee functionality following ACL reconstruction. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2008, 26, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Palmieri-Smith, R.M.; Thomas, A.C.; Wojtys, E.M. Maximizing quadriceps strength after ACL reconstruction. Clin. Sports Med. 2008, 27, 405–424. [Google Scholar] [CrossRef]
- Comfort, P.; Stewart, A.; Bloom, L.; Clarkson, B. Relationships between strength, sprint, and jump performance in well-trained youth soccer players. J. Strength Cond. Res. 2014, 28, 173–177. [Google Scholar] [CrossRef]
- Pääsuke, M.; Ereline, J.; Gapeyeva, H. Knee extension strength and vertical jumping performance in nordic combined athletes. J. Sports Med. Phys. Fit. 2001, 41, 354–361. [Google Scholar]
- Alexander, M.J. The relationship between muscle strength and sprint kinematics in elite sprinters. Can. J. Sport Sci. J. Can. Sci. Sport 1989, 14, 148–157. [Google Scholar]
- LaStayo, P.C.; Woolf, J.M.; Lewek, M.D.; Snyder-Mackler, L.; Reich, T.; Lindstedt, S.L. Eccentric Muscle Contractions: Their Contribution to Injury, Prevention, Rehabilitation, and Sport. J. Orthop. Sports Phys. Ther. 2003, 33, 557–571. [Google Scholar] [CrossRef]
- Ando, R.; Saito, A.; Umemura, Y.; Akima, H. Local architecture of the vastus intermedius is a better predictor of knee extension force than that of the other quadriceps femoris muscle heads. Clin. Physiol. Funct. Imaging 2015, 35, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, E.; Strzelczyk, P.; Herrmann, M.; Scuderi, G. Cadaveric study on static medial patellar stabilizers: The dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2006, 14, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Watanabe, K.; Akima, H. The highest antagonistic coactivation of the vastus intermedius muscle among quadriceps femoris muscles during isometric knee flexion. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2013, 23, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.A.; Rine, R.M. Central somatosensory changes associated with improved dynamic balance in subjects with anterior cruciate ligament deficiency. Gait Posture 2006, 24, 190–195. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H.; Lee, D.-H. Proprioception in Patients With Anterior Cruciate Ligament Tears: A Meta-analysis Comparing Injured and Uninjured Limbs. Am. J. Sports Med. 2017, 45, 2916–2922. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Saithna, A.; Quelard, B.; Daggett, M.; Borade, A.; Ouanezar, H.; Thaunat, M.; Blakeney, W.G. Arthrogenic muscle inhibition after ACL reconstruction: A scoping review of the efficacy of interventions. Br. J. Sports Med. 2019, 53, 289–298. [Google Scholar] [CrossRef]
- Koyonos, L.; Owsley, K.; Vollmer, E.; Limpisvasti, O.; Gambardella, R. Preoperative cryotherapy use in anterior cruciate ligament reconstruction. J. Knee Surg. 2014, 27, 479–484. [Google Scholar]
- Van Melick, N.; van Cingel, R.E.H.; Brooijmans, F.; Neeter, C.; van Tienen, T.; Hullegie, W.; der Sanden, M.W.G.N. Evidence-based clinical practice update: Practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br. J. Sports Med. 2016, 50, 1506–1515. [Google Scholar] [CrossRef]
- Sharma, M.; McFarlane, C.; Kambadur, R.; Kukreti, H.; Bonala, S.; Srinivasan, S. Myostatin: Expanding horizons. IUBMB Life 2015, 67, 589–600. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Signaling in Muscle Atrophy and Hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.H.; Prince, S. Decorin: A Growth Factor Antagonist for Tumor Growth Inhibition. BioMed. Res. Int. 2015, 2015, 654765. [Google Scholar] [CrossRef] [PubMed]
- Ouchi Noriyuki; Walsh Kenneth A Novel Role for Adiponectin in the Regulation of Inflammation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1219–1221. [CrossRef]
- Giles, L.S.; Webster, K.E.; McClelland, J.A.; Cook, J. Can ultrasound measurements of muscle thickness be used to measure the size of individual quadriceps muscles in people with patellofemoral pain? Phys. Ther. Sport 2015, 16, 45–52. [Google Scholar] [CrossRef]
- Miao, P.; Xu, Y.; Pan, C.; Liu, H.; Wang, C. Vastus medialis oblique and vastus lateralis activity during a double-leg semisquat with or without hip adduction in patients with patellofemoral pain syndrome. BMC Musculoskelet. Disord. 2015, 16, 289. [Google Scholar] [CrossRef]
- Wurtzel, C.N.W.; Gumucio, J.P.; Grekin, J.A.; Khouri, R.K.; Davis, C.S.; Russell, A.J.; Bedi, A.; Mendias, C.L. Pharmacological inhibition of myostatin protects against skeletal muscle atrophy and weakness after anterior cruciate ligament tear. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 2499–2505. [Google Scholar] [CrossRef]
- Akima, H.; Saito, A. Inverse activation between the deeper vastus intermedius and superficial muscles in the quadriceps during dynamic knee extensions. Muscle Nerve 2013, 47, 682–690. [Google Scholar] [CrossRef]
- Peck, B.D.; Brightwell, C.R.; Johnson, D.L.; Ireland, M.L.; Noehren, B.; Fry, C.S. Anterior Cruciate Ligament Tear Promotes Skeletal Muscle Myostatin Expression, Fibrogenic Cell Expansion, and a Decline in Muscle Quality. Am. J. Sports Med. 2019, 47, 1385–1395. [Google Scholar] [CrossRef]
- Mendias, C.L.; Lynch, E.B.; Davis, M.E.; Sibilsky Enselman, E.R.; Harning, J.A.; Dewolf, P.D.; Makki, T.A.; Bedi, A. Changes in circulating biomarkers of muscle atrophy, inflammation, and cartilage turnover in patients undergoing anterior cruciate ligament reconstruction and rehabilitation. Am. J. Sports Med. 2013, 41, 1819–1826. [Google Scholar] [CrossRef]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Pathol. 2001, 54, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Lyras, D.N.; Kazakos, K.; Agrogiannis, G.; Verettas, D.; Kokka, A.; Kiziridis, G.; Chronopoulos, E.; Tryfonidis, M. Experimental study of tendon healing early phase: Is IGF-1 expression influenced by platelet rich plasma gel? Orthop. Traumatol. Surg. Res. OTSR 2010, 96, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Alejandro-Osorio, A.L.; Grorud, K.W.; Martinez, D.A.; Vailas, A.C.; Grindeland, R.E.; Vanderby, R. Systemic administration of IGF-I enhances healing in collagenous extracellular matrices: Evaluation of loaded and unloaded ligaments. BMC Physiol. 2007, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Suzuki, S.; Kato, C.; Kato, T. Atrophy of individual thigh muscles measured by MRI in older adults with knee osteoarthritis: A cross-sectional study. Ann. Phys. Rehabil. Med. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Norte, G.E.; Knaus, K.R.; Kuenze, C.; Handsfield, G.G.; Meyer, C.H.; Blemker, S.S.; Hart, J.M. MRI-based assessment of lower-extremity muscle volumes in patients before and after ACL reconstruction. J. Sport Rehabil. 2018, 27, 201–212. [Google Scholar] [CrossRef]
| Characteristic | |
|---|---|
| Age, year | 30.4 ± 5.9 |
| Height, cm | 170.8 ± 8.0 |
| Weight, kg | 69.9 ± 10.8 |
| Body mass index, kg/m2 | 23.8 ± 2.3 |
| Days between injury and reconstruction, days | 38.4 ± 76.7 |
| PRE | PO1 | PO2 | F | p | η2p | |
|---|---|---|---|---|---|---|
| RF (mm) | 20.72 ± 4.28 | 19.24 ± 4.28 * | 18.28 ± 18.28 * | 7.30 | 0.009 | 0.36 |
| VI (mm) | 20.45 ± 6.82 | 16.05 ± 6.13 * | 13.18 ± 4.7 *,# | 59 | <0.001 | 0.819 |
| VL (mm) | 22.7 ± 4.86 | 20.37 ± 3.8 * | 19.97 ± 3.48 * | 2.96 | 0.094 | 0.186 |
| VM (mm) | 31.02 ± 8.76 | 27.57 ± 7.24 * | 26.13 ± 6.93 * | 15.1 | <0.001 | 0.537 |
| VMO (mm) | 16.91 ± 3.98 | 14.12 ± 3.01 * | 13.77 ± 3.16 * | 23.7 | <0.001 | 0.646 |
| PRE vs. PO1 | PRE vs. PO2 | PO1 vs. PO2 | |
|---|---|---|---|
| Myostatin | 0.15 | 0.55 | 0.46 |
| TGF-beta 1 | 0.25 | 0.36 | 0.08 |
| TNF-alpha | 0.26 | 0.29 | 0.05 |
| Decorin | 0.43 | 0.28 | 0.12 |
| Adiponectin | 0.02 | 0.03 | 0.01 |
| IGF-1 | 0.84 | 0.82 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.-H.; Eun, S.-P.; Park, D.-H.; Kwak, H.-B.; Chang, E. The Effects of Anterior Cruciate Ligament Reconstruction on Individual Quadriceps Muscle Thickness and Circulating Biomarkers. Int. J. Environ. Res. Public Health 2019, 16, 4895. https://doi.org/10.3390/ijerph16244895
Yang J-H, Eun S-P, Park D-H, Kwak H-B, Chang E. The Effects of Anterior Cruciate Ligament Reconstruction on Individual Quadriceps Muscle Thickness and Circulating Biomarkers. International Journal of Environmental Research and Public Health. 2019; 16(24):4895. https://doi.org/10.3390/ijerph16244895
Chicago/Turabian StyleYang, Jae-Ho, Seung-Pyo Eun, Dong-Ho Park, Hyo-Bum Kwak, and Eunwook Chang. 2019. "The Effects of Anterior Cruciate Ligament Reconstruction on Individual Quadriceps Muscle Thickness and Circulating Biomarkers" International Journal of Environmental Research and Public Health 16, no. 24: 4895. https://doi.org/10.3390/ijerph16244895
APA StyleYang, J.-H., Eun, S.-P., Park, D.-H., Kwak, H.-B., & Chang, E. (2019). The Effects of Anterior Cruciate Ligament Reconstruction on Individual Quadriceps Muscle Thickness and Circulating Biomarkers. International Journal of Environmental Research and Public Health, 16(24), 4895. https://doi.org/10.3390/ijerph16244895





