Degradation of Bisphenol A by CeCu Oxide Catalyst in Catalytic Wet Peroxide Oxidation: Efficiency, Stability, and Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Catalyst
2.3. Characterizations of the Catalyst
2.4. Catalytic Degradation Experiments and Analytical Methods
2.5. Quality Control
3. Results and Discussion
3.1. Characterizations of the Catalysts
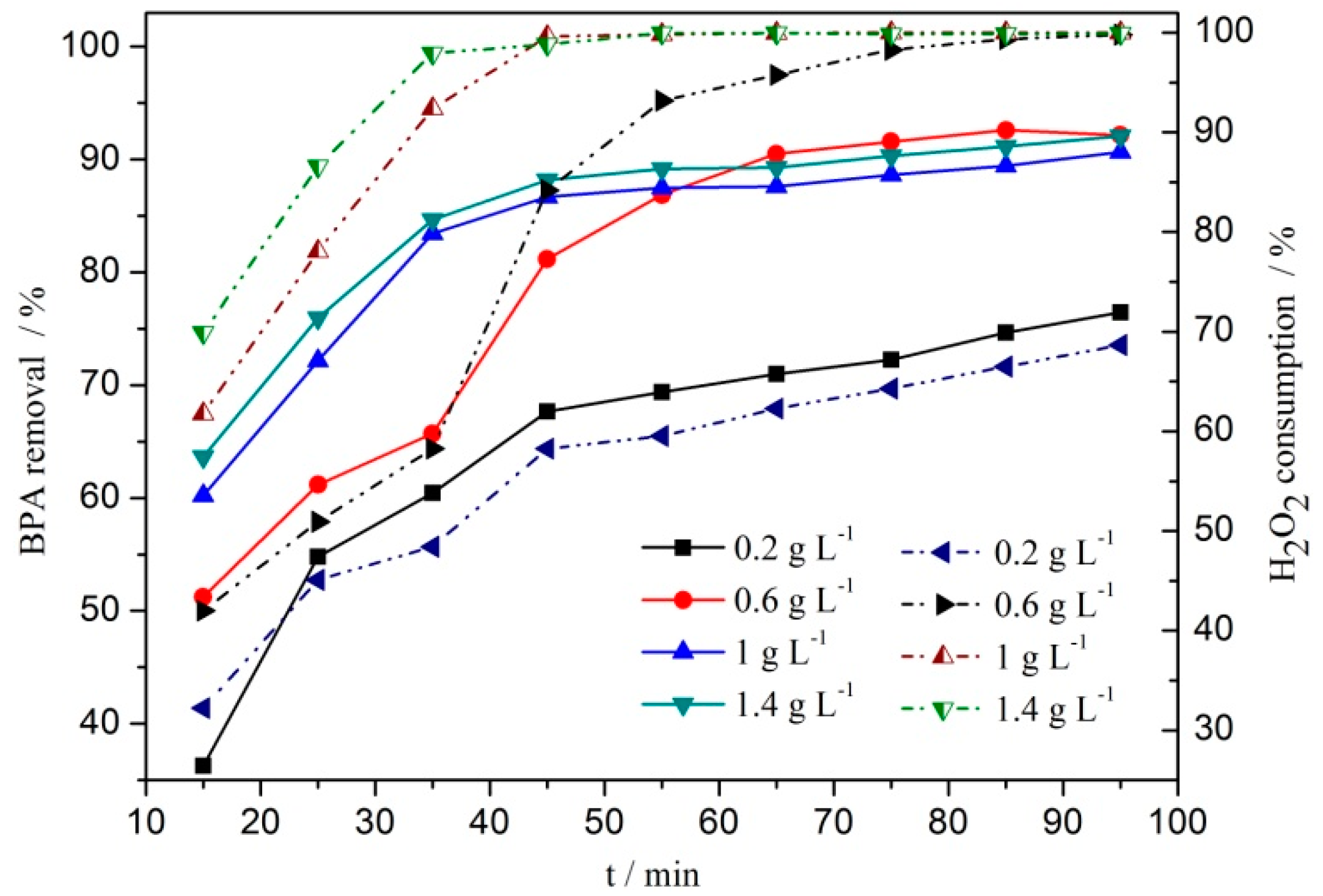
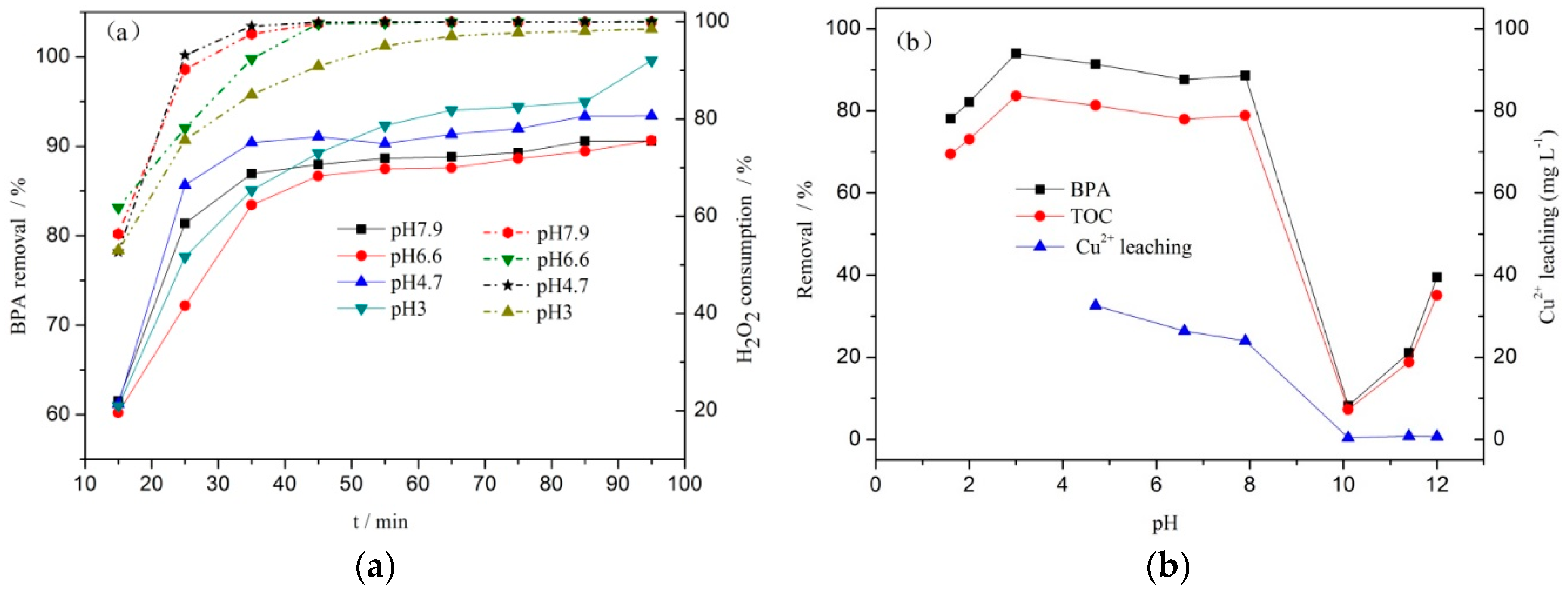
3.2. Catalytic Performances of the Catalysts
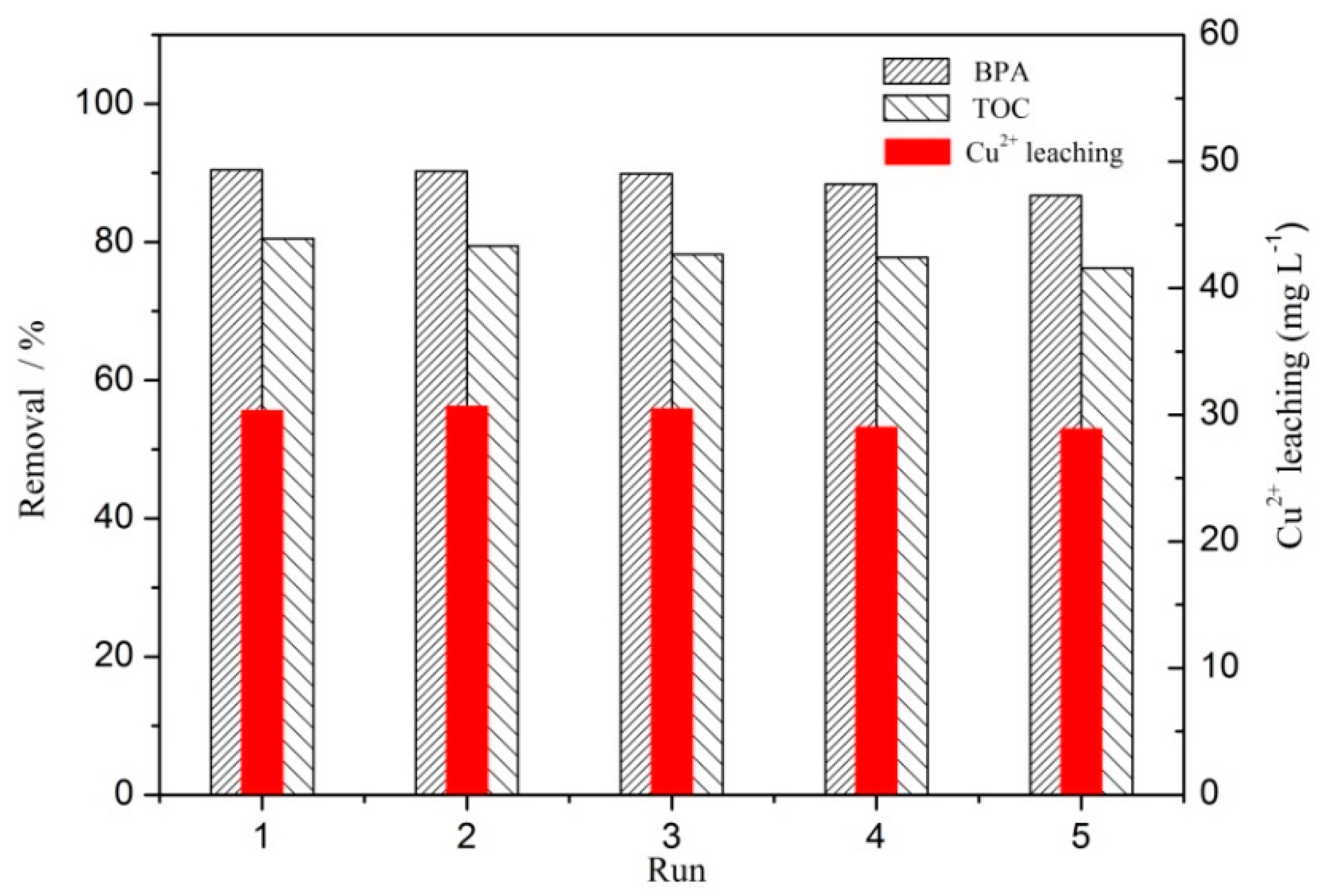
3.3. Stability of the CC450 Catalyst
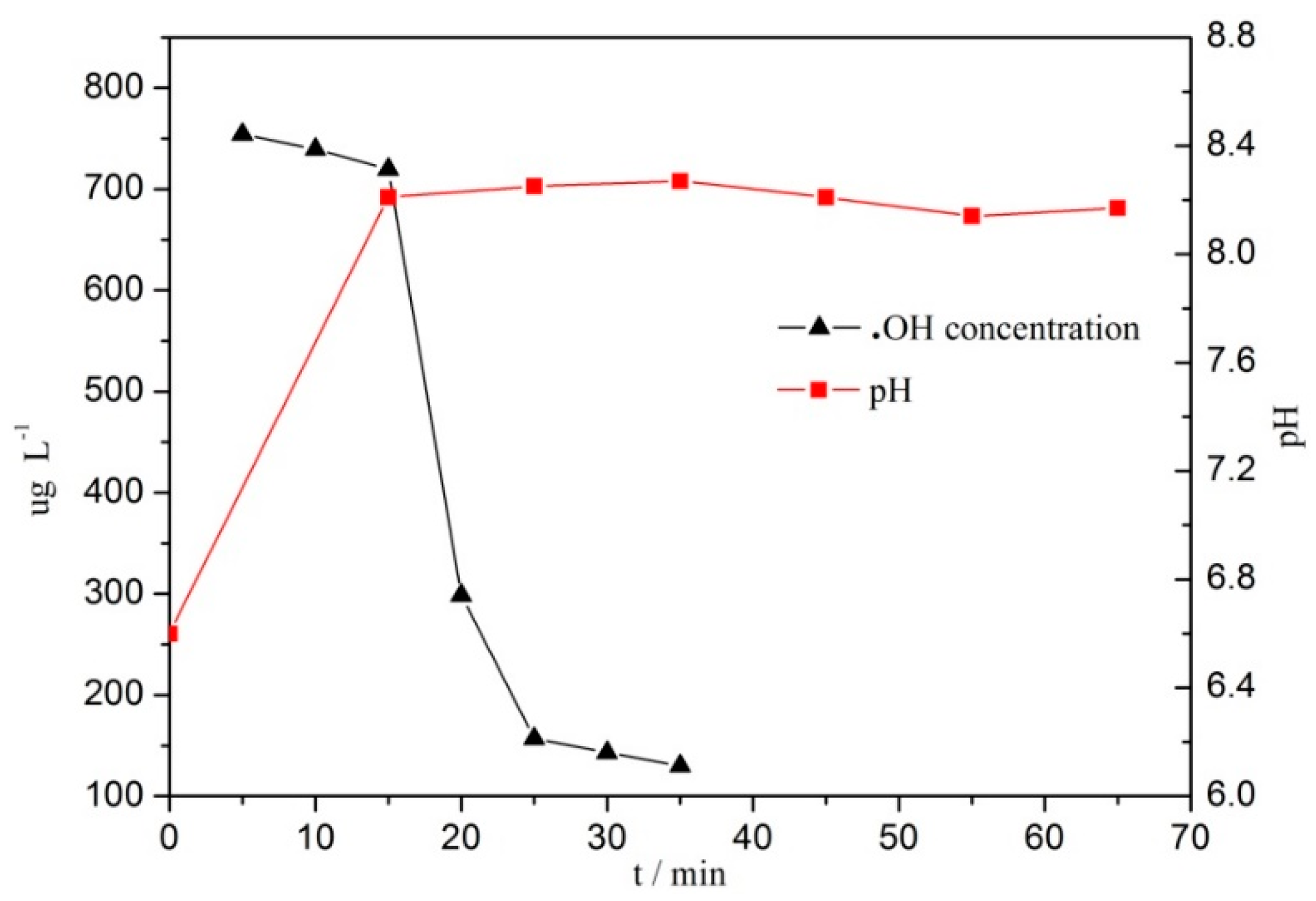
3.4. Hydroxyl Radical and pH Changes in the Reaction
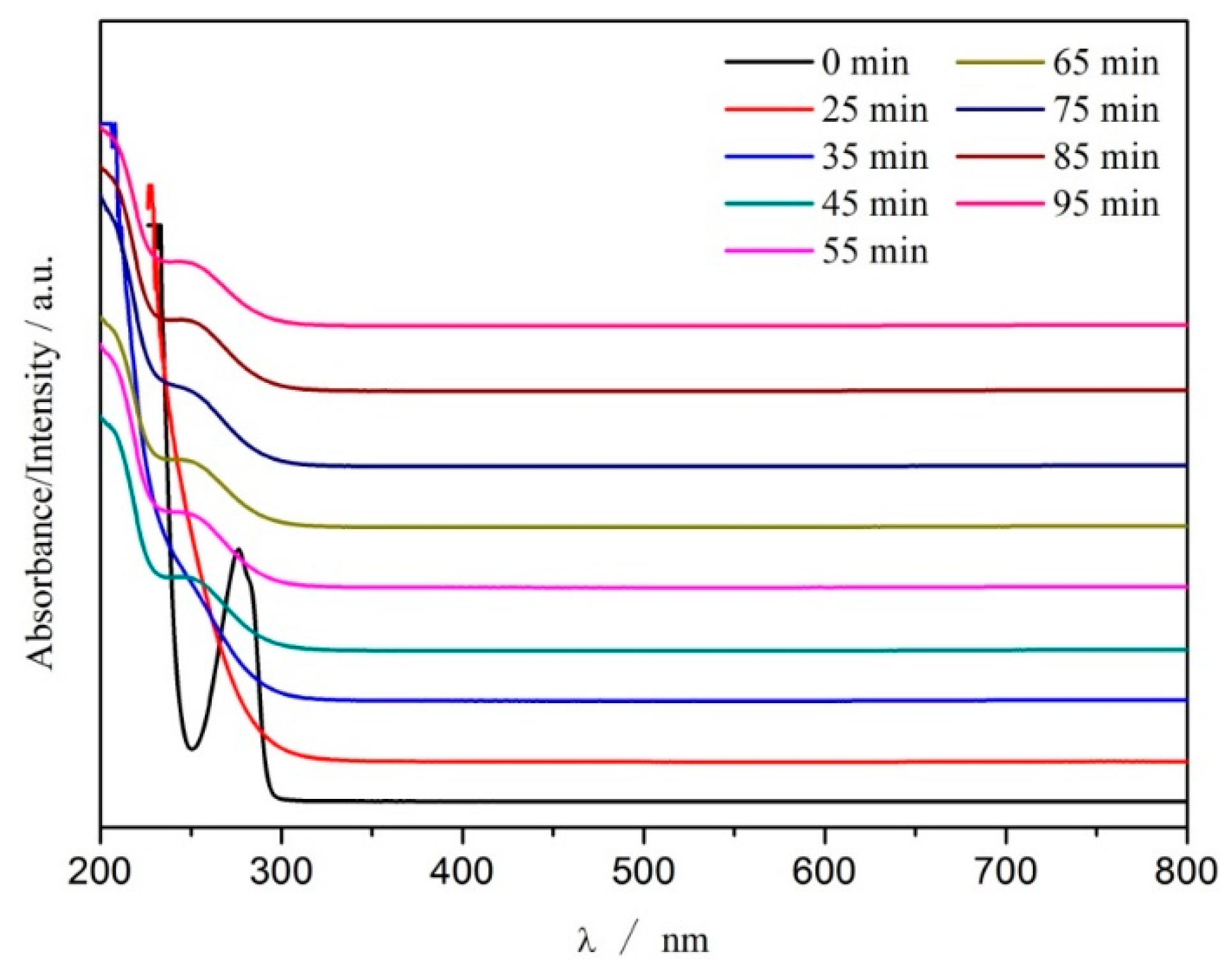
3.5. UV-VIS Spectra Change in CWPO Reaction System
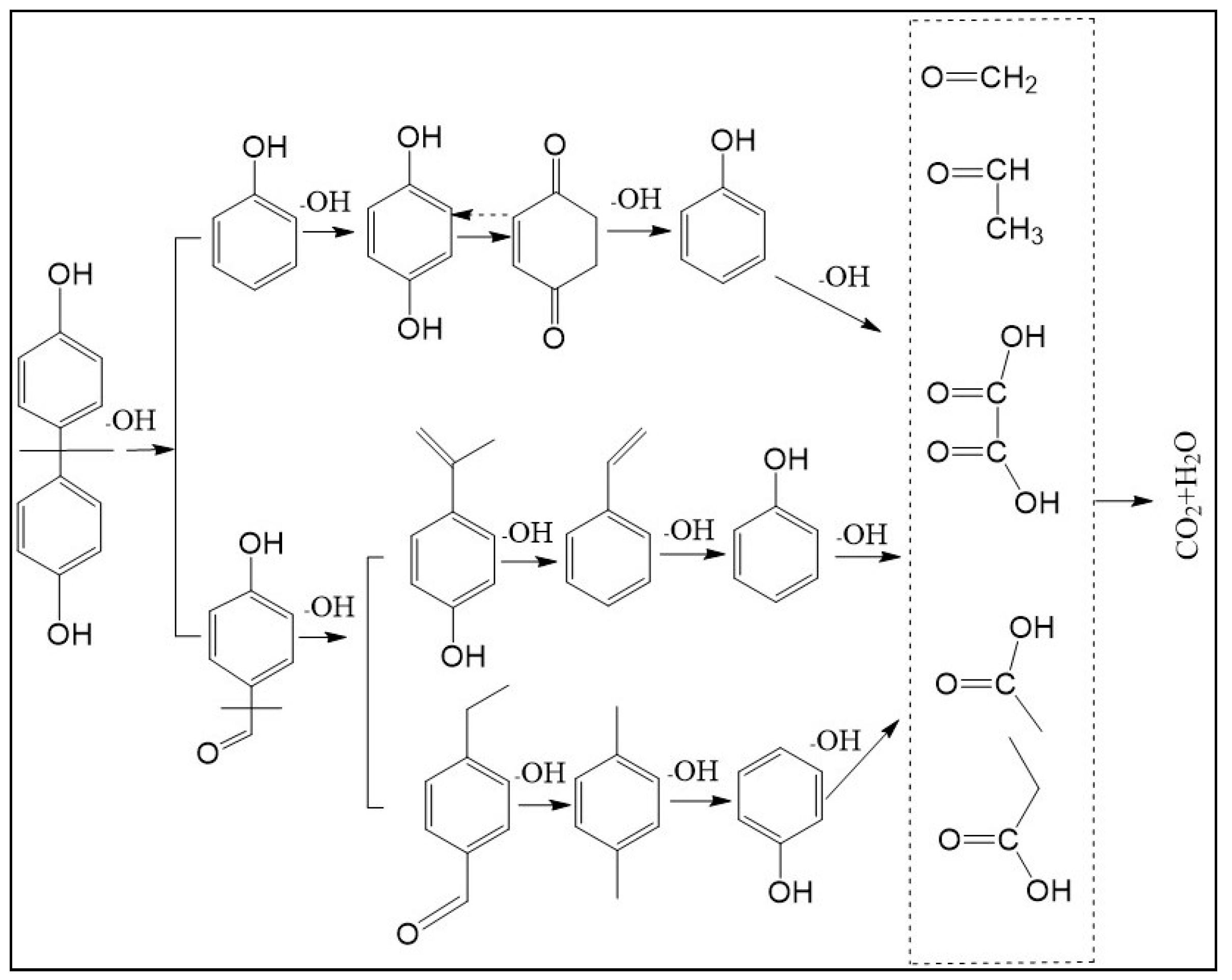
3.6. Catalytic Mechanism and Degradation Pathway
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noszczyńska, M.; Piotrowska-Seget, Z. Bisphenols: Application, occurrence, safety, and biodegradation mediated by bacterial communities in wastewater treatment plants and rivers. Chemosphere 2018, 201, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, Z.; Shi, Q.M.; Ge, X.; Wang, H.X.; Li, M.X.; Chen, G.; Wang, Q.; Ju, Q.; Zhang, J.P.; et al. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology 2017, 387, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, B.; Andrei, V.; Tudorache, M.; Lin, F.; Cadigan, C.; Richards, R.; Parvulescu, V.I. Enhanced photo-degradation of bisphenol pollutants onto gold-modified photocatalysts. Catal. Today 2017, 284, 153–159. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Kim, K.; Kavitha, B.; Kumar, V.; Raza, N.; Kalagara, S. Photocatalytic degradation of bisphenol A in aqueous media: A review. J. Environ. Manag. 2018, 213, 189–205. [Google Scholar] [CrossRef]
- Chen, Q.; Lan, Y.; Shi, J.; Liu, W.J.; Zhu, B.; Sun, D.; Duan, S.S. Levels of NP and BPA in the Pearl River Estuary, China: Fluctuations with Country Policy Changes over the Past 40 Years. Int. J. Environ. Res. Public Health 2019, 16, 4100. [Google Scholar] [CrossRef]
- Vijayalakshmi, V.; Senthilkumar, P.; Mophin-Kani, K.; Sivamani, S.; Sivarajasekar, N.; Vasantharaj, S. Bio-degradation of Bisphenol A by Pseudomonas aeruginosa PAb1 isolated from effluent of thermal paper industry: Kinetic modeling and process optimization. J. Radiat. Res. Appl. Sci. 2018, 11, 56–65. [Google Scholar] [CrossRef]
- Heidari, H.; Sedighi, M.; Zamir, S.M.; Shojaosadati, S.A. Bisphenol A degradation by Ralstonia eutropha in the absence and presence of phenol. Int. Biodeter. Biodegr. 2017, 119, 37–42. [Google Scholar] [CrossRef]
- Fang, L.; Hong, R.; Gao, J.; Gu, C. Degradation of bisphenol A by nano-sized manganese dioxide synthesized using montmorillonite as templates. Appl. Clay Sci. 2016, 132–133, 155–160. [Google Scholar] [CrossRef]
- Choong, C.E.; Ibrahim, S.; Yoon, Y.; Jang, M. Removal of lead and bisphenol A using magnesium silicate impregnated palm-shell waste powdered activated carbon: Comparative studies on single and binary pollutant adsorption. Ecotoxicol. Environ. Saf. 2018, 148, 142–151. [Google Scholar] [CrossRef]
- Acosta, R.; Nabarlatz, D.; Sánchez-Sánchez, A.; Jagiello, J.; Gadonneix, P.; Celzard, A.; Fierro, V. Adsorption of Bisphenol A on KOH-activated tyre pyrolysis char. J. Environ. Chem. Eng. 2018, 6, 823–833. [Google Scholar] [CrossRef]
- Syranidou, E.; Christofilopoulos, S.; Politi, M.; Weyens, N.; Venieri, D.; Vangronsveld, J.; Kalogerakis, N. Bisphenol-A removal by the halophyte Juncus acutus in a phytoremediation pilot: Characterization and potential role of the endophytic community. J. Hazard. Mater. 2017, 323, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.; Lok, L.; Veksha, A.; Giannis, A.; Lim, T.T. Enhanced photocatalytic degradation of bisphenol A with Ag-decorated S-doped g-C3N4 under solar irradiation: Performance and mechanistic studies. Chem. Eng. J. 2018, 333, 739–749. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Zhu, R.L.; Xi, Y.F.; Xu, T.Y.; Yan, L.X.; Zhu, J.X.; Zhu, G.Q.; He, H.P. Heterogeneous photo-Fenton degradation of bisphenol A over Ag/AgCl/ferrihydrite catalysts under visible light. Chem. Eng. J. 2018, 346, 567–577. [Google Scholar] [CrossRef]
- Tan, X.Q.; Wan, Y.H.; Huang, Y.J.; He, C.; Zhang, Z.L.; He, Z.Y.; Hu, L.L.; Zeng, J.W.; Shu, D. Three-dimensional MnO2 porous hollow microspheres for enhanced activity as ozonation catalysts in degradation of bisphenol A. J. Hazard. Mater. 2017, 321, 162–172. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.J.; Wu, Q.S.; Li, J. Degradation and mechanism analysis of bisphenol A in aqueous solutions by pulsed discharge plasma combined with activated carbon. Sep. Purif. Technol. 2018, 190, 288–296. [Google Scholar] [CrossRef]
- Lee, G.; Ibrahim, S.; Kittappa, S.; Park, H.; Park, C.M. Sonocatalytic activity of a heterostructured β-Bi2O3/Bi2O2CO3 nanoplate in degradation of bisphenol A. Ultrason. Sonochem. 2018, 44, 64–72. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Dolatabadi, M. Modeling and kinetics study of electrochemical peroxidation process for mineralization of bisphenol A; a new paradigm for groundwater treatment. J. Mol. Liq. 2018, 254, 76–82. [Google Scholar] [CrossRef]
- Nadejde, C.; Neamtu, M.; Hodoroaba, V.D.; Schneider, R.J.; Paul, A.; Ababei, G.; Panne, U. Green Fenton-like magnetic nanocatalysts: Synthesis, characterization and catalytic application. Appl. Catal. B Environ. 2015, 176–177, 667–677. [Google Scholar] [CrossRef]
- Žerjav, G.; Kaplan, R.; Pintar, A. Catalytic wet air oxidation of bisphenol A aqueous solution in trickle-bed reactor over single TiO2 polymorphs and their mixtures. J. Environ. Chem. Eng. 2018, 6, 2148–2158. [Google Scholar] [CrossRef]
- Mena, I.F.; Diaz, E.; Rodriguez, J.J.; Mohedano, A.F. CWPO of bisphenol A with iron catalysts supported on microporous carbons from grape seeds activation. Chem. Eng. J. 2017, 318, 153–160. [Google Scholar] [CrossRef]
- Yang, S.S.; Wu, P.X.; Yang, Q.L.; Zhu, N.W.; Lu, G.N.; Dang, Z. Regeneration of iron-montmorillonite adsorbent as an efficient heterogeneous Fenton catalytic for degradation of Bisphenol A: Structure, performance and mechanism. Chem. Eng. J. 2017, 328, 737–747. [Google Scholar] [CrossRef]
- Cleveland, V.; Bingham, J.; Kan, E. Heterogeneous Fenton degradation of bisphenol A by carbon nanotube-supported Fe3O4. Sep. Purif. Technol. 2014, 133, 388–395. [Google Scholar] [CrossRef]
- Pachamuthu, M.P.; Karthikeyan, S.; Maheswari, R.; Lee, A.F.; Ramanathan, A. Fenton-like degradation of Bisphenol A catalyzed by mesoporous Cu/TUD-1. Appl. Surf. Sci. 2017, 393, 67–73. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, D.; Hu, C.; Shi, Y.L. Framework Cu-doped AlPO4 as an effective Fenton-like catalyst for bisphenol A degradation. Appl. Catal. B Environ. 2017, 207, 9–16. [Google Scholar] [CrossRef]
- Inchaurrondo, N.; Cechini, J.; Font, J.; Haure, P. Strategies for enhanced CWPO of phenol solutions. Appl. Catal. B Environ. 2012, 111–112, 641–648. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ding, Y.B.; Tang, H.Q.; Han, X.Y.; Zhu, L.H.; Wang, N. Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: Efficiency, stability and mechanism. Chem. Eng. J. 2014, 236, 251–262. [Google Scholar] [CrossRef]
- Tomul, F.; Turgut Basoglu, F.; Canbay, H. Determination of adsorptive and catalytic properties of copper, silver and iron contain titanium-pillared bentonite for the removal bisphenol A from aqueous solution. Appl. Surf. Sci. 2016, 360, 579–593. [Google Scholar] [CrossRef]
- Park, C.M.; Heo, J.; Yoon, Y. Oxidative degradation of bisphenol A and 17α-ethinyl estradiol by Fenton-like activity of silver nanoparticles in aqueous solution. Chemosphere 2017, 168, 617–622. [Google Scholar] [CrossRef]
- Jiao, Z.J.; Zhou, G.L.; Zhang, H.D.; Shen, Y.; Zhang, X.M.; Li, J.X.; Gao, X. Effect of Calcination Temperature on Catalytic Performance of CeCu Oxide in Removal of Quinoline by Wet Hydrogen Peroxide Oxidation from Water. J. Brazil. Chem. Soc. 2018, 29, 2233–2243. [Google Scholar] [CrossRef]
- Benipal, G.; Harris, A.; Srirajayatsayai, C.; Tate, A.; Topalidis, V.; Eswani, Z.; Qureshi, M.; Hardaway, C.J.; Galiotos, J.; Douvris, C. Examination of Al, As, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Pb, Sb, Se, V, and Zn in sediments collected around the downtown Houston, Texas area, using inductively coupled plasma-optical emission spectroscopy. Microchem. J. 2017, 130, 255–262. [Google Scholar] [CrossRef]
- Bussan, D.; Harris, A.; Douvris, C. Monitoring of selected trace elements in sediments of heavily industrialized areas in Calcasieu Parish, Louisiana, United States by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Microchem. J. 2019, 144, 51–55. [Google Scholar] [CrossRef]
- Liu, T.X.; Lan, H.R.; Zeng, Y.M.; Hong, L.; Zhang, H.X. Applied research of nano cerium oxide in the catalytic oxidation of preparing aromatic compounds from lignin. J. Mol. Catal. 2017, 31, 372–381. [Google Scholar]
- Liu, Y.; He, H.P.; Wu, D.L.; Zhang, Y.L. Heterogeneous catalytic ozonation reaction mechanism. Prog. Chem. 2016, 28, 1112–1120. [Google Scholar]
- Guan, X.H.; Xie, P. Literature review of combined pollution of metal ions and organic pollutants. J. Civil Environ. Eng. 2019, 41, 120–128. [Google Scholar]
- Niu, L.; Zhao, B.X.; Ye, W.; Qiu, S.; Guan, W.B.; Liu, N.; Zhang, X.L. Fe-Zn-Al Pillared Montmorillonite for Catalytic Wet Peroxide Oxidation Degradation of Orange Acid II: Preparation Methods and Properties. J. Chin. Ceram. Soc. 2016, 44, 1501–1508. [Google Scholar]
- Chen, W.J.; Zou, C.J.; Liu, Y.; Li, X.K. The experimental investigation of bisphenol A degradation by Fenton process with different types of cyclodextrins. J. Ind. Eng. Chem. 2017, 56, 428–434. [Google Scholar] [CrossRef]
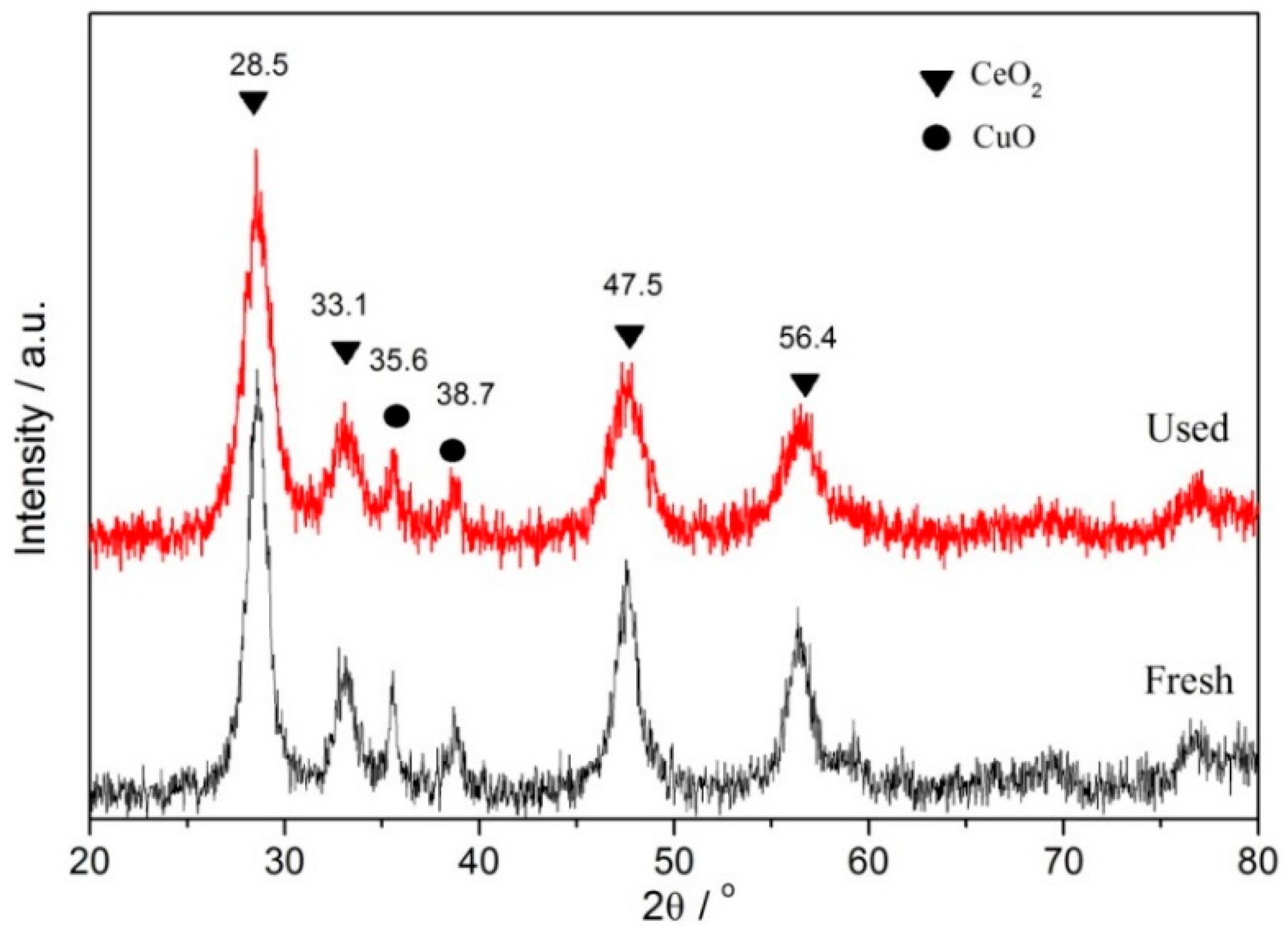

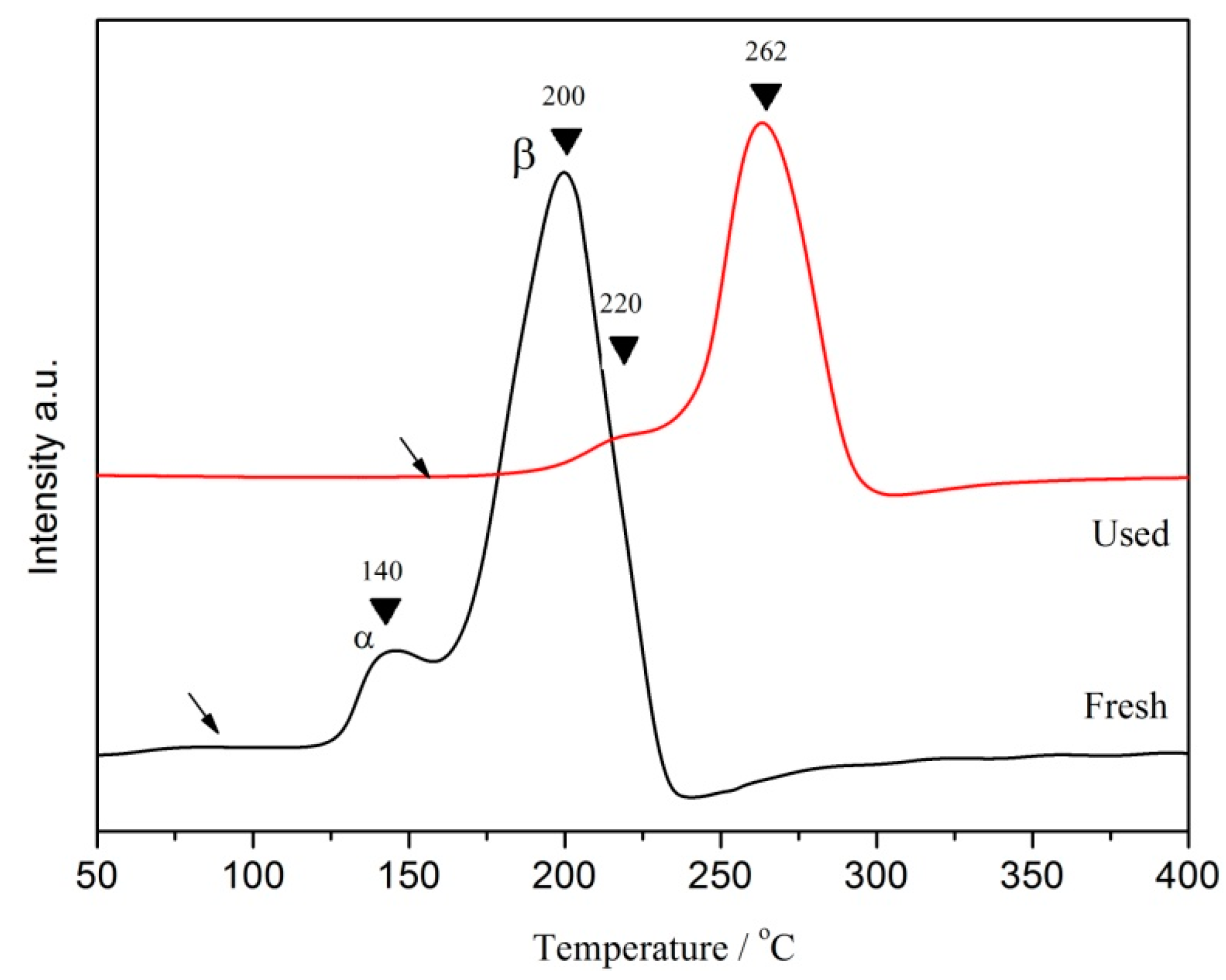
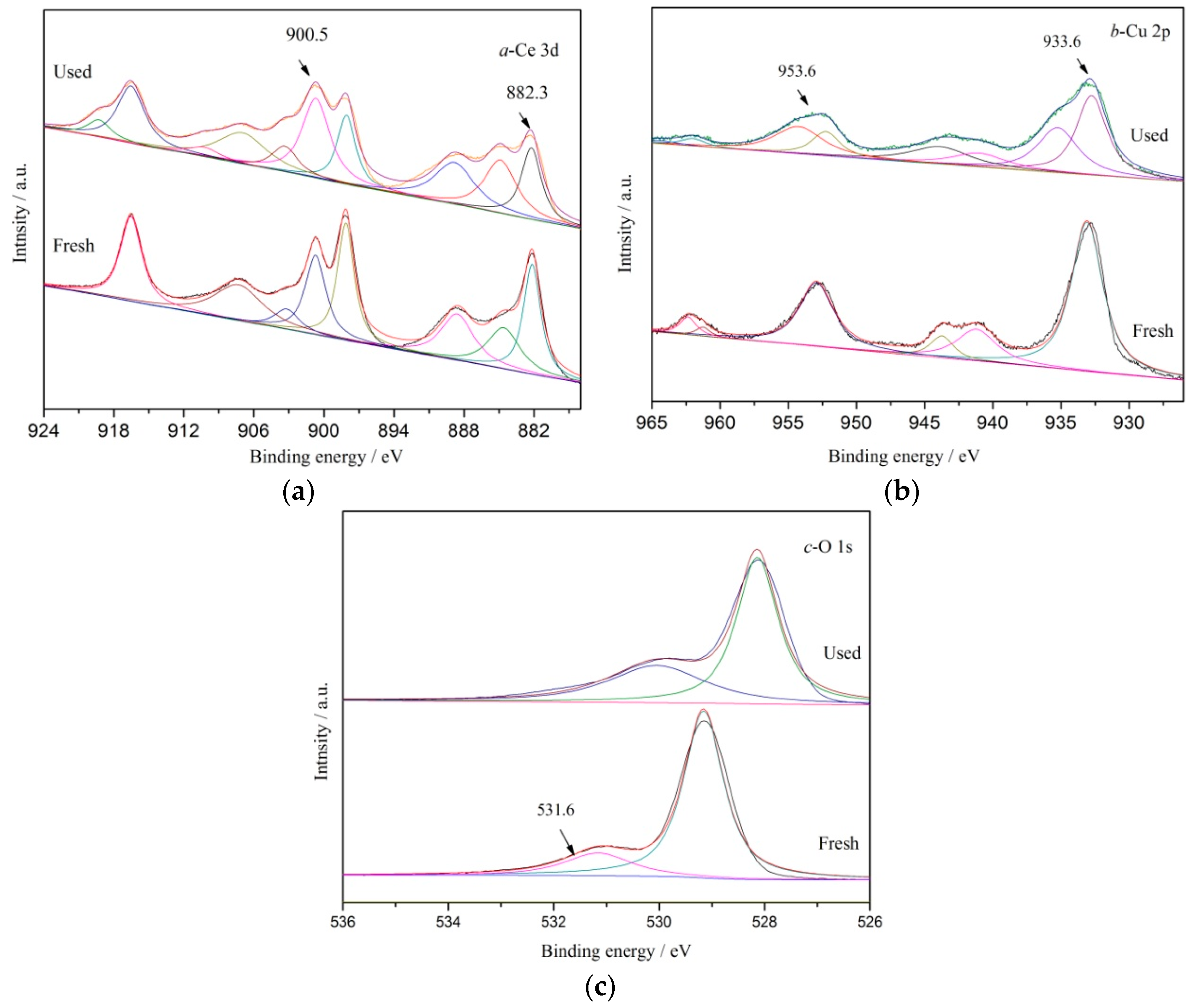
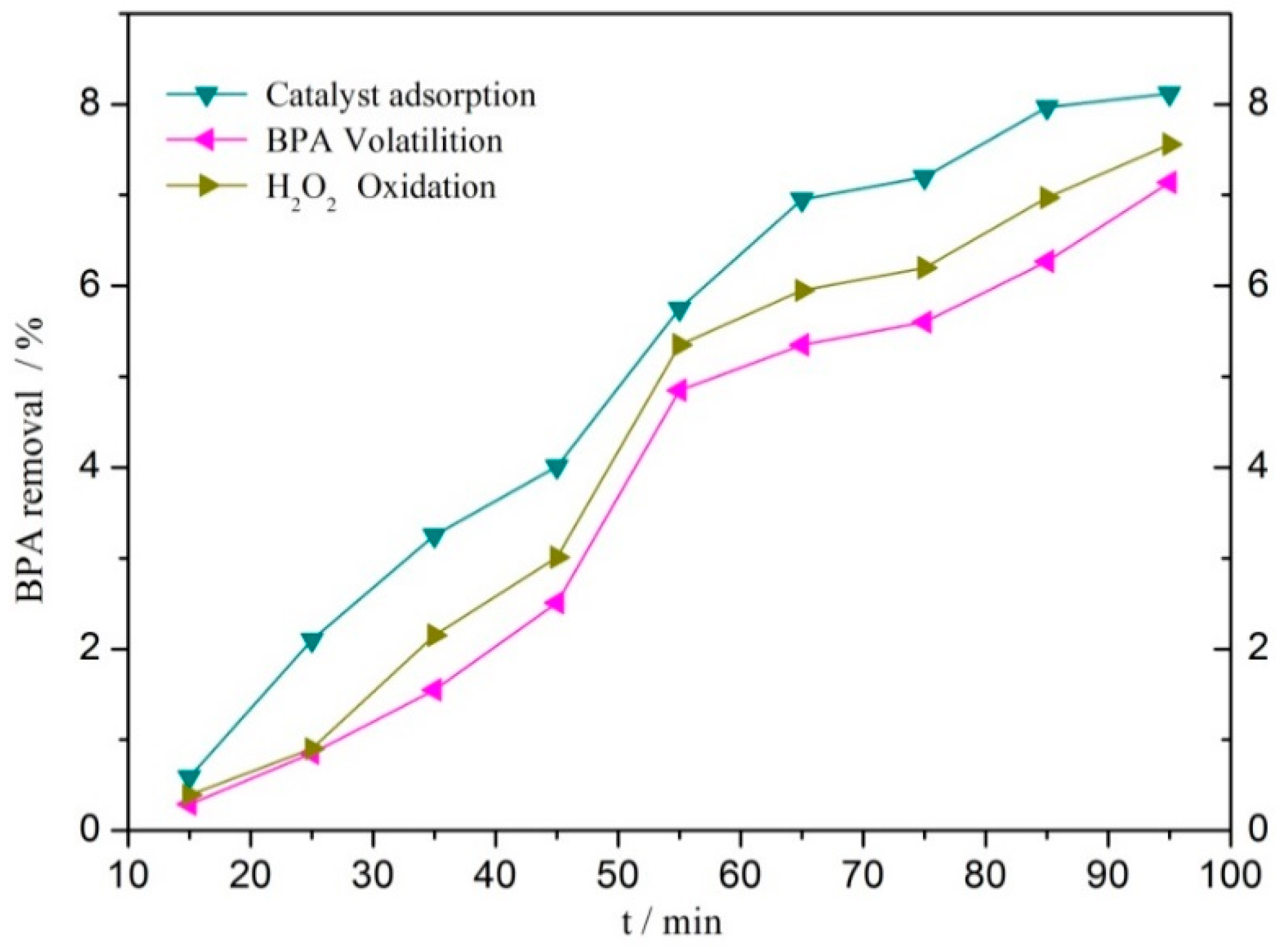
| Catalyst | BPA or TOC Removal (%) | Conditions | Ref. |
|---|---|---|---|
| Fe-C (G S) | XBPA = 100%, TOC = 60% | 2.8% wt Fe, 100 mg L−1 BPA, 530 mg L−1 H2O2, pH = 3, 80 °C | [21] |
| Fe-Mt-TC-C (Iron-pillared montmorillonite Tetracycline carbon) | XBPA = 100%, TOC = 78.3% | 0.4 g L−1 Fe-Mt-TC-C, 0.4 mmol L−1 BPA, 15 mmol L−1 H2O2, pH = 3 | [22] |
| Fe3O4-MWCNT (Multi-walled carbon nanotubes) | XBPA = 97% | 0.5 g L−1 Fe3O4-MWCNT, 0.3 mmol L−1 BPA, 1.2 mmol L−1 H2O2, pH = 3 | [23] |
| Cu-TUD (Technische Universiteit Delft)-1 | XBPA = 90.4% | 0.1 g 2.5wt% Cu/TUD-1, 100 ppm BPA, 90 mM H2O2, pH = 3.5 | [24] |
| Cu-AlPO4 | XBPA = 88% | 1 g L−1 Cu-AlPO4, 25 mg L−1 BPA, 10 mM H2O2, pH = 7 | [25] |
| CuO-Al2O3 | XBPA = 100%, TOC = 91% | 25 g L−1 CuO-Al2O3, 1 g L−1 BPA, 3.3 mL H2O2, pH = 2.4–7.2 | [26] |
| CuFeO2 | XBPA = 100%, TOC = 85% | 1 g L−1 CuFeO2, 0.1 mmol L−1 BPA, 20 mmol L−1 H2O2, pH = 5 | [27] |
| (P, Cu, Ag, Fe, P, Cu)-Ti-PILC (Pillared clays) | XBPA ≥ 87% | 5 g L−1 mcat, 20 ppm BPA, 1360 ppm H2O2, pH = 4 | [28] |
| PVP (polyvinylpyrrolidone)-AgNP | XBPA = 95.5% | 10 mg L−1 [AgNP]0, 0.4 mmol L−1 H2O2, pH = 4 | [29] |
| Ag-AgCl-Fh | XBPA = 100%, TOC = 92% | 1 g L−1 Ag/AgCl/Fh, 30 mg L−1 BPA, 10 mM H2O2, pH = 3 | [14] |
| Parameter | Value |
|---|---|
| RF (Radio frequency) generator power (kW) | 0.9 |
| Frequency of RF generator/MHz | 27.12 |
| Nebulizer | Cross-flow |
| Plasma gas flow rate (L min−1) | 12 |
| Auxiliary gas flow rate (L min−1) | 0.6 |
| Nebulizer gas flow rate (L min−1) | 0.6 |
| Replicates | 3 |
| Integration time (s) | 0.5 |
| Wavelength (nm) | Cu, 224.70 nm Ce, 413.38 nm |
| Limit of detection (μg L−1) | 1 |
| Limit of quantification (μg L−1) | Cu, 3; Ce, 5 |
| Repeatability | Relative standard deviation ≤ 1.5% |
| Stability | Relative standard deviation ≤ 2% |
| R2 | Cu, 0.99997; Ce, 0.99994 |
| Compound | Molecular Weight | Tentative Structure |
|---|---|---|
| Benzaldehyde, 4-ethyl- | 134 | 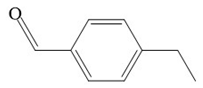 |
| p-Benzoquinone | 108 | 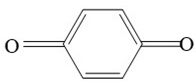 |
| Phenol | 94 |  |
| Styrene | 104 | 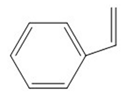 |
| p-Isopropenylphenol | 134 | 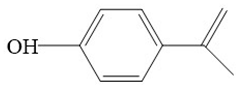 |
| p-xylene | 106 | 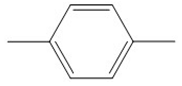 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Z.; Chen, L.; Zhou, G.; Gong, H.; Zhang, X.; Liu, Y.; Gao, X. Degradation of Bisphenol A by CeCu Oxide Catalyst in Catalytic Wet Peroxide Oxidation: Efficiency, Stability, and Mechanism. Int. J. Environ. Res. Public Health 2019, 16, 4675. https://doi.org/10.3390/ijerph16234675
Jiao Z, Chen L, Zhou G, Gong H, Zhang X, Liu Y, Gao X. Degradation of Bisphenol A by CeCu Oxide Catalyst in Catalytic Wet Peroxide Oxidation: Efficiency, Stability, and Mechanism. International Journal of Environmental Research and Public Health. 2019; 16(23):4675. https://doi.org/10.3390/ijerph16234675
Chicago/Turabian StyleJiao, Zhaojie, Ligong Chen, Guilin Zhou, Haifeng Gong, Xianming Zhang, Yunqi Liu, and Xu Gao. 2019. "Degradation of Bisphenol A by CeCu Oxide Catalyst in Catalytic Wet Peroxide Oxidation: Efficiency, Stability, and Mechanism" International Journal of Environmental Research and Public Health 16, no. 23: 4675. https://doi.org/10.3390/ijerph16234675
APA StyleJiao, Z., Chen, L., Zhou, G., Gong, H., Zhang, X., Liu, Y., & Gao, X. (2019). Degradation of Bisphenol A by CeCu Oxide Catalyst in Catalytic Wet Peroxide Oxidation: Efficiency, Stability, and Mechanism. International Journal of Environmental Research and Public Health, 16(23), 4675. https://doi.org/10.3390/ijerph16234675





