Vitamin D Deficiency and Unclear Abdominal Pain in Patients from Low- and Middle-Income Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
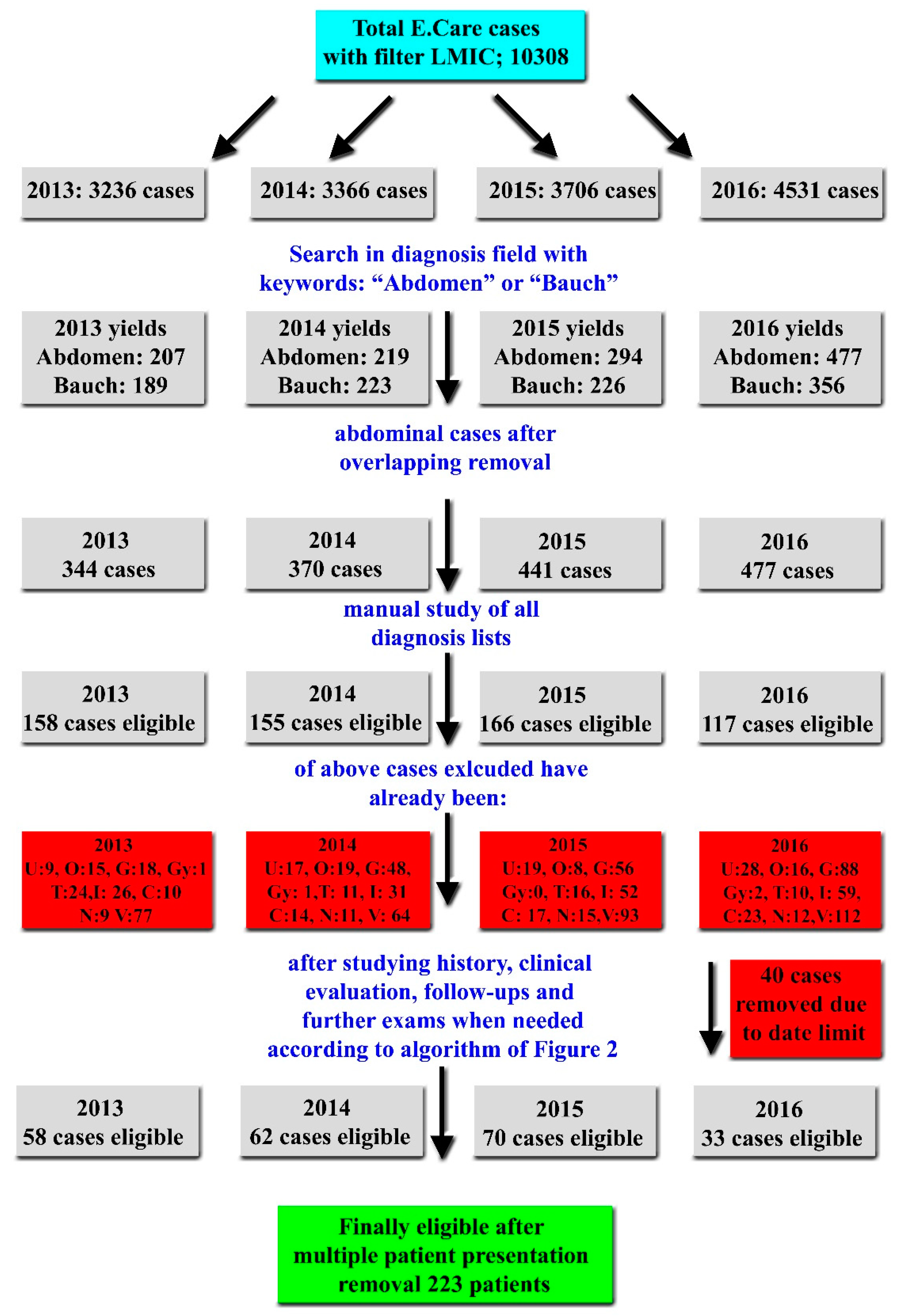
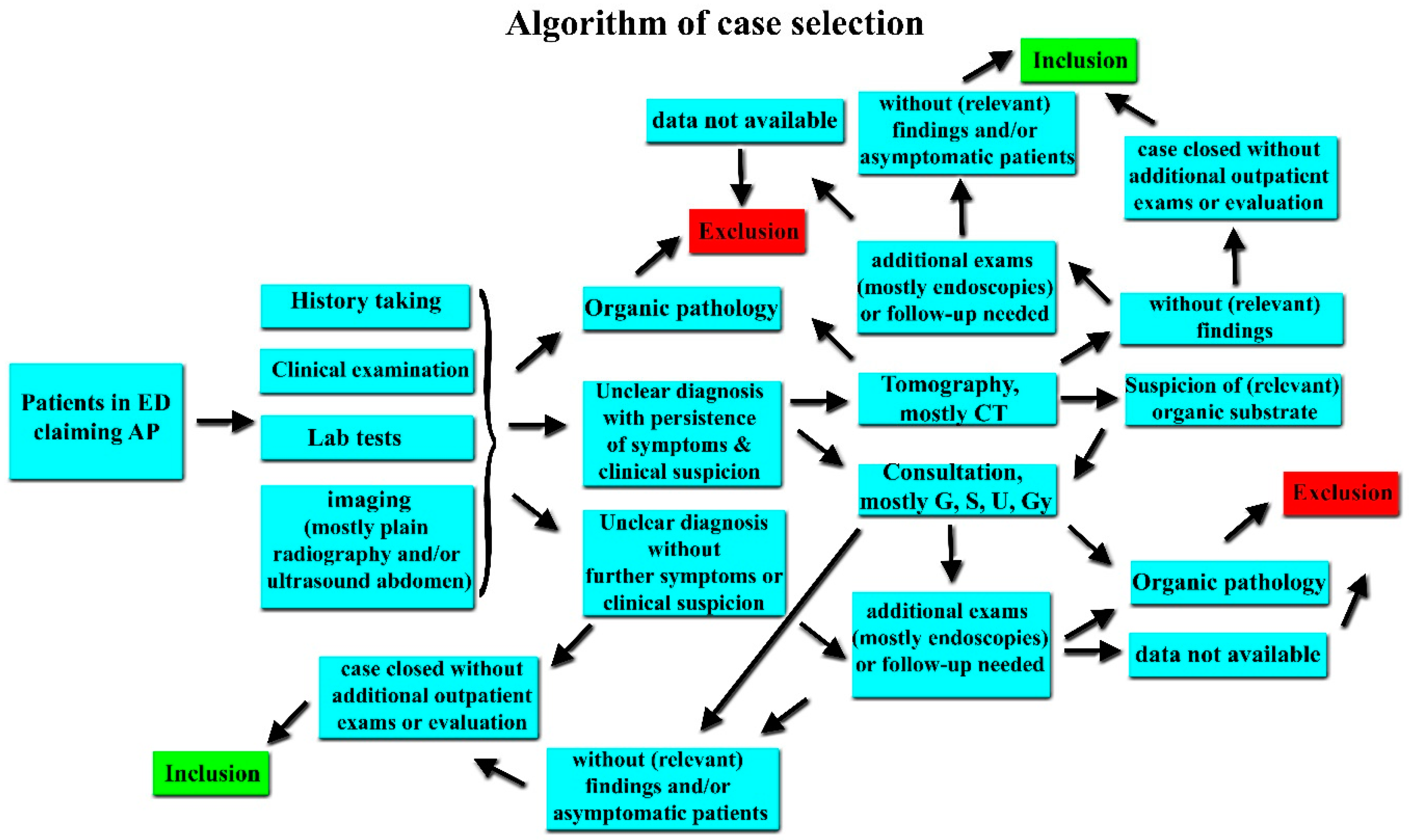
Inclusion and Exclusion Criteria
2.2. Data Collection and Extraction
2.3. Definition of Economies
2.4. Vitamin D Estimation
2.5. Ethical Considerations
2.6. Statistical Analysis
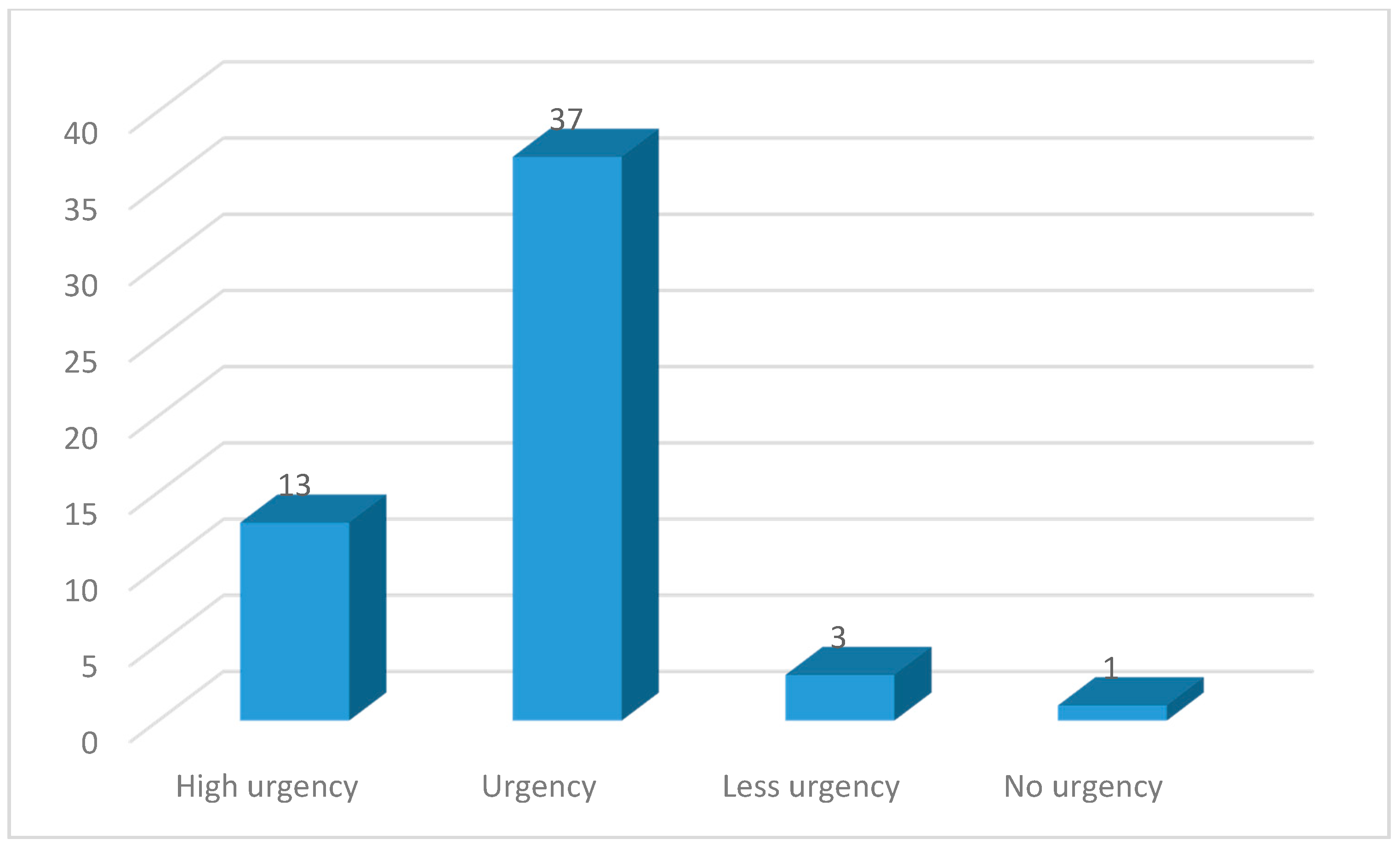
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
STROBE Statement
References
- Exadaktylos, A.K.; Sadowski-Cron, C.; Mäder, P.; Weissmann, M.; Dinkel, H.P.; Negri, M.; Zimmermann, H. Decision making in patients with acute abdominal pain at a university and at a rural hospital: Does the value of abdominal sonography differ? World J. Emerg. Surg. 2008, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.d.C.; Craig, K.D. Updating the definition of pain. Pain 2016, 157, 2420–2423. [Google Scholar] [CrossRef] [PubMed]
- Gans, S.L.; Pols, M.A.; Stoker, J.; Boermeester, M.A. Guideline for the Diagnostic Pathway in Patients with Acute Abdominal Pain. Dig. Surg. 2015, 32, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Korterink, J.; Devanarayana, N.M.; Rajindrajith, S.; Vlieger, A.; Benninga, M.A. Childhood functional abdominal pain: Mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 159–171. [Google Scholar] [CrossRef]
- Velissaris, D.; Karanikolas, M.; Pantzaris, N.; Kipourgos, G.; Bampalis, V.; Karanikola, K.; Fafliora, E.; Apostolopoulou, C.; Gogos, C. Acute Abdominal Pain Assessment in the Emergency Department: The Experience of a Greek University Hospital. Orig. Artic. J. Clin. Med. Res. 2017, 9, 987–993. [Google Scholar] [CrossRef]
- Macaluso, C.R.; McNamara, R.M. Evaluation and management of acute abdominal pain in the emergency department. Int. J. Gen. Med. 2012, 5, 789–797. [Google Scholar] [CrossRef]
- Marincek, B. Nontraumatic abdominal emergencies: Acute abdominal pain: Diagnostic strategies. Eur. Radiol. 2002, 12, 2136–2150. [Google Scholar] [CrossRef]
- Breidthardt, T.; Brunner-Schaub, N.; Balmelli, C.; Insenser, J.J.S.; Burri-Winkler, K.; Geigy, N.; Mundorff, L.; Exadaktylos, A.; Scholz, J.; Haaf, P.; et al. Inflammatory Biomarkers and Clinical Judgment in the Emergency Diagnosis of Urgent Abdominal Pain. Clin. Chem. 2019, 65, 302–312. [Google Scholar] [CrossRef]
- Cervellin, G.; Lippi, G. Abdominal migraine in the differential diagnosis of acute abdominal pain. Am. J. Emerg. Med. 2015, 33, 864.e3–864.e5. [Google Scholar] [CrossRef]
- Numeroso, F. Emergency physician’s perception of cultural and linguistic barriers in immigrant care: Results of a multiple-choice questionnaire in a large Italian urban emergency department. World J. Emerg. Med. 2015, 6, 111. [Google Scholar] [CrossRef]
- Abbas, M.; Aloudat, T.; Bartolomei, J.; Carballo, M.; Durieux-Paillard, S.; Gabus, L.; Jablonka, A.; Jackson, Y.; Kaojaroen, K.; Koch, D.; et al. Migrant and refugee populations: A public health and policy perspective on a continuing global crisis. Antimicrob. Resist. Infect. Control 2018, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Klukowska-Röetzler, J.; Eracleous, M.; Müller, M.; Srivastava, D.S.; Krummrey, G.; Keidar, O.; Exadaktylos, A.K. Increased urgent care center visits by southeast european migrants: A retrospective, controlled trial from Switzerland. Int. J. Environ. Res. Public Health 2018, 15, 1857. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Klingberg, K.; Srivastava, D.; Exadaktylos, A.K. Consultations by asylum seekers: Recent trends in the Emergency Department of a Swiss University Hospital. PLoS ONE 2016, 11, e0155423. [Google Scholar] [CrossRef] [PubMed]
- Braun, C.T.; Gnägi, C.R.; Klingberg, K.; Srivastava, D.; Ricklin, M.E.; Exadaktylos, A.K. Migrationsspezifische Aspekte des Patientengutes einer grossen europäischen universitären Notaufnahme über einen Zeitraum von zehn Jahren. Praxis 2017, 106, 409–414. [Google Scholar] [CrossRef]
- Buri, C.; Laederach, K. Kultur-und migrationsspezifische Aspekte funktioneller Bauchschmerzen. Ther. Umschau 2011, 68, 421–433. [Google Scholar] [CrossRef]
- Nwosu, B.U.; Maranda, L.; Candela, N. Vitamin D status in pediatric irritable bowel syndrome. PLoS ONE 2017, 12, e0172183. [Google Scholar]
- El Amrousy, D.; Hassan, S.; El Ashry, H.; Yousef, M.; Hodeib, H. Vitamin D supplementation in adolescents with irritable bowel syndrome: Is it useful? A randomized controlled trial. Saudi J. Gastroenterol. 2018, 24, 109. [Google Scholar] [CrossRef]
- Jalili, M.; Hekmatdoost, A.; Vahedi, H.; Poustchi, H.; Khademi, B.; Saadi, M.; Zemestani, M.; Janani, L. Co-Administration of soy isoflavones and Vitamin D in management of irritable bowel disease. PLoS ONE 2016, 11, e0158545. [Google Scholar] [CrossRef]
- Khayyat, Y.; Attar, S. Vitamin D Deficiency in Patients with Irritable Bowel Syndrome: Does it exist? Oman Med. J. 2015, 30, 115–118. [Google Scholar] [CrossRef]
- Tazzyman, S.; Richards, N.; Trueman, A.R.; Evans, A.L.; Grant, V.A.; Garaiova, I.; Plummer, S.F.; Williams, E.A.; Corfe, B.M. Vitamin D associates with improved quality of life in participants with irritable bowel syndrome: Outcomes from a pilot trial. BMJ Open Gastroenterol. 2015, 2, e000052. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Amani, R.; Hasanvand, A.; Yousefi Rad, E.; Alipour, M.; Saboori, S.; Choghakhori, R. Association of Serum Vitamin D Concentration With Clinical Symptoms and Quality of Life in Patients With Irritable Bowel Syndrome. J. Am. Coll. Nutr. 2018, 38, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Buyukuslu, N.; Esin, K.; Hizli, H.; Sunal, N.; Yigit, P.; Garipagaoglu, M. Clothing preference affects vitamin D status of young women. Nutr. Res. 2014, 34, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S. Vitamin D and African Americans. J. Nutr. 2006, 136, 1126–1129. [Google Scholar] [CrossRef] [PubMed]
- Kagotho, E.; Omuse, G.; Okinda, N.; Ojwang, P. Vitamin D status in healthy black African adults at a tertiary hospital in Nairobi, Kenya: A cross sectional study. BMC Endocr. Disord. 2018, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Junaid, K.; Rehman, A.; Jolliffe, D.A.; Wood, K.; Martineau, A.R. High prevalence of vitamin D deficiency among women of child-bearing age in Lahore Pakistan, associating with lack of sun exposure and illiteracy. BMC Women’s Health 2015, 15, 83. [Google Scholar] [CrossRef][Green Version]
- Jazayeri, M.; Moradi, Y.; Rasti, A.; Nakhjavani, M.; Kamali, M.; Baradaran, H.R. Prevalence of vitamin D deficiency in healthy Iranian children: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2018, 32, 83. [Google Scholar] [CrossRef]
- Bern, I.U. Inselspital Bern. Skywork Inspir. 2017, 1, 1. [Google Scholar]
- Rutschmann, O.T.; Sieber, R.S.; Hugli, O.W. Recommandations de la Société Suisse de Médecine d’Urgence et de Sauvetage pour le triage dans les services d’urgences hospitaliers en Suisse. Bull. Méd. Suisses 2009, 90, 1789–1790. [Google Scholar] [CrossRef]
- Mackway-Jones, K. Emergency Triage: Manchester Triage Group; BMJ Publishing Group: London, UK, 1997. [Google Scholar]
- The World Bank. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 29 January 2019).
- Composition of Macro Geographical (Continental) Regions, Geographical Sub-Regions, and Selected Economic and Other Groupings Web Site. Available online: http://millenniumindicators.un.org/unsd/methods/m49/m49regin.htm (accessed on 3 November 2010).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Talachian, E.; Bidari, A.; Zahmatkesh, H. Abdominal pain-related functional gastrointestinal disorders based on Rome III criteria in a pediatric gastroenterology clinic. Med. J. Islam. Repub. Iran 2015, 29, 247. [Google Scholar] [PubMed]
- Buono, J.L.; Mathur, K.; Averitt, A.J.; Andrae, D.A. Economic Burden of Irritable Bowel Syndrome with Diarrhea: Retrospective Analysis of a U.S. Commercially Insured Population. J. Manag. Care Spec. Pharm. 2017, 23, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Kehler, L.; Verma, S.; Krone, R.; Roper, E. Vitamin D deficiency in children presenting to the emergency department: A growing concern. Vitamin D deficiency in Birmingham’s children: Presentation to the emergency department. Emerg. Med. J. 2013, 30, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Panarese, A.; Pesce, F.; Porcelli, P.; Riezzo, G.; Iacovazzi, P.A.; Leone, C.M.; De Carne, M.; Rinaldi, C.M.; Shahini, E. Chronic functional constipation is strongly linked to vitamin D deficiency. World J. Gastroenterol. 2019, 25, 1729–1740. [Google Scholar] [CrossRef]
- Whitehurst, J.L.; Reid, C.M. Vitamin D deficiency as a cause of chronic pain in the palliative medicine clinic: Two case reports. Palliat. Med. 2014, 28, 87–89. [Google Scholar] [CrossRef]
- Alkhatatbeh, M.J.; Amara, N.A.; Abdul-Razzak, K.K. Association of 25-hydroxyvitamin D with HDL-cholesterol and other cardiovascular risk biomarkers in subjects with non-cardiac chest pain. Lipids Health Dis. 2019, 18, 27. [Google Scholar] [CrossRef]
- Alkhatatbeh, M.J.; Abdul-Razzak, K.K.; Amara, N.A.; Al-Jarrah, M. Non-cardiac Chest Pain and Anxiety: A Possible Link to Vitamin D and Calcium. J. Clin. Psychol. Med. Settings 2019, 26, 194–199. [Google Scholar] [CrossRef]
- Gazerani, P.; Fuglsang, R.; Pedersen, J.G.; Sørensen, J.; Kjeldsen, J.L.; Yassin, H.; Nedergaard, B.S. A randomized, double-blinded, placebo-controlled, parallel trial of vitamin D 3 supplementation in adult patients with migraine. Curr. Med. Res. Opin. 2019, 35, 715–723. [Google Scholar] [CrossRef]
- Rapisarda, L.; Mazza, M.R.; Tosto, F.; Gambardella, A.; Bono, F.; Sarica, A. Relationship between severity of migraine and vitamin D deficiency: A case-control study. Neurol. Sci. 2018, 39, 167–168. [Google Scholar] [CrossRef]
- Song, T.-J.; Chu, M.-K.; Sohn, J.-H.; Ahn, H.-Y.; Lee, S.H.; Cho, S.-J. Effect of Vitamin D Deficiency on the Frequency of Headaches in Migraine. J. Clin. Neurol. 2018, 14, 366. [Google Scholar] [CrossRef]
- Togha, M.; Razeghi Jahromi, S.; Ghorbani, Z.; Martami, F.; Seifishahpar, M. Serum Vitamin D Status in a Group of Migraine Patients Compared With Healthy Controls: A Case–Control Study. Headache 2018, 58, 1530–1540. [Google Scholar] [CrossRef] [PubMed]

| Group | Total | ||||
|---|---|---|---|---|---|
| Controls | Patients | ||||
| Gender | Women | Count | 13 | 17 | 30 |
| % within gender | 43.3% | 56.7% | 100.0% | ||
| % within group | 48.1% | 63.0% | 55.6% | ||
| Men | Count | 14 | 10 | 24 | |
| % within gender | 58.3% | 41.7% | 100.0% | ||
| % within group | 51.9% | 37.0% | 44.4% | ||
| Total | Count | 27 | 27 | 54 | |
| % within gender | 50.0% | 50.0% | 100.0% | ||
| % within group | 100.0% | 100.0% | 100.0% | ||
| Group | n | Mean | Std. Deviation | Std. Error Mean | p-Value | |
|---|---|---|---|---|---|---|
| Age | Controls | 27 | 47.70 | 13.088 | 2.519 | 0.085 |
| Patients | 27 | 41.30 | 13.708 | 2.638 | ||
| BMI | Controls | 27 | 26.163 | 5.6835 | 1.0938 | 0.186 |
| Patients | 27 | 24.259 | 4.7021 | 0.9049 | ||
| 25OHD* (nmol/L) | Controls | 27 | 53.67 | 22.940 | 4.415 | 0.060 |
| Patients | 27 | 41.26 | 24.513 | 4.718 |
| Group | Total | |||||
|---|---|---|---|---|---|---|
| Controls | Patients | |||||
| 25OHD a nmol/L | ≤25 | Count | 1 | 14 | 15 | |
| % within group | 3.7% | 51.9% | 27.8% | |||
| >25 | Count | 26 | 13 | 39 | ||
| % within group | 96.3% | 48.1% | 72.2% | |||
| Total | Count | 27 | 27 | 54 | ||
| % within group | 100.0% | 100.0% | 100.0% | |||
| 25OHD b nmol/L | ≤50 | Count | 13 | 21 | 34 | |
| % within group | 48.1% | 77.8% | 63.0% | |||
| >50 | Count | 14 | 6 | 20 | ||
| % within group | 51.9% | 22.2% | 37.0% | |||
| Total | Count | 27 | 27 | 54 | ||
| % within group | 100.0% | 100.0% | 100.0% | |||
| Frequency | Percent | Valid Percent | Cumulative Percent | |
|---|---|---|---|---|
| South America | 3 | 5.6 | 5.6 | 5.6 |
| Eastern Africa | 3 | 5.6 | 5.6 | 11.1 |
| Northern Africa | 6 | 11.1 | 11.1 | 22.2 |
| Caribbean | 1 | 1.9 | 1.9 | 24.1 |
| Eastern Asia | 5 | 9.3 | 9.3 | 33.3 |
| Southern Asia | 9 | 16.7 | 16.7 | 50.0 |
| South-eastern Asia | 2 | 3.7 | 3.7 | 53.7 |
| Southern Europe | 11 | 20.4 | 20.4 | 74.1 |
| Western Asia | 6 | 11.1 | 11.1 | 85.2 |
| Eastern Europe | 7 | 13.0 | 13.0 | 98.1 |
| Northern Europe | 1 | 1.9 | 1.9 | 100.0 |
| Total | 54 | 100.0 | 100.0 |
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
|---|---|---|---|---|---|
| Admission | Police | 1 | 1.9 | 1.9 | 1.9 |
| Self-admission | 38 | 70.4 | 70.4 | 72.2 | |
| Ambulance | 5 | 9.3 | 9.3 | 81.5 | |
| Private (GP) | 3 | 5.6 | 5.6 | 87.0 | |
| Primary utilization | 3 | 5.6 | 5.6 | 92.6 | |
| Other or not specified | 4 | 7.4 | 7.4 | 100.0 | |
| Total | 54 | 100.0 | 100.0 | ||
| Discharge | At home | 42 | 77.8 | 77.8 | 77.8 |
| Inpatient | 9 | 16.7 | 16.7 | 94.4 | |
| Other or not specified | 3 | 5.6 | 5.6 | 100.0 | |
| Total | 54 | 100.0 | 100.0 | ||
| Group | n | Mean | Std. Deviation | Std. Error Mean | p-Value | |
|---|---|---|---|---|---|---|
| Age | Controls | 13 | 43.31 | 13.073 | 3.626 | 0.179 |
| Patients | 17 | 36.65 | 13.124 | 3.183 | ||
| BMI | Controls | 13 | 26.908 | 5.8273 | 1.6162 | 0.194 |
| Patients | 17 | 24.329 | 4.7864 | 1.1609 | ||
| 25OHD (nmol/L) | Controls | 13 | 53.31 | 25.316 | 7.021 | 0.037 |
| Patients | 17 | 36.59 | 16.382 | 3.973 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doulberis, M.; Papaefthymiou, A.; Kountouras, J.; Polyzos, S.A.; Srivastava, S.; Klukowska-Rötzler, J.; Perrig, M.; Papoutsi, S.; Exadaktylos, A.K.; Srivastava, D.S. Vitamin D Deficiency and Unclear Abdominal Pain in Patients from Low- and Middle-Income Countries. Int. J. Environ. Res. Public Health 2019, 16, 4607. https://doi.org/10.3390/ijerph16234607
Doulberis M, Papaefthymiou A, Kountouras J, Polyzos SA, Srivastava S, Klukowska-Rötzler J, Perrig M, Papoutsi S, Exadaktylos AK, Srivastava DS. Vitamin D Deficiency and Unclear Abdominal Pain in Patients from Low- and Middle-Income Countries. International Journal of Environmental Research and Public Health. 2019; 16(23):4607. https://doi.org/10.3390/ijerph16234607
Chicago/Turabian StyleDoulberis, Michael, Apostolis Papaefthymiou, Jannis Kountouras, Stergios A. Polyzos, Simone Srivastava, Jolanta Klukowska-Rötzler, Martin Perrig, Sylvana Papoutsi, Aristomenis K. Exadaktylos, and David Shiva Srivastava. 2019. "Vitamin D Deficiency and Unclear Abdominal Pain in Patients from Low- and Middle-Income Countries" International Journal of Environmental Research and Public Health 16, no. 23: 4607. https://doi.org/10.3390/ijerph16234607
APA StyleDoulberis, M., Papaefthymiou, A., Kountouras, J., Polyzos, S. A., Srivastava, S., Klukowska-Rötzler, J., Perrig, M., Papoutsi, S., Exadaktylos, A. K., & Srivastava, D. S. (2019). Vitamin D Deficiency and Unclear Abdominal Pain in Patients from Low- and Middle-Income Countries. International Journal of Environmental Research and Public Health, 16(23), 4607. https://doi.org/10.3390/ijerph16234607








