Pyruvate Kinase M2 Expression: A Potential Metabolic Biomarker to Differentiate Endometrial Precancer and Cancer that is Associated with Poor Outcomes in Endometrial Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Microarray
2.2. Immunohistochemistry Stain
2.3. Statistical Analysis
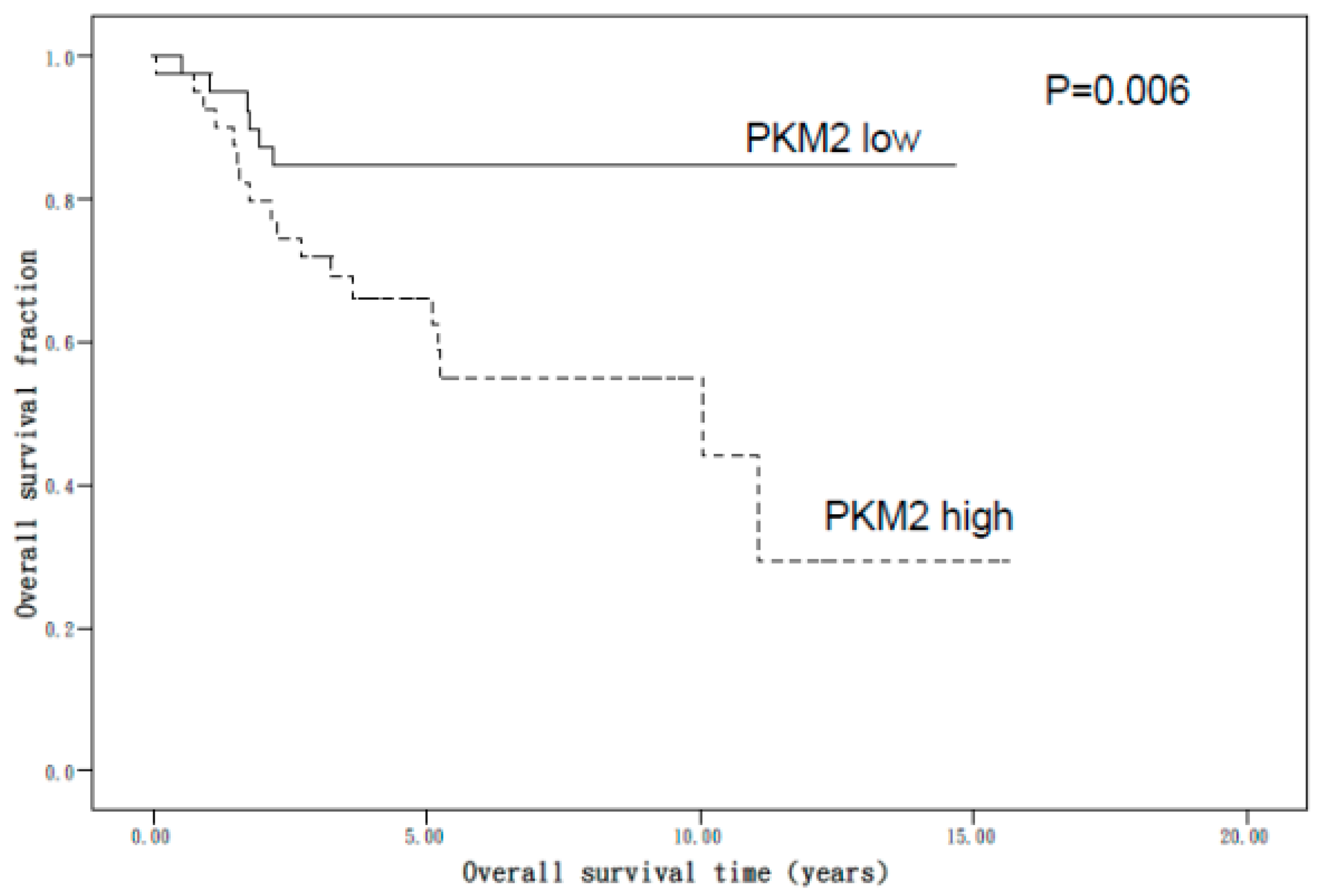
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emons, G.; Fleckenstein, G.; Hinney, B.; Huschmand, A.; Heyl, W. Hormonal interactions in endometrial cancer. Endocr. Relat. Cancer 2000, 7, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Kurman, R.J.; Kaminski, P.F.; Norris, H.J. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer 1985, 56, 403–412. [Google Scholar] [CrossRef]
- Mutter, G.L. Histopathology of genetically defined endometrial precancers. Int. J. Gynecol. Pathol. 2000, 19, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Mutter, G.L. Diagnosis of premalignant endometrial disease. J. Clin. Pathol. 2002, 55, 326–331. [Google Scholar] [CrossRef]
- Silverberg, S.G. Problems in the Differential Diagnosis of Endometrial Hyperplasia and Carcinoma. Mod. Pathol. 2000, 13, 309–327. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Critchley, H.O.; Williams, A.R.; Arends, M.J.; Saunders, P.T. New concepts for an old problem: The diagnosis of endometrial hyperplasia. Hum. Reprod. Update 2017, 23, 232–254. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Noguchi, T.; Inoue, H.; Tanaka, T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J. Boil. Chem. 1986, 261, 13807–13812. [Google Scholar]
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233. [Google Scholar] [CrossRef]
- Anastasiou, D.; Yu, Y.; Israelsen, W.J.; Jiang, J.K.; Boxer, M.B.; Hong, B.S.; Tempel, W.; Dimov, S.; Shen, M.; Jha, A.; et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 2012, 8, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.-K.; Huang, T.-S.; Liao, Y.-P.; Huang, R.-L.; Su, P.-H.; Shen, H.-Y.; Lai, H.-C.; Wang, Y.-C. Pyruvate kinase M2 is a poor prognostic marker of and a therapeutic target in ovarian cancer. PLoS ONE 2017, 12, e0182166. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Piña, P.; Guerrero, G.; Lazos, M.; Salcedo, M. A simple method for the construction of small format tissue arrays. J. Clin. Pathol. 2003, 56, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Scully, R.E.; Bonfiglio, T.A.; Kurman, R.J.; Silverberg, S.G.; Wilkinson, E.J. Uterine Corpus. Histological Typing of Female Genital Tract Tumours, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1994; pp. 13–30. [Google Scholar]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-S.; Hsieh, D.-S.; Loh, S.-H.; Chen, A.; Yao, C.-W.; Yen, C.-Y. Increasing expression of serine protease matriptase in ovarian tumors: Tissue microarray analysis of immunostaining score with clinicopathological parameters. Mod. Pathol. 2006, 19, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, S.; Izadi-Mood, N.; Sotoudeh, K.; Tavangar, S.M. Altered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium. Diagn. Pathol. 2009, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Reed, S.D.; Voigt, L.F.; Jordan, C.D.; Newton, K.M.; Garcia, R.L. Diagnosing endometrial hyperplasia: Why is it so difficult to agree? Am. J. Surg. Pathol. 2008, 32, 691–698. [Google Scholar] [CrossRef]
- Kendall, B.S.; Ronnett, B.M.; Isacson, C.; Cho, K.R.; Hedrick, L.; Diener-West, M.; Kurman, R.J. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am. J. Surg. Pathol. 1998, 22, 1012–1019. [Google Scholar] [CrossRef]
- Suh-Burgmann, E.; Hung, Y.Y.; Armstrong, M.A. Complex atypical endometrial hyperplasia: The risk of unrecognized adenocarcinoma and value of preoperative dilation and curettage. Obstet. Gynecol. 2009, 114, 523–529. [Google Scholar] [CrossRef]
- Taylor, P.J.; Gomel, V. 2 Endometrial ablation: Indications and preliminary diagnostic hysteroscopy. Baillière’s Clin. Obstet. Gynaecol. 1995, 9, 251–260. [Google Scholar] [CrossRef]
- Teng, Y.; Ai, Z.; Wang, Y.; Wang, J.; Luo, L. Proteomic identification of PKM2 and HSPA5 as potential biomarkers for predicting high-risk endometrial carcinoma. J. Obstet. Gynaecol. Res. 2013, 39, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Matias-Guiu, X.; Catasus, L.; Bussaglia, E.; Lagarda, H.; Garcia, A.; Pons, C.; Muñoz, J.; Argüelles, R.; Machin, P.; Prat, J. Molecular pathology of endometrial hyperplasia and carcinoma. Hum. Pathol. 2001, 32, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.L.; Mutter, G.L. Molecular and Pathologic Aspects of Endometrial Carcinogenesis. J. Clin. Oncol. 2006, 24, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.-C.; Meinel, A.; Handzel, R.; Einenkel, J. Histopathology of endometrial hyperplasia and endometrial carcinoma. Ann. Diagn. Pathol. 2007, 11, 297–311. [Google Scholar] [CrossRef]
- Banno, K.; Kisu, I.; Yanokura, M.; Tsuji, K.; Masuda, K.; Ueki, A.; Kobayashi, Y.; Yamagami, W.; Nomura, H.; Tominaga, E.; et al. Biomarkers in endometrial cancer: Possible clinical applications (Review). Oncol. Lett. 2012, 3, 1175–1180. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Christofk, H.R.; Vander Heiden, M.G.; Wu, N.; Asara, J.M.; Cantley, L.C. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 2008, 452, 181–186. [Google Scholar] [CrossRef]
- Liu, L.; Ulbrich, J.; Müller, J.; Wüstefeld, T.; Aeberhard, L.; Kress, T.R.; Muthalagu, N.; Rycak, L.; Rudalska, R.; Moll, R.; et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature 2012, 483, 608–612. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.K.; Han, Y.-M.; Kim, J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int. J. Biochem. Cell Boil. 2008, 40, 1043–1054. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yoon, S.O.; Seol, S.Y.; Hong, S.W.; Kim, J.W.; Choi, S.H.; Cho, J.Y. Overexpression of the M2 isoform of pyruvate kinase is an adverse prognostic factor for signet ring cell gastric cancer. World J. Gastroenterol. 2012, 18, 4037–4043. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Jackson, K.; Shi, R.; Zhao, Y. Sensitizing the Therapeutic Efficacy of Taxol with Shikonin in Human Breast Cancer Cells. PLoS ONE 2014, 9, e94079. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Yang, Y.C.; Wang, H.P.; Tien, Y.W.; Shun, C.T.; Huang, H.Y.; Hsiao, M.; Hua, K.T. Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene 2018, 37, 1730–1742. [Google Scholar] [CrossRef]
- Wong, N.; De Melo, J.; Tang, D. PKM2, a Central Point of Regulation in Cancer Metabolism. Int. J. Cell Boil. 2013, 2013, 242513. [Google Scholar] [CrossRef]
- Hosseini Nasab, S.; Jooya, N.; Esmaeili, A.; Zarrin Khameh, N.; Diaz-Arrastia, C.; Momeni, M. Using Pyruvate Kinase as a Predictor for Patient with Endometrial Cancer Having Complex Hyperplasia with Atypia to Prevent Hysterectomy and Preserve Fertility: Retrospective Immunohistochemical Study. Reprod. Sci. 2018, 25, 1286–1291. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Z. Pyruvate kinase M2 at a glance. J. Cell Sci. 2015, 128, 1–6. [Google Scholar] [CrossRef]
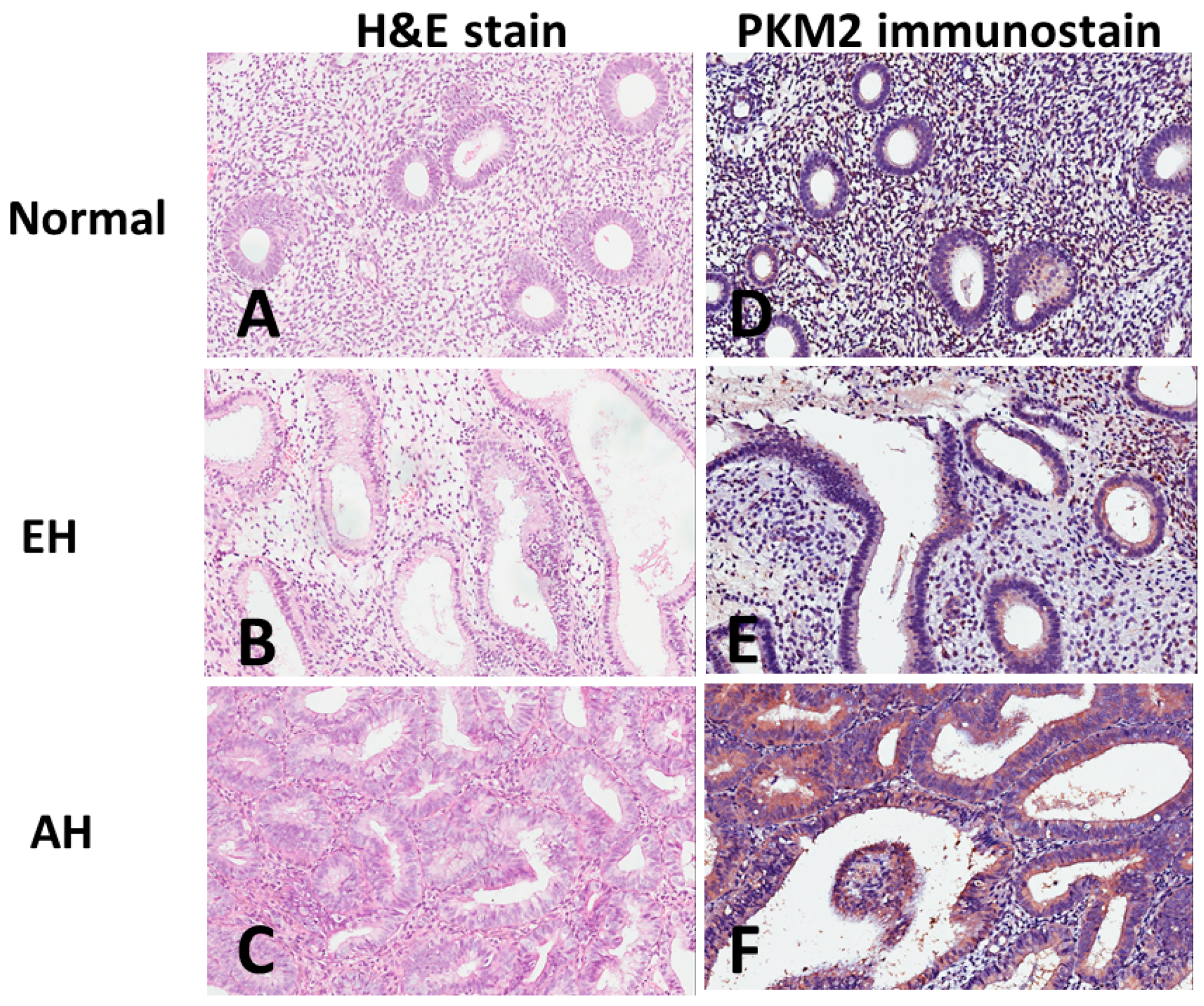
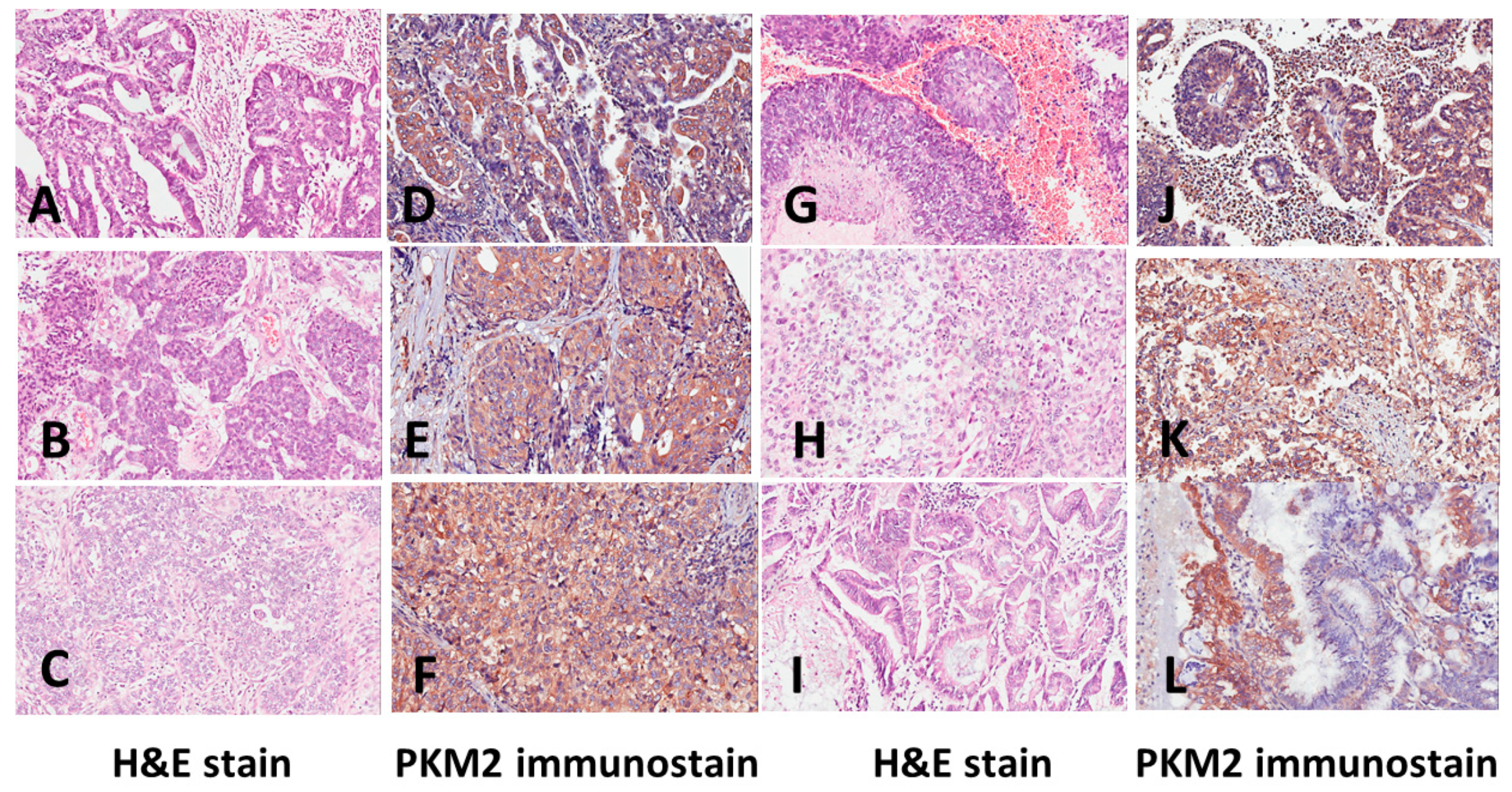
| PKM2 low n. (%) | PKM2 high n. (%) | p Value | |
|---|---|---|---|
| <0.001 | |||
| Normal | 30 (100.0) | 0(0) | |
| EH | 36 (100.0) | 0(0) | |
| AH | 28 (87.5) | 4 (12.5) | |
| EC | 54 (50.0) | 54 (50.0) |
| PKM2 Expression Low | PKM2 Expression High | p Value | |
|---|---|---|---|
| Characteristic | 0+, 1+ | 2+, 3+, 4+, 6+ | |
| Patients (no.) | 42 | 43 | |
| Age (years) | 0.009 | ||
| Range | 21–81 | 32–89 | |
| Mean ± SEM | 52.21 ± 1.60 | 58.77 ± 1.85 | |
| FIGO Stage [no. (%)] | 0.712 | ||
| I, II | 28 (51.9) | 26 (48.1) | |
| III, IV | 14 (45.2) | 17 (54.8) | |
| Nuclear grade [no. (%)] | |||
| G1 | 24 (58.5) | 17 (41.5) | 0.202 |
| G2 | 9 (47.4) | 10 (52.6) | |
| G3 | 9 (36.0) | 16 (64.0) | |
| Histological type [no. (%)] | 0.135 a | ||
| CC | 1 (12.5) | 7 (87.5) | |
| EmAC | 34 (53.1) | 30 (46.9) | |
| MC | 1 (50.0) | 1 (50) | |
| SC | 6 (54.5) | 5 (45.5) | |
| Histological type [no. (%)] | 0.345 | ||
| EmAC | 34 (53.1) | 30 (46.9) | |
| Non- EmAC | 8 (38.1) | 13 (61.9) |
| Variable | Univariate Analysis Crude HR (95% CI) | Multivariate Adjusted HR (95% CI) |
|---|---|---|
| Age (years) | 1.04 (1.00–1.07) * | 1.04 (1.00–1.08) |
| PKM2 expression a | ||
| Low | 1.00 (Ref.) | 1.00 (Ref.) |
| High | 3.40 (1.35–8.56) * | 1.96 (0.71–5.37) |
| FIGO Stage | ||
| I, II | 1.00 (Ref.) | 1.00 (Ref.) |
| III, IV | 8.41 (3.28–21.58) * | 7.97 (2.71–23.48) * |
| Nuclear grade | ||
| G1 | 1.00 (Ref.) | 1.00 (Ref.) |
| G2 | 1.54 (0.47–5.07) | 0.89 (0.24–3.28) |
| G3 | 4.78 (1.79–12.76) * | 4.04 (0.96–16.99) |
| Histological type | ||
| EmAC | 1.00 (Ref.) | 1.00 (Ref.) |
| Non-EmAC | 2.90 (1.27–6.63) * | 0.32 (0.08–1.30) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-J.; Chou, Y.-C.; Lin, Y.-J.; Yu, M.-H.; Ou, Y.-C.; Chu, P.-W.; Wu, C.-C.; Wang, Y.-C.; Chao, T.-K. Pyruvate Kinase M2 Expression: A Potential Metabolic Biomarker to Differentiate Endometrial Precancer and Cancer that is Associated with Poor Outcomes in Endometrial Carcinoma. Int. J. Environ. Res. Public Health 2019, 16, 4589. https://doi.org/10.3390/ijerph16234589
Lai Y-J, Chou Y-C, Lin Y-J, Yu M-H, Ou Y-C, Chu P-W, Wu C-C, Wang Y-C, Chao T-K. Pyruvate Kinase M2 Expression: A Potential Metabolic Biomarker to Differentiate Endometrial Precancer and Cancer that is Associated with Poor Outcomes in Endometrial Carcinoma. International Journal of Environmental Research and Public Health. 2019; 16(23):4589. https://doi.org/10.3390/ijerph16234589
Chicago/Turabian StyleLai, Yu-Ju, Yu-Ching Chou, Yi-Jia Lin, Mu-Hsien Yu, Yu-Che Ou, Po-Wei Chu, Chia-Chun Wu, Yu-Chi Wang, and Tai-Kuang Chao. 2019. "Pyruvate Kinase M2 Expression: A Potential Metabolic Biomarker to Differentiate Endometrial Precancer and Cancer that is Associated with Poor Outcomes in Endometrial Carcinoma" International Journal of Environmental Research and Public Health 16, no. 23: 4589. https://doi.org/10.3390/ijerph16234589
APA StyleLai, Y.-J., Chou, Y.-C., Lin, Y.-J., Yu, M.-H., Ou, Y.-C., Chu, P.-W., Wu, C.-C., Wang, Y.-C., & Chao, T.-K. (2019). Pyruvate Kinase M2 Expression: A Potential Metabolic Biomarker to Differentiate Endometrial Precancer and Cancer that is Associated with Poor Outcomes in Endometrial Carcinoma. International Journal of Environmental Research and Public Health, 16(23), 4589. https://doi.org/10.3390/ijerph16234589




