Abstract
Non-typhoidal salmonellosis is a leading cause of foodborne zoonosis. To better understand the epidemiology of human salmonellosis, this study aimed to determine the prevalence, antimicrobial resistance and sequence types of Salmonella in retail food and wild birds (proximity to humans) in Singapore. We analyzed 21,428 cooked and ready-to-eat food and 1,510 residual faecal samples of wild birds collected during 2010–2015. Thirty-two Salmonella isolates from food and wild birds were subjected to disc diffusion and multi-locus sequence typing (MLST). Salmonella was isolated from 0.08% (17/21,428) of food and 0.99% (15/1510) of wild birds. None of the isolates from wild birds (n = 15) exhibited phenotypic resistance, while the isolates from food (47.1%, 8/17) showed a high prevalence of phenotypic resistance to, at least, one antimicrobial. These findings suggested that the avian Salmonella isolates had been subjected to less antimicrobial selection pressure than those from food samples. MLST revealed specific sequence types found in both food and wild birds. The study can guide future studies with whole-genome analysis on a larger number of isolates from various sectors for public health measures.
1. Introduction
Salmonellosis, caused by Gram-negative bacteria of the genus Salmonella, is a leading type of foodborne infection in humans worldwide [1,2]. Contaminated food is likely the primary cause of salmonellosis. Besides foodborne transmission, the literature has shown several human salmonellosis outbreaks that have been associated with wild birds, suggesting a role for zoonotic transmission [3]. In addition to their ability to cause infection, there has been an increasing number of Salmonella strains reported as being resistant to commonly used antimicrobials [4,5]. This may significantly affect human health by limiting the choice of antimicrobials for treating severe salmonellosis cases in humans.
In order to strategize public health measures for controlling indigenous cases of salmonellosis, it is important to gather information on the occurrence and distribution of Salmonella serovars in a particular setting. Multi-locus Sequence Typing (MLST) was chosen instead of conventional Kauffman-White serotyping, as it is more discriminating and reproducible [6]. When coupled with antimicrobial susceptibility profiles, a greater discrimination between strains can be obtained, in order to provide useful information for surveillance and epidemiological investigations.
In Singapore, non-typhoidal salmonellosis is a leading cause of foodborne diseases [7]. Over the decade since the disease has been made notifiable in 2008, the incidence of non-typhoidal salmonellosis has increased [8]. However, information on the prevalence and characteristics of Salmonella species, in cooked or ready-to-eat food and wild birds in Singapore, is limited. In addition, there is a limited number of reports globally describing the prevalence and characteristics of Salmonella in wild birds, especially with respect to those in food chain. Although, wild birds would not directly connect to the food chain, there may be potential links between the Salmonella strains from food and wild birds. Such information would provide an insight in better understanding the epidemiology of Salmonella in the larger environmental ecosystem in relation to human health.
This study, thus, aimed to identify and report on the prevalence, antimicrobial resistance, and sequence types of Salmonella species isolated from cooked and ready-to-eat retail food and wild birds. Such information is imperative in guiding further investigations and public health risk management strategies in Singapore and elsewhere.
2. Materials and Methods
Two independent epidemiological studies for food and wild birds were conducted during the same study period (2010–2015) as follow.
Isolation of Salmonella in cooked and ready-to-eat food: A cross-sectional study was carried out to determine the prevalence of Salmonella in cooked and ready-to-eat food. A total of 21,428 cooked or ready-to-eat food samples were analyzed in this study. The food samples were collected by the Food and Water Sampling Unit (FWSU) of the National Environment Agency (NEA) from 2010 to 2015 (study period). The samples were convenience-randomly collected from different types of retail food premises (hawker centers, restaurants, caterers, food courts), across various regions of Singapore, as part of FWSU’s routine food surveillance program. The categories and types of food sampled are shown in Table 1. For each sample, at least 100 g of cooked and ready-to-eat food were collected in either a sterile bag or in its original packaging. Upon collection, all samples were placed in cooler bags with ice and transported to a commercial laboratory accredited under the Singapore Accreditation Council Singapore Laboratory Accreditation (SAC-Singlas) Scheme. All food samples were tested for the presence (per 25 g of food) of Salmonella using methods specified in the U.S. Food and Drug Administration Bacteriological Analytical Manual (FDA-BAM) Chapter 5 [9].

Table 1.
Prevalence of Salmonella in cooked or ready-to-eat food and wild birds.
Isolation of Salmonella in faecal samples from wild birds: A cross-sectional study was carried out to determine the prevalence of Salmonella in wild birds. A total of 1510 residual faecal samples from wild birds were collected for the isolation of Salmonella. Bird (injured) carcasses were conveniently received frozen by the Environmental Health Institute of the National Environment Agency, Singapore, from 2010 to 2015 (study period) as part of the zoonotic disease surveillance program. These birds were primarily resident birds collected from urban areas and recreational parks. Approximately 1g of faecal matter, after dissection, was incubated with 9 mL of Universal Pre-enrichment Broth at 35 ± 1 °C for 18–24 h. The enriched samples were streaked onto Xylose Lysine Desoxycholate (XLD) agar (Oxoid, UK) and incubated at 35 ± 1 °C for another 18–24 h. Presumptive Salmonella colonies were confirmed biochemically using API 20E (bioMérieux, France) and serological latex agglutination tests (Oxoid, UK).
Biobank of Salmonella isolates: Salmonella isolates obtained from cooked and ready-to-eat food and wild birds were subsequently subjected to antimicrobial susceptibility testing and multi-locus sequence typing (MLST), as described below. All Salmonella isolates were stored in Brain Heart Infusion broth (Acumedia, US) with 15% glycerol at −80 °C and were freshly sub-cultured on Tryptone Soy Agar (Oxoid, UK) before antimicrobial susceptibility testing and multi-locus sequence typing (MLST).
Antimicrobial susceptibility testing of Salmonella isolates: Susceptibility tests were performed by using the disk diffusion method according to the Clinical and Laboratory Standards Institute guideline (CLSI, 2013) with 11 antimicrobial agents of eight classes; namely amikacin 30 µg (AK30), amoxycillin-clavulanic acid 20/10 µg (AMC30), ampicillin 10 µg (AMP10), ceftriaxone 30 µg (CRO30), ciprofloxacin 5 µg (CIP5), chloramphenicol 30 µg (C30), gentamicin 10 µg (CN10), nalidixic acid 30 µg (NA30), norfloxacin 10 µg (NOR10), sulphamethoxazole-trimethoprim 23.75/1.25 µg (SXT25) and tetracycline 30 µg (TE30) (Oxoid, Basingstoke, UK) [10]. The antibiotics were selected based on their public health importance. Isolates were classified as sensitive (S), intermediate (I) or resistant (R). Multidrug-resistant (MDR) strains were defined as such by phenotypic resistance to three or more antimicrobial classes.
Multi-locus sequence typing (MLST) of Salmonella isolates: Salmonella DNA was extracted from isolates by using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. PCR amplifications were then carried out using primers targeting the gene loci (aroC, dnaN, hemD, hisD, purE, sucA, thrA) described in the MLST database (http://mlst.warwick.ac.uk/mlst/dbs/Senterica/documents/primersEnterica_html) [11]. All PCRs were carried out in a final reaction volume of 50 µl. The reaction mix contained 5X reaction buffer (Thermo Scientific, Vilnius, Lithuania), 1 U of DNA polymerase (Thermo Scientific, Lithuania), 0.2 mM of dNTP Mix (1st BASE, Seri Kembangan, Malaysia), 1 μL (10 μM) of each primer (Integrated DNA Technologies, Singapore) and 5 μL of DNA template. The PCR protocol was as follows: initial denaturation at 98 °C for 30 s, followed by 35 cycles of 98 °C for 10 sec, 55 °C for 30 sec, 72 °C for 30 s, with a final extension at 72 °C for 10 min. The amplified fragments were visualised on 2% agarose gels and subsequently; purified and sequenced using BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA). Raw sequences were then assembled in Lasergene Software version 8.0 (DNASTAR, Madison, WI, USA) and the consensus sequences were compared with those available in the MLST database (http://enterobase.warwick.ac.uk/species/senterica/allele_st_search) to determine allelic numbers and sequence types. Salmonella serovars were predicted based on sequence types of strains available in the MLST database [12].
Statistical analysis: The 95% confidence intervals of proportions were calculated using http://vassarstats.net/prop1.html. Z-scores for two-population proportions were calculated using http://www.socscistatistics.com/tests/Default.aspx.
3. Results
3.1. Prevalence of Salmonella in Cooked or Ready-to-Eat Food and Wild Birds
From the 21,428 cooked or ready-to-eat food samples tested, 17 (0.08%) were positive for Salmonella species (Table 1). Nine of these 17 were poultry/egg dishes (Table 1). Of 1510 wild bird carcasses tested, Salmonella species were detected in 15 (0.99%) faecal samples. Those birds belonged to Columbiformes (n = 3), Passeriformes (8), Pelecaniformes, and (3) Strigiformes (1). The details of wild birds detected with Salmonella are shown in Table 1.
3.2. Antimicrobial Resistance in Salmonella Isolated from Food and Wild Birds
Nearly half of Salmonella isolates from food (47.1%, 8/17) samples were resistant to at least one of the antimicrobials tested in this study (Table 2). In contrast, none of the Salmonella isolates from wild birds (n = 15) showed phenotypic resistance to any of the antimicrobials. The proportion of Salmonella isolates, resistant to at least one antimicrobial, from food samples (47.1%, Z-score 3.0679, p < 0.05) was significantly higher than that of isolates from wild birds (0.0%) (Table 2). One of 17 Salmonella isolates from a food sample (5.9%) was resistant to three or more antimicrobial classes and thus was considered a MDR strain (ST3633, S. Albany) (Table 3).

Table 2.
Percentage of Salmonella isolates from food and wild birds resistant to at least one antimicrobial.

Table 3.
Percentage of antimicrobial susceptibility in Salmonella isolated from cooked or ready-to-eat food and wild birds.
All nalidixic acid-resistant isolates found were considered as having reduced susceptibility to ciprofloxacin. In addition, we found Salmonella isolates with directly-measured intermediate susceptibility to ciprofloxacin in food (52.9%, 9/17) and wild birds (60.0%, 9/15) samples. On the other hand, none of the isolates were phenotypically resistant to ciprofloxacin or norfloxacin (fluoroquinolones), ceftriaxone (third generation cephalosporin), or amikacin (aminoglycoside) (Table 3).
3.3. Distribution of Sequence Types of Salmonella Isolates in Food and Wild Birds
Nineteen different sequence types belonged to 16 predicted serovars were identified in this study. Of 19 sequence types identified, 17 sequence types were found only in either food or wild birds samples (Figure 1). In contrast, 2 sequence types were found in both food and wild birds samples (ST19, ST365) (Table 4).
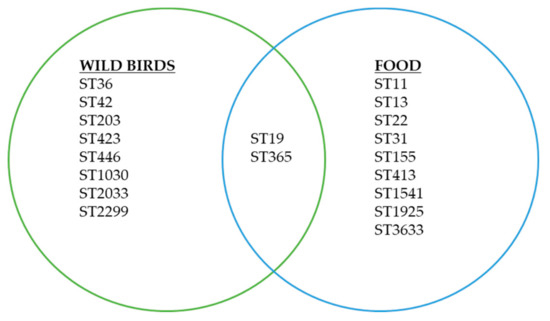
Figure 1.
Sequence types and cross sectorial distributions of Salmonella isolates in food and wild birds found in this study.

Table 4.
Characteristics of Salmonella isolates from food and wild birds (ST: Sequence type, MLST: Multi locus sequence typing, AK, Amikacin; AMP, Ampicillin; AMC, Amoxycillin-Clavulanic acid; C, Chloramphenicol; CRO, Ceftriaxone; CIP, Ciprofloxacin; CN, Gentamicin; NA, Nalidixic acid; NOR, Norfloxacin; SXT, Trimethoprim-Sulphamethoxazole; TE, Tetracycline). S, Sensitive (Green); I, Intermediate (Yellow); R, Resistant (Red).
4. Discussion
To the best of our knowledge, this is the first study to estimate the prevalence of Salmonella in cooked or ready-to-eat food sold at retail food premises in Singapore. While, a direct comparison of prevalence data between studies was technically challenging, due to the difference in sampling and laboratory methods, the prevalence of Salmonella in retail cooked or ready-to-eat food in this study (0.08%) was relatively lower than, or comparable to, that of Salmonella reported in overseas countries (Malaysia (17%), Ireland (0.06%–0.1%), Palestine (0.0%), Spain (1.2%–11.1%), Greece (17.9%), Iran (14.0%) and China (1.0%)) [13,14,15,16,17,18,19,20]. Nevertheless, as a large majority (60%) of residents in Singapore dine out at retail food premises at least four times a week, the detection of Salmonella in about 1 in 1000 food dishes may constitute a food safety concern [21]. From the Salmonella-positive food samples, the majority (9/17) were poultry- or egg-containing cooked dishes. This observation highlights that poultry and eggs, as food ingredients, may be at relatively high risk for Salmonella contamination. This suggests that improper cooking and post-cooking contamination are likely contributing factors for the contamination in cooked food.
The majority of wild bird species positive for Salmonella in this study were birds well adapted to the urban environments. Wild birds can be natural reservoirs of Salmonella [22,23]. Humans can acquire the Salmonella infection directly via contact with bird droppings or indirectly via ingestion of contaminated food and food-producing animals [24,25]. Several human salmonellosis outbreaks associated with wild birds have been reported [26,27]. The present study, therefore, reiterates a possible risk of humans acquiring zoonotic salmonellosis through contact with wild birds and their droppings, especially where wild birds are in close proximity to humans.
In addition to their propensity to cause foodborne illnesses, the presence of antimicrobial-resistant Salmonella has been increasingly reported around the world [5,28,29]. In this study, nearly half of Salmonella isolates from food were resistant to, at least, one of eleven antimicrobials tested. The misuse of antimicrobial agents in food is a key contributing factor for the emergence of resistant pathogens [29,30,31]. Resistant pathogens can further spread to susceptible bacterial populations in the environment through horizontal gene transfer [31]. In contrast, all 15 Salmonella isolates from wild birds did not show phenotypic resistance to any of the antibiotics tested. This finding was significant in comparison to the number of resistant isolates from food in this study. The absence of phenotypic antimicrobial resistance in Salmonella from wild birds suggests that the birds isolates have not been subjected to antimicrobial selection pressure as much as food isolates [32].
In this study, we detected Salmonella isolates from food samples that were resistant to ampicillin, amoxicillin/clavulanic acid, and tetracycline (Table 3). They were susceptible to ceftriaxone, which can be used for the treatment of invasive infections or infections from bacteria resistant to other antimicrobials. It was evident that Salmonella isolates from food were resistant to some drugs of choice for the empirical treatment of Salmonella or other infections in humans (Table 4). The proportion of food Salmonella isolates resistant to these drugs ranged from 5.9% to 35.3%, depending on the type of antimicrobials. The proportion of isolates resistant to nalidixic acid (quinolone, an indicator for reduced susceptibility to fluoroquinolones) was 35.3% (6/17) in food samples. Although, no isolate was resistant to fluroquinolones (ciprofloxacin and norfloxacin), in this study, the finding of Salmonella isolates resistant to quinolone (nalidixic acid), as well as isolates with intermediate susceptibility to ciprofloxacin in local food and wild birds samples may be a tell-tale sign for the emergence of fluroquinolones resistance in the local environment (Table 3).
We detected one MDR Salmonella isolate (ST3633 S. Albany) in a food sample (steamed chicken). MDR Salmonella infection is a public health concern, as it can be associated with high morbidity and mortality, which in turn contributes to high healthcare costs and economic burden [33,34]. Although, the prevalence of MDR Salmonella strains in this study (5.88%, 1/17 food) was relatively lower than in some overseas studies (32.7%–90.9%), close monitoring is needed for further assessment of the situation. [35,36,37].
Through the application of MLST, we observed that the majority of the sequence types (n = 17/19) found in food and wild birds did not overlap. In Singapore, more than 90% of food is imported, and therefore, the sequence types of Salmonella found in food, in this study, may represent the introduction of Salmonella strains from elsewhere other than from the local environment. Whereas, wild birds most likely acquire Salmonella from the local environment through contact with other wild birds’ droppings, or as a result of feeding in contaminated water, or eating Salmonella-carrying preys [22].
We detected ST11 (S. Entertidis) and ST1925 (S. Entertidis) in food samples (ST11 in steamed chicken, sugarcane juice, noodle dark sweet soy-sauce; and ST1925 in chocolate cake, mushroom salad, nasi padang). Both sequence types are known to be geographically widespread and had previously been reported in various sectors, including food, humans, or animals [11]. ST1925 was isolated in an avian sample from a slaughter house in Malaysia in 2012 and from human cases associated with foodborne outbreaks in Singapore (2013–2017) [11,38]. Most of these strains were isolated from ready-to-eat food dishes, which are largely assorted in nature, and thus the ability to track the origins of contamination was limited. Nevertheless, the observations of the Salmonella isolates with clinically relevant sequence types (ST1925) in retail food samples suggest that such foods could have been the source of human salmonellosis.
We detected ST42 and ST423 (S. Paratyphi B var Java monophasic) isolates in wild birds (black bittern and crow) samples respectively. S. Paratyphi B causes paratyphoid fever [39]. Animals, besides human, can be reservoirs of S. Paratyphi B [39]. In addition, S. Paratyphi B, in particular ST42, has been found in food, feed, fertilizer, and reptiles [40,41]. While, not all variants of S. Paratyphi B are capable of causing enteric fever, S. Paratyphi B primarily causes gastroenteritis [42], similar to the non-typhoidal strains. The detection of S. Paratyphi B in the bird population suggests that wild birds may play a role in the epidemiology of paratyphoidal salmonellosis in Singapore and elsewhere.
Two sequence types, namely ST19 (S. Typhimurium) and ST365 (S. Weltevreden), were found in both sample types (food and wild birds) suggesting their ability to adapt to, and sustain in, different hosts or types of samples. Besides, the isolates belonged to ST19 and ST365 demonstrated similar antimicrobial susceptibility profiles suggesting the possibility of strain relatedness, common origin, and transmission between food and wild birds. ST19 (S. Typhimurium) has previously been found in wild birds and the variant definitive type (DT) 160 was reportedly associated with a prolonged transmission over a 14-year period across different hosts in New Zealand [43,44,45,46,47]. Other variants of S. Typhimurium ST19 (DT40 and DT56) isolated from wild birds were genetically similar to isolates from livestock and human cases [26,48]. S. Weltevreden is one of the most common serovars reported to be associated with human salmonellosis in tropical countries [7,49,50]. It is largely a monophyletic serovar belonging to the singleton node of sequence type (ST) 365, based on MLST data, and is rarely carrying antimicrobial resistance traits [6,51,52]. S. Weltevreden was previously isolated from seafood, shrimps and duck, suggesting an aquatic environment as its potential source of origin [53,54,55,56].
This study analyzed all samples received by the NEA between 2010 and 2015 through the national surveillance program. Although, the sample size of isolates was relatively small. Most Salmonella strains in this study were isolated from assorted retail food, and thus, the ability to track the sources of contamination from original ingredients was limited. In addition, the use of MLST provides limited discriminatory power; isolates with identical ST may be distantly related. This warrants future studies by whole-genome analysis of a larger number of isolates from various sources, including human clinical samples.
5. Conclusions
This study provides useful information on the characteristics, such as sequence types and antimicrobial resistance profiles, of Salmonella in food and wild birds in Singapore. Findings from this study identify specific sequence types of Salmonella from food and wild birds that are possibly interrelated, in shaping the epidemiology of salmonellosis, as a basis for further investigations and risk management.
Author Contributions
Conceptualization, K.T.A., H.M.Y., T.B., R.A.G., and L.C.N.; data curation, K.T.A., H.J.C., X.F.L., M.H., C.C., G.Y., J.Q.O., and V.M.; formal analysis, K.T.A.; funding acquisition, L.C.N.; investigation, K.T.A., H.J.C., M.L.C., G.Y., X.F.L., M.H., C.C., G.Y., H.M.Y., J.Q.O., and V.M.; methodology, H.C.H., M.M., N.W.S.T., T.B., T.H.K., and J.S.; project administration, K.T.A., G.Y., H.M.Y., R.A.G., and L.C.N.; resources, M.M., N.W.S.T., and T.B.; supervision, H.C.H., R.A.G., J.S., and L.C.N.; writing—original draft, K.T.A.; Writing—review and editing, K.T.A., M.L.C., M.H., H.C.H., M.M., T.B., T.H.K., R.A.G., J.S., and L.C.N. All authors approved the manuscript.
Funding
This study was funded by the National Environment Agency, Singapore.
Acknowledgments
The authors thank the Food and Water Sampling and Enforcement Unit, of the North West Regional Office, National Environment Agency, for the collection of retail food samples and for sharing of historical data for analysis. In addition, the authors thank collaborators from the National Parks Board Singapore, and the Wildlife Reserves Singapore who provided the wild birds samples (carcasses).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N.; et al. World Health Organization global esimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Boore, A.L.; Hoekstra, M.R.; Iwamoto, M.; Fields, P.I.; Bishop, R.D.; Swerdlow, D.L. Salmonella enterica infections in the United States and assessment of coefficients of variation: A novel approach to identify epidemiologic characteristics of individual serotypes, 1996–2011. PLoS ONE 2015, 10, e0145416. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, E. Antimicrobial drug resistance in Salmonella: Problems and perspectives in food- and water-borne infections. FEMS Microbiol. Rev. 2002, 26, 141–148. [Google Scholar] [CrossRef]
- Su, L.-H.; Chiu, C.-H.; Chu, C.; Ou, J.T. Antimicrobial Resistance in Nontyphoid Salmonella Serotypes: A Global Challenge. Clini. Infect. Dis. 2004, 39, 546–551. [Google Scholar] [CrossRef]
- Patchanee, P.; Boonkhot, P.; Kittiwan, N.; Tadee, P.; Chotinun, S. Dissemination of Salmonella enterica sequence types among ASEAN economic community countries. Southeast. Asian J. Trop. Med. Public Health 2015, 46, 707–719. [Google Scholar]
- Ministry of Health. Weekly Infectious Diseases Bulletin. Available online: https://www.moh.gov.sg/resources-statistics/infectious-disease-statistics/2018/weekly-infectious-diseases-bulletin (accessed on 2 September 2018).
- Lin, Y.N.; Fong, R.; Leo, J.; Kwan, W.W.; Ye, A.; Chan, P.P.; Chong, N.; Lim, G.; Octavia, S.; Lin, M.; et al. Distribution of Salmonella spp. along the food chain in Singapore, 2008–2016. ENB Q. 2019, 45, 44–54. [Google Scholar]
- Andrew, W.H.; Wang, H.; Jacobson, A.; Hammack, T. Bacteriological Analytical Manual (BAM) Chapter 5: Salmonella. Available online: https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam-chapter-5-salmonella (accessed on 22 July 2019).
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 24th Informational Supplement (M100-S24); CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- Alikhan, N.; Zhou, Z.; Sergeant, M.J.; Achtman, M. A genomic overview of the population structure of Salmonella. PLoS Genet. 2018, 14, e1007261. [Google Scholar] [CrossRef]
- Achman, M.; Wain, J.; Weill, F.; Nair, S.; Zhou, Z.; Sangal, V.; Krauland, M.G.; Hale, J.L.; Harbottle, H.; Uebeck, A.; et al. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog. 2012, 8, e1002776. [Google Scholar]
- Arumugaswamy, R.; Rusul, G.; Abdul Hamid, S.; Cheah, C. Prevalence of Salmonella in raw and cooked foods in Malaysia. Food Microbiol. 1995, 12, 3–8. [Google Scholar] [CrossRef]
- Food Safety Authority of Ireland. Survey of the Microbiological Safety of Ready-to-Eat, Pre-Cut and Pre-Packaged Fresh Herbs and Salad Leaves from Retail Estabalishments in Ireland (13NS7). Available online: https://www.fsai.ie/publications_survey_salad_leaves/ (accessed on 6 July 2018).
- Issa, Y.; Abu-Rayyan, A.; Hemidat, S. Prevalence of Salmonella in different poultry and meat food products in Hebron district: A prevalence study. Lancet 2017, 390, S33. [Google Scholar] [CrossRef]
- Duggan, S.; Jordan, E.; Gutierrez, M.; Barrett, G.; O’Brien, T.; Hand, D.; Kenny, K.; Fanning, J.; Leonard, N.; Egan, J. Salmonella in meats, water, fruit and vegetables as disclosed from testing undertaken by food business operators in Ireland from 2005 to 2009. Ir. Vet. J. 2012, 65, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Cabedo, L.; Picart, i.B.L.; Teixido, I.C.A. Prevalence of Listeria monocytogenes and Salmonella in ready-to-eat food in Catalonia, Spain. J. Food Protect. 2008, 71, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Kotzekidou, P. Microbiological examination of ready-to-eat foods and ready-to-bake frozen pastries from university canteens. Food Microbiol. 2013, 34, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Ansari, C.B. Bacteriological examination of ready-to-eat foods (RTE) products of Tehran province, Iran. Adv. Food Sci. Technol. 2015, 3, 328–331. [Google Scholar]
- Zhang, H.; Hou, P.; Chen, Y.; Ma, Y.; Li, X.; Lv, H.; Wang, M.; Tan, H.; Bi, Z. Prevalence of foodborne pathogens in cooked meat and seafood from 2010 to 2013 in Shandong Province, China. Iran. J. Public Health 2016, 45, 1577–1585. [Google Scholar]
- The Health Promotion Board (HPB). The National Nutrition Survey. 2010. Available online: https://www.hpb.gov.sg/article/health-promotion-board’s-food-strategy-aims-to-change-the-way-singaporeans-eat-at-home-and-eat-out (accessed on 6 July 2018).
- Tizard, I. Salmonellosis in wild birds. Semin. Avian Exot. Pet. Med. 2004, 13, 50–66. [Google Scholar] [CrossRef]
- Chomel, B.B.; Belotto, A.; Meslin, F. Wildlife, exotic pets, and emerging zoonoses. Emerg. Infect. Dis. 2007, 13, 6–11. [Google Scholar] [CrossRef]
- Andres-Barranco, S.; Vico, J.P.; Garrido, V.; Samper, S.; Herrera-Leon, S.; De Frutos, C.; Mainar-Jaime, R.C. Role of wild bird and rodents in the epidemiology of subclinical salmonellosis in finishing pigs. Foodborne Pathog. Dis. 2014, 11, 689–697. [Google Scholar] [CrossRef]
- Vico, J.; Mainar-Jaime, R. Salmonellosis in wild birds and its relationship with the infection in finishing pigs. In Proceedings of the 9th International Conference on the Epidemiology and Control of biological, chemical and physical hazards in pigs and pork: SAFEPORK 2011, International Conference, Maastricht, The Neherlands, 19–22 June 2011; pp. 264–267. [Google Scholar]
- Alley, M.R.; Connolly, J.; Fenwick, S.; Mackereth, G.; Leyland, M.; Rogers, L.E.; Haycock, M.; Nicol, C.; Reed, C. An epidemic of salmonellosis caused by Salmonella Typhimurium DT160 in wild birds and humans in New Zealand. N. Z. Vet. J. 2002, 50, 170–176. [Google Scholar] [CrossRef]
- Hernandez, S.M.; Keel, K.; Sanchez, S.; Trees, E.; Gerner-Smidt, P.; Adams, J.K.; Cheng, Y.; Ray, A.; Martin, G.; Presotto, A.; et al. Epidemiology of a Salmonella enterica subsp. enterica serovar Typhimurium strain associated with a songbird outbreak. Appl. Environ. Microbiol. 2012, 78, 7290–7298. [Google Scholar] [CrossRef] [PubMed]
- Varma, J.K.; Molbak, K.; Barrett, T.J.; Beebe, J.L.; Jones, T.F.; Rabatsky-Ehr, T.; Smith, K.E.; Vugia, D.J.; Chang, H.-G.H.; Angulo, F.J. Antimicrobial-resistant nontyphoidal Salmonella is associated with excess bloodstream infections and hospitalizations. J. Infect. Dis. 2005, 191, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance in the United States. Available online: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 7 July 2018).
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Shopland, S.; Wigley, P.; Bradon, H.; Leatherbarrow, H.A.; Willams, N.J.; Bennett, M.; De Pinna, E.; Lawson, B.; Cunningham, A.A.; et al. Characterisation of Salmonella enterica serotype Typhimurium isolates from wild birds in northern England from 2005–2006. BMC Vet. Res. 2008, 4, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Barza, M.; Travers, K. Excess infections due to antimicrobial resistance: The “attributable fraction”. Clin. Infect. Dis. 2002, 34, S126–S130. [Google Scholar] [CrossRef]
- Martin, L.J.; Fyfe, M.; Dore, K.; Buxton, J.A.; Pollari, F.; Henry, B.; Middleton, D.; Ahmed, R.; Jamieson, F.; Ciebin, B.; et al. Increased burden of illness associated with antimicrobial-resistant Salmonella enterica serotype Typhimurium infections. J. Infect. Dis. 2004, 189, 377–384. [Google Scholar] [CrossRef][Green Version]
- Jamali, H.; Radmehr, B.; Ismail, S. Prevalence and antimicrobial resistance of Listeria, Salmonella, and Yersinia species isolates in ducks and geese. Poult. Sci. 2014, 93, 1023–1030. [Google Scholar] [CrossRef]
- Medeiros, M.A.; De Oliveira, D.C.; Rodrigues, D.; De Freitas, D.R. Prevalence and antimicrobial resistance of Salmonella in chicken carcasses at retail in 15 Brazilian cities. Rev. Panam. Salud Publica 2011, 30, 555–560. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, H.; Sun, J.; Liu, Y.; Zhou, X.; Beier, R.; Wu, G.; Hou, X. Characterization of multidrug-resistant Salmonella enterica serovars Indiana and Enteritidis from chickens in Eastern China. PLoS ONE 2014, 9, e96050. [Google Scholar] [CrossRef]
- Octavia, S.; Ang, M.L.; La, M.-V.; Zulaina, S.; Saat, Z.; Saat, Z.A.; Tien, W.S.; Han, H.K.; Ooi, P.L.; Cui, L.; et al. Retrospective genome-wide comparisons of Salmonella enterica serovar Enteritidis from suspected outbreaks in Singapore. Infect. Genet. Evol. 2018, 61, 229–233. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Facts about Typhoid and Paratyphoid Fever. Available online: https://ecdc.europa.eu/en/typhoid-and-paratyphoid-fever/facts (accessed on 2 September 2018).
- Toboldt, A.; Tietze, E.; Helmuth, R.; Junker, E.; Fruth, A.; Malorny, B. Population structure of Salmonella enterica serovar 4,[5],12:b:- strains and likely sources of human infection. Appl. Environ. Microbiol. 2013, 79, 5121–5129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xie, J.; Wu, F.; Xu, X.; Yang, X.; Zhao, R.; Ma, Q.; Li, P.; Wang, L.; Hao, R.; Jia, L.; et al. Antibiotic resistance and molecular characterization of the hydrogen sulfide-negative phenotype among diverse Salmonella serovars in China. BMC Infect. Dis. 2018, 18, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Chart, H. The pathogenicity of strains of Salmonella paratyphi B and Salmonella java. J. Appl. Microbiol. 2003, 94, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lawson, B.; De Pinna, E.; Horton, R.A.; Macgregor, S.K.; John, S.K.; Chantrey, J.; Duff, P.J.; Kirkwood, J.K.; Simpson, V.R.; Robinson, R.A.; et al. Epidemiological evidence that garden birds are a source of human salmonellosis in England and Wales. PLoS ONE 2014, 9, e88968. [Google Scholar] [CrossRef]
- Penfold, J.B.; Amery, H.C.; Peet, P.J. Gastroenteritis Associated with Wild Birds in A Hospital Kitchen. Br. Med. J. 1979, 2, 802. [Google Scholar]
- Tizard, I.; Fish, N.; Harmeson, J. Free flying sparrows as carriers of salmonellosis. Can. Vet. J. 1979, 20, 143–144. [Google Scholar]
- Mather, A.E.; Lawson, B.; De Pinna, E.; Wigley, P.; Parkhill, J.; Thomson, N.R.; Page, A.J.; Holmes, M.A.; Paterson, G.K. Genomic analysis of Salmonella enterica serovar Typhimurium from wild passerines in England and Wales. Appl. Environ. Microbiol. 2016, 82, 6728–6735. [Google Scholar] [CrossRef]
- Bloomfield, S.J.; Benschop, J.; Biggs, P.J.; Marshall, J.C.; Hayman, D.T.; Carter, P.E.; Midwinter, A.C.; Mather, A.E.; French, N.P. Genomic analysis of Salmonella enterica serovar Typhimurium DT160 associated with a 14-Year Outbreak, New Zealand, 1998–2012. Emerg. Infect. Dis. 2017, 23, 906–913. [Google Scholar] [CrossRef]
- Horton, R.; Wu, G.; Speed, K.; Kidd, S.; Davies, R.; Coldham, N.; Duff, J. Wild birds carry similar Salmonella enterica serovar Typhimurium strains to those found in domestic animals and livestock. Res. Vet. Sci. 2013, 95, 45–48. [Google Scholar] [CrossRef]
- Basu, S.; Sood, L. Salmonella weltevreden: A sero-type of increasing public health importance in India. Trop. Geogr. Med. 1975, 27, 387–394. [Google Scholar] [PubMed]
- Thong, K.; Goh, Y.; Radu, S.; Noorzaleha, S.; Yasin, R.; Koh, Y.; Lim, V.K.; Rusul, G.; Puthucheary, S. Genetic diversity of clinical and environmental strains of Salmonella enterica serotype Weltevreden isolated in Malaysia. J. Clin. Microbiol. 2002, 40, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Nandy, S.; Bharadwaj, R.; Niyogi, S.K.; Dutta, S. Salmonella enterica serovar Weltevreden ST1500 associated foodborne outbreak in Pune, India. Indian J. Med. Res. 2015, 141, 239–241. [Google Scholar] [PubMed]
- Makendi, C.; Page, A.J.; Wren, B.W.; Phuong, T.L.; Clare, S.; Hale, C.; Goulding, D.; Klemm, E.J.; Pickard, D.; Okoro, C.; et al. A phylogenetic and phenotypic analysis of Salmonella enterica serovar Weltevreden, an regions. PLoS Negl. Trop. Dis. 2016, 10, e0004446. [Google Scholar] [CrossRef] [PubMed]
- Bangtrakulnonth, A.; Pornreongwong, S.; Pulsrikarn, C.; Sawanpanyalert, P.; Hendriksen, R.S.; Wong, D.M.; Aarestrup, F.M. Salmonella serovars from humans and other sources in Thailand, 1993–2002. Emerg. Infect. Dis. 2004, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Heinitz, M.L.; Ruble, R.D.; Wagner, D.E.; Tatini, S.R. Incidence of Salmonella in fish and seafood. J. Food Protect. 2000, 63, 579–592. [Google Scholar] [CrossRef]
- Kumar, R.; Surendran, P.; Thampuran, N. Distribution and genotypic characterization of Salmonella serovars isolated from tropical seafood of Cochin, India. J. Appl. Microbiol. 2009, 106, 515–524. [Google Scholar] [CrossRef]
- Noor, U.G.; Larsen, M.H.; Barco, L.; Minh, P.T.; Dalsgaard, A. Clonal occurrence of Salmonella Weltevreden in cultured shrimp in the Mekong Delta, Vietnam. PLoS ONE 2015, 10, e0134252. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).