Potentially Harmful Element Concentrations in the Vegetables Cultivated on Arable Soils, with Human Health-Risk Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetable Sampling and Preparation
2.2. Sample Analyses
2.3. Quality Control
2.4. Statistical Analyses
2.5. Soil-To-Plant Transfer Indices
2.6. Human Health Risk Assessment
3. Results and Discussion
3.1. Abundance of PHEs in Vegetables
3.2. Statistical Analyses
3.3. Soil-To-Plant Transfer Indices
3.4. Human Health Risk Assessment
3.4.1. Daily Intake Rates
3.4.2. Non-Carcinogenic Risk of PHEs
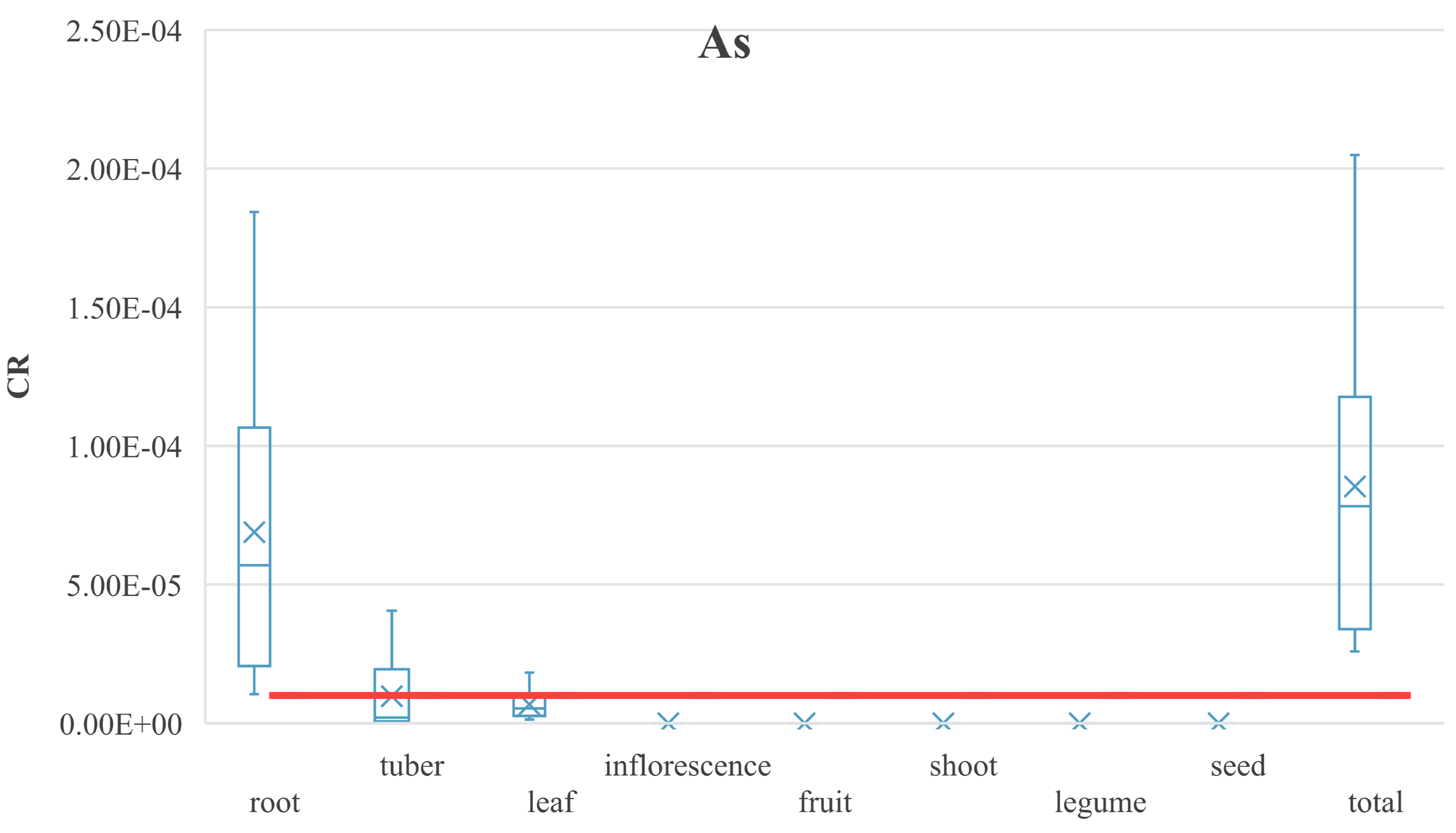
3.4.3. Carcinogenic Risk of PHEs
3.4.4. Margin of Exposure to Pb
3.4.5. Uncertainties in HHRA
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Guenther, P.M.; Dodd, K.W.; Reedy, J.; Krebs-Smith, S.M. Most Americans eat much less than recommended amounts of fruits and vegetables. J. Am. Diet. Assoc. 2006, 106, 1371–1379. [Google Scholar] [CrossRef]
- Fuller, R.; Rahona, E.; Fisher, S.; Caravanos, J.; Webb, D.; Kass, D.; Matte, T.; Landrigan, P.J. Pollution and non-communicable disease: Time to end the neglect. Lancet Planet Health 2018, 2, e96–e98. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Khan, S.; Shah, M.T.; Brusseau, M.L.; Khan, S.A.; Mainhagu, J. Transfer of heavy metals from soils to vegetables and associated human health risks at selected sites in Pakistan. Pedosphere 2018, 28, 666–679. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Boersch, J.; Frohne, T.; Du Laing, G.; Rinklebe, J. Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J. Environ. Manag. 2017, 186, 192–200. [Google Scholar] [CrossRef]
- Gabari, V.; Fernández-Caliani, J.C. Assessment of trace element pollution and human health risk associated with cultivation of mine soil: A case study in the Iberian Pyrite Belt. Hum. Ecol. Risk Assess. 2017. [Google Scholar] [CrossRef]
- Liu, C.; Chang, C.; Fei, Y.; Li, Y.; Wang, Q.; Zhai, G.; Lei, J. Cadmium accumulation in edible flowering cabbages in the Peral River Delta, China: Critical soil factors and enrichment models. Environ. Pollut. 2018, 233, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Matsumoto, M.; Ozaki, A.; van Tinh, N.; Kurosawa, K. Heavy Metal Contamination of irrigation water, soil, and vegetables and the difference between dry and wet seasons near a multi-industry zone in Bangladesh. Water 2019, 11, 583. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, J.; Du, B.; Liu, H.; Zhang, W.; Liang, J.; Zhang, W.; You, L.; Zhou, J. Health risks to local residents from the exposure of heavy metals around the largest copper smelter in China. Ecotoxicol. Environ. Safe 2019, 171, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Baghaie, A.R.; Fereydoni, M. The potential risk of heavy metals on human health due to the daily consumption of vegetables. Environ. Health Eng. Manag. 2019, 6, 11–16. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Merendino, N. Health risk assessment of potentially toxic elements in vegetables grown under the impact of Kajaran Mining Complex. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Bini, C.; Bech, J. PHEs, Environment and Human Health. Potentially Harmful Elements in the Environment and the Impact on Human Health; Springer: Dordrecht, Germany, 2014. [Google Scholar]
- Mallik, A.; Dutta, J.; Sultana, P.; Mitra, A. Bioaccumulation pattern of heavy metals in vegetables collected from selected areas in and around Kolkata city (India). Int. J. High. Educ. Res. 2017, 7, 121–134. [Google Scholar]
- Rahman, M.; Islam, M.A. Concentrations and health risk assessment of trace elements in cereals, fruits, and vegetables of Bangladesh. Biol. Trace Elem. Res. 2019, 191, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Use of Grounds and Surface Area in the Year 2015; Central Statistical Office: Warszawa, Poland, 2016. Available online: https://stat.gov.pl/download/gfx/portalinformacyjny/pl/defaultaktualnosci/5507/8/10/1/uzytkowanie_gruntow_i_powierzchnia_zasiewow_w_2015_r_zm.pdf (accessed on 19 May 2019).
- Szczepańska, J.; Twardowska, I. Distribution and environmental impact of coal-mining wastes in Upper Silesia, Poland. Environ. Geol. 1999, 38, 249–258. [Google Scholar] [CrossRef]
- Jończy, I.; Gawor, Ł. Coal mining and post-metallurgic dumping grounds and their connections with exploitation of raw materials in the region of Ruda Śląska. Arch. Min. Sci. 2017, 62, 301–311. [Google Scholar]
- Krzaklewski, W.; Barszcz, J.; Małek, S.; Kozioł, K.; Pietrzykowski, M. Contamination of forest soils in the vicinity of the sedimentation pond after zinc and lead ore flotation (in the region of Olkusz, southern Poland). Water Air Soil Pollut. 2004, 159, 151–164. [Google Scholar] [CrossRef]
- Postawa, A.; Motyka, J. Selected trace elements and metals in groundwater within Permian sediments near Olkusz (Zn-Pb ore mining region, S Poland). Environ. Sci. Pollut. Res. 2019, 26, 34–43. [Google Scholar] [CrossRef]
- Potysz, A.; Kierczak, J.; Pietranik, A.; Kądziołka, K. Mineralogical, geochemical, and leaching study of historical Cu-slags issued from processing of the Zechstein formation (Old Copper Basin, south-western Poland). Appl. Geochem. 2018, 98, 22–35. [Google Scholar] [CrossRef]
- Dudka, S.; Piotrowska, M.; Chlopecka, A.; Witek, T. Trace metal contamination of soils and crop plants by the mining and smelting industry in Upper Silesia, South Poland. J. Geochem. Explor. 1995, 52, 237–250. [Google Scholar] [CrossRef]
- Gzyl, J. Lead and cadmium contamination of soil and vegetables in the Upper Silesia region in Poland. Sci. Total Environ. 1990, 96, 199–209. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A.; Baran, A.; Wdowin, M.; Mazur-Kajta, K.; Czech, T. The contents of the potentially harmful elements in the arable soils of southern Poland, with the assessment of ecological and health risks: A case study. Environ. Geochem. Health 2019. [Google Scholar] [CrossRef]
- Gruszecka-Kosowska, A.; Baran, A.; Wdowin, M.; Mazur-Kajta, K.; Czech, T. Geochemical fractions of the agricultural soils of southern Poland and the assessment of the Potentially Harmful Element mobility. Submitted. 2019. [Google Scholar]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Marcante, N.C.; Canniti-Brazaca, S.G. Hevy metals in vegetables and potential risk for human health. Sci. Agric. 2010, 69, 54–60. [Google Scholar] [CrossRef]
- Method 6020B: Inductively Coupled Plasma—Mass Spectrometry. Revision 2; USEPA: Washington, DC, USA, 1998. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/6020b.pdf (accessed on 15 January 2019).
- PN-EN ISO 17294-2. In Water Quality—Use of Inductively Induced Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of 62 Elements; Polish Committee for Standardization: Warsaw, Poland, 2006.
- Dziubanek, G.; Piekut, A.; Rusin, M.; Baranowska, R.; Hajok, I. Contamination of food crops grown on soils with elevated heavy metals content. Ecotoxicol. Environ. Safe 2015, 118, 183–189. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.; Shayler, H.A.; Spliethoff, H.M.; Mitchell, R.G.; Marquez-Bravo, L.G.; Ferenz, G.S.; Russell-Anelli, J.M.; Casey, L.; Bachman, S. Concentrations of lead, cadmium and barium in urban garden-grown vegetables: The impact of soil variables. Environ. Pollut. 2014, 194, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Exposure Factors Handbook: 2011 Edition; EPA/600/R-09/052F, US Environmental Protection Agency; National Center for Environmental Assessment: Washington, DC, USA, 2011. Available online: http://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=522996 (accessed on 3 March 2019).
- PN-EN ISO 17294-1. In Water Quality—Induced Plasma Mass Spectrometry (ICP-MS)—Part 1: General Guidelines; Polish Committee for Standardization: Warsaw, Poland, 2007.
- PN-EN ISO 11885. In Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); Polish Committee for Standardization: Warsaw, Poland, 2009.
- WHO. GEMS/Food-EURO Second Workshop on Reliable Evaluation of Low-Level Contamination of Food: Report on a Workshop in the Frame of GEMS Food-EURO, Kulmbach, Germany, 26–27 May 1995; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.H. Heavy metals in food and crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Inacio, M.; Neves, O.; Pereira, V.; da Silva, E.F. Levels of selected potential harmful elements (PHEs) in soils and vegetables used in diet of the population living in the surroundings of the Estarreja Chemical Complex (Portugal). Appl. Geochem. 2014, 44, 38–44. [Google Scholar] [CrossRef]
- Wang, G.; Su, M.Y.; Chen, Y.H.; Lin, F.F.; Luo, D.; Gao, S.F. Transfer characteristic of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ. Pollut. 2006, 144, 127–135. [Google Scholar] [CrossRef]
- Risk Assessment Guidance for Superfund, Vol. 1: Human Health Evaluation Manual, Part A; Interim final; Office of Emergency and Remedial Response. US Environmental Protection Agency: Washington, DC, USA, 1989. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf (accessed on 11 April 2019).
- WHO. Dietary Exposure Assessment of Chemicals in Food. Report of Joint FAO/WHO Consultation; WHO Library: Annapolis, MD, USA, 2005. [Google Scholar]
- Seremak-Bulge, J. Fruits and Vegetables Market State and Perspectives. No 48. Institute of Agricultural and Food Economics—National Research Institute; IERiGŻ-PIB: Warsaw, Poland, 2016. [Google Scholar]
- Seremak-Bulge, J. Potato Market State and Perspectives. No 43. Institute of Agricultural and Food Economics—National Research Institute; IERiGŻ-PIB: Warsaw, Poland, 2016. [Google Scholar]
- Risk Assessment Guidance for Superfund, Vol. 3: Part A, Process for Conducting Probabilistic Risk Assessment; Office of Emergency and Remedial Response, US Environmental Protection Agency: Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags3adt_complete.pdf (accessed on 11 April 2019).
- Regional Screening Level (RSL) Summary Table (TR=1E-06, HQ=1), April 2019. US Environmental Protection Agency: Washington DC, USA, 2019. Available online: https://semspub.epa.gov/src/document/HQ/199432 (accessed on 21 May 2019).
- Panel, E.C. Scientific opinion on lead in food—EFSA panel on contaminants in the food chain. Eur. Food Saf. Auth. J. 2010, 8, 151. [Google Scholar]
- European Food Safety Authority. Opinion of the scientific committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. Eur. Food Saf. Auth. J. 2005, 10, 282. [Google Scholar]
- Wang, M.; Liang, B.; Zhang, W.; Chen, K.; Zhang, Y.; Zhou, H.; Cheng, Y.; Liu, H.; Zhong, X.; Li, Y.; et al. Dietary lead exposure and associated health risks in Guangzhou, China. Int. J. Environ. Res. Public Health 2019, 16, 1417. [Google Scholar] [CrossRef]
- Dziubanek, G.; Baranowska, R.; Ćwieląg-Drabek, M.; Spychała, A.; Piekut, A.; Rusin, M.; Hajok, I. Cadmium in edible plants from Silesia, Poland, and its implications for health risk in populations. Ecotoxicol. Environ. Safe 2017, 142, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Figurska-Ciura, D.; Łoźna, K.; Styczyńska, M. Cadmium, lead, zinc and copper contents in selected vegetables and fruit from garden allotments of the south-western Poland. Pol. J. Food Nutr. Sci. 2007, 57, 137–143. [Google Scholar]
- Singh, S.; Zacharias, M.; Kalpana, S.; Mishra, S. Heavy metals accumulation and distribution pattern in different vegetable crops. J. Environ. Chem. Ecotoxicol. 2012, 4, 75–81. [Google Scholar] [CrossRef]
- Liu, X.; Song, Q.; Tang, Y.; Wanlu, L.; Xu, J.; Wu, J.; Wang, F.; Brookes, P.C. Human health risk assessment of heavy metals in soil-vegetable system: A multi-medium analysis. Sci. Total Environ. 2013, 463, 530–540. [Google Scholar] [CrossRef]
- Chang, C.Y.; Yu, H.Y.; Chen, J.J.; Li, F.B.; Zhang, H.H.; Liu, C.P. Accumulation of heavy metals in leaf vegetables from agricultural soils and associated potential health risks in the Pearl River Delta, South China. Environ. Monit. Assess. 2014, 186, 1547–1560. [Google Scholar] [CrossRef]
- Chefri, A.; Abdoun, S.; Gaci, O. Food survey: Levels and potential health risk of chromium, lead, zinc and copper content in fruits and vegetables consumed in Algeria. Food Chem. Toxicol. 2014, 70, 48–53. [Google Scholar]
- Li, N.; Kang, Y.; Pan, W.; Zeng, L.; Zhang, Q.; Luo, J. Concentration and transportation of heavy metals in vegetables and risk assessment of human exposure to bioaccessible heavy metals in soils near a waste-incinerator site, South China. Sci. Total Environ. 2015, 521, 144–151. [Google Scholar] [CrossRef]
- Baldantoni, D.; Morra, L.; Zaccardelli, M.; Alfani, A. Cadmium accumulation in leaves of leafy vegetables. Ecotoxicol. Environ. Safe 2016, 123, 89–94. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.T.; Zhou, X.; Liu, L.; Gu, J.F.; Wang, W.L.; Zou, J.L.; Tian, T.; Peng, P.Q.; Liao, B.H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plant nutrients, and associated health risk: A review. Environ. Sci. Pollut. Res. 2015, 22, 13772–13799. [Google Scholar] [CrossRef]
- Chaves, L.H.G.; Estrela, M.A.; Sena de Souza, R. Effect on plant growth and heavy metal accumulation by sunflower. J. Phytol. 2011, 3, 4–9. [Google Scholar]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Translocation, bioaccumulation, toxicity and amelioration—A review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Rehman, S.; Khan, A.Z.; Khan, M.A.; Shah, M.T. Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotox Environ. Safe 2010, 73, 1820–1827. [Google Scholar] [CrossRef]
- Alam, M.; Khan, M.; Khan, A.; Zeb, S.; Khan, M.A.; ul Amin, N.; Sajid, M.; Khattak, A.M. Concentrations, dietary exposure, and human health risk assessment of heavy metals in market vegetables of Peshawar, Pakistan. Environ. Monit. Assess. 2018, 190, 505. [Google Scholar] [CrossRef] [PubMed]
- Ávila, P.F.; da Silva, E.F.; Candeias, C. Health risk assessment through consumption of vegetables rich in heavy metals: The case study of the surrounding villages from Panasqueira mine, Central Portugal. Environ. Geochem. Health 2017, 39, 565–589. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Parra, F.; Izquierdo-Díaz, M.; Dominguez-Castillo, A.; Medina, R.; De Miguel, E. Human-health probabilistic risk assessment: The role of exposure factors in an urban garden scenario. Landsc. Urban Plan. 2019, 185, 191–199. [Google Scholar] [CrossRef]
- Eliku, T.; Leta, S. Heavy metals bioconcentration from soil to vegetables and appraisal of health risk in Koka and Wonji farms, Ethiopia. Environ. Sci. Pollut. Res. 2017, 24, 11807–11815. [Google Scholar] [CrossRef]
- Ferri, R.; Hashim, D.; Smith, D.R.; Guazzetti, S.; Donna, F.; Ferretti, E.; Curatolo, M.; Moneta, C.; Beone, G.M.; Lucchini, R.G. Metal contamination of home garden soils and cultivated vegetables in the province of Brescia, Italy: Implications for human exposure. Sci. Total Environ. 2015, 518, 507–517. [Google Scholar] [CrossRef]
- Latif, A.; Bilal, M.; Asghar, W.; Azeem, M.; Ahmad, M.I.; Abbas, A.; Ahmad, M.Z.; Shahzad, T. Heavy metal accumulation in vegetables and assessment of their potential health risk. J. Environ. Anal. Chem. 2018, 5, 234. [Google Scholar] [CrossRef]
- Kananke, T.; Wansapala, J.; Gunarante, A. Detection of Ni, Cd, and Cu in green leafy vegetables collected from different cultivation areas in and around Colombo District Sri Lanka. Environ. Monit. Assess. 2016, 188, 187. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Gong, W.; Li, B.; Zuo, J.; Pan, L.; Liu, X. Analysis of heavy metals in foodstuffs and an assessment of the health risks to the general public via consumption in Beijing, China. Int. J. Environ. Res. Public Health 2019, 16, 909. [Google Scholar] [CrossRef] [PubMed]
- Margenat, A.; Matamoros, V.; Díez, S.; Cañameras, N.; Comas, J. Occurrence and human health implications of chemical contaminants in vegetables grown in peri-urban agriculture. Environ. Int. 2019, 124, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Pipoyan, D.; Beglaryan, M.; Sireyan, L.; Merendino, N. Exposure assessment of potentially toxic trace elements via consumption of fruits and vegetables grown under the impact of Alaverdi’s mining complex. Hum. Ecol. Risk Assess. 2019, 25, 819–834. [Google Scholar] [CrossRef]
- Mihaileanu, R.G.; Neamtiu, I.A.; Fleming, M.; Pop, C.; Bloom, M.S.; Roba, C.; Surcel, M.; Stamatian, F.; Gurzau, E. Assessment of heavy metals (total chromium, lead, and manganese) contamination of residential soil and homegrown vegetables near a former chemical manufacturing facility in Tarnaveni, Romania. Environ. Monit. Assess. 2019, 191, 8. [Google Scholar] [CrossRef]
- Shaheen, N.; Irfan, N.F.; Khan, I.N.; Islam, S.; Islam, M.S.; Ahmed, M.K. Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere 2016, 152, 431–438. [Google Scholar] [CrossRef]
- Wachirawongsakorn, P. Health risk assessment via consumption of Pb and Cd contaminated vegetables collected from fresh markets in the lower north of Thailand. Hum. Ecol. Risk Assess. 2016, 22, 611–622. [Google Scholar] [CrossRef]
- Zhong, T.; Xue, D.; Zhao, L.; Zhang, X. Concentration of heavy metals in vegetables and potential health risk assessment in China. Environ. Geochem. Health 2018, 40, 313. [Google Scholar] [CrossRef]
- EC 1881/2006. Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuff. Off. J. Eur. Union 2006, 364, 5–24.
- Kumar, V.; Sharma, A.; Dhunna, G.; Chawla, A.; Bhardwaj, R.; Thukral, A.K. A tabulated review on distribution of heavy metals in various plants. Environ. Sci. Pollut. Res. 2017, 24, 2210–2260. [Google Scholar] [CrossRef]
- Marles, R.J. Mineral nutrient composition of vegetables, fruits and grains: The context of reports of apparent historical declines. J. Food Compos. Anal. 2017, 56, 93–103. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press, Taylor &Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Jolly, Y.N.; Islam, A.; Akbar, S. Transfer of metals from soil to vegetables and possible health risk assessment. SpringerPlus 2013, 2, 385. [Google Scholar] [CrossRef] [PubMed]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Yabanli, M.; Yozukmaz, A.; Sel, F. Heavy metal accumulation in the leaves, stem and root of the invasive submerged macrophyte Myriophyllum spicatum L. (Haloragaceae): An example of Kadin Creek (Mugla, Turkey). Braz. Arch. Biol. Technol. 2014, 57, 434–440. [Google Scholar] [CrossRef]
- Youssef, M.A.; Abd El-Gawad, A.M. Accumulation and translocation of heavy metals in eggplant (Solanum melongena L.) grown in a contaminated soil. J. Energy Environ. Chem. Eng. 2018, 3, 9–18. [Google Scholar] [CrossRef]
- Joint, FAO/WHO Expert Committee on Food Additives, and World Health Organization. Evaluation of Certain Food Additives and Contaminants. Thirty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series, No. 776; Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland, 1989. [Google Scholar]
- Joint, FAO/WHO Expert Committee on Food Additives, and World Health Organization. Summary and Conclusions of the Seventy Third Meeting of the Joint FAO/WHO Expert Committee on Food Additive; JECFA/73/SC; Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland, 2010. [Google Scholar]
- Baars, A.J.; Theelen, R.M.C.; Janssen, P.J.C.M.; Hesse, J.M.; van Apeldoorn, M.E.; Meijerink, M.C.M.; Verdam, L.; Zeilmaker, M.J. Re-Evaluation of Human-Toxicological Maximum Permissible Risk Levels. RIVM Report 711701 025; National Institute of Public Health and the Environment: Bilthoven, The Netherlands, 2001. [Google Scholar]
- Joint, FAO/WHO Expert Committee on Food Additives, and World Health Organization. Food Additives and Contaminants. Codex Alimentarius Commission. Joint FAO/WHO Food Standards Program, ALI-NORM01/12A; Joint FAO/WHO Expert Committee on Food Additives: The Hague, The Netherlands, 2001. [Google Scholar]
- Joint, FAO/WHO Expert Committee on Food Additives, and World Health Organization. Safety Evaluation of Certain Contaminants in Food. Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Mercury (Addendum); Food and Agriculture Organization of the United Nations: Rome, Italy; Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. Guideline for Drinking Water Quality, Third Edition, Incorporating First and Second Addenda. Volume 1 Recommendations; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Sander, S.; Kappenstein, O.; Ebner, I.; Fritsch, K.A.; Schmidt, R.; Pfaff, K.; Luch, A. Release of aluminium and thallium ions from uncoated food contact materials made of aluminium alloys into food and food simulant. PLoS ONE 2018. [Google Scholar] [CrossRef]
- Dzwonkowski, W. Evolution of potato production in Poland and the EU. Scientific Journals of the Warsaw University of Life Sciences in Warsaw. Probl. World Agric. 2017, 17, 71–80. [Google Scholar]
- Regulation of the Minister of the Environment of 1 September 2016 on the conduct of the assessment of contamination of the surface of the earth. J. Lows 2016, 1396, 1–86.
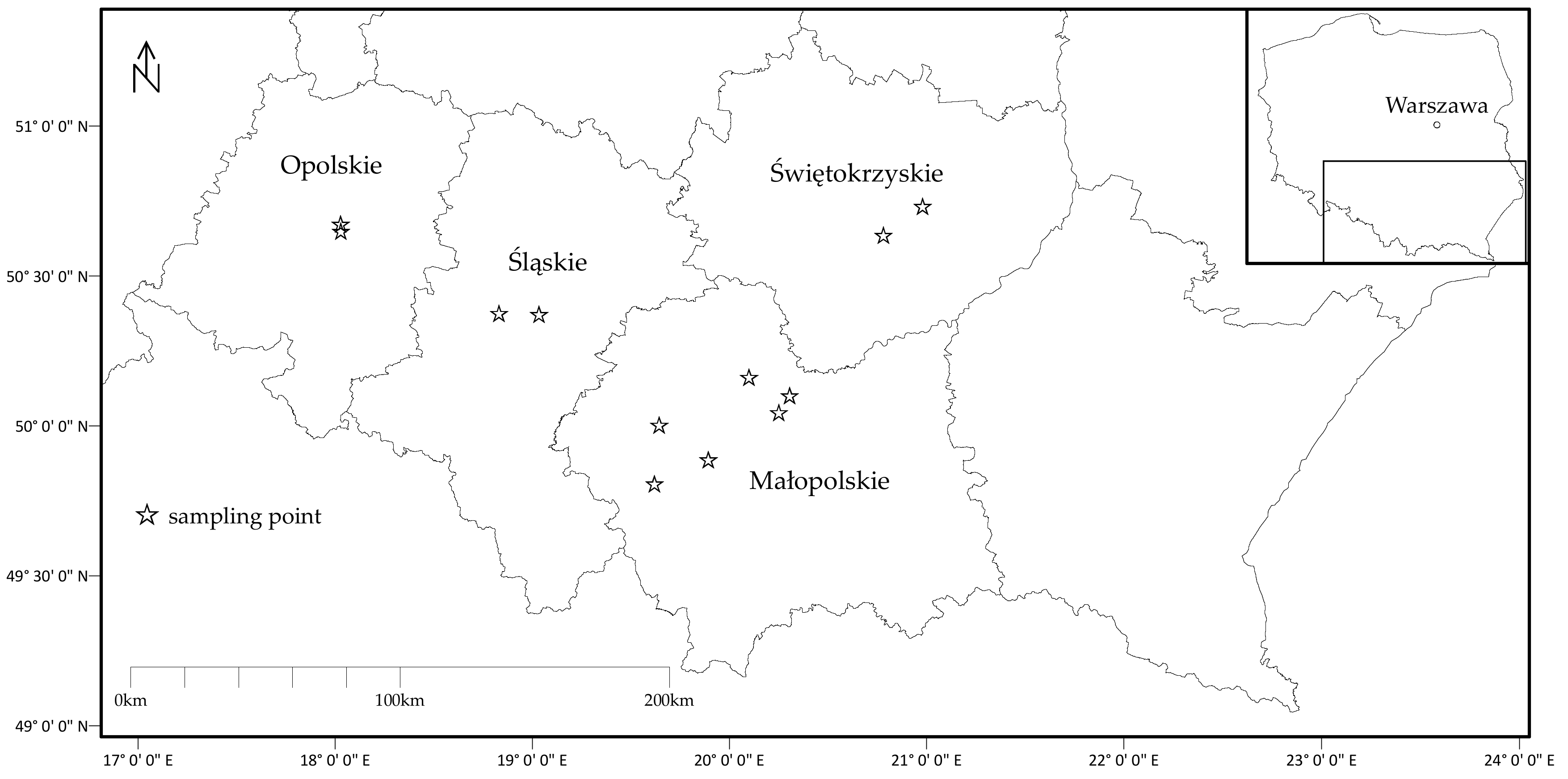
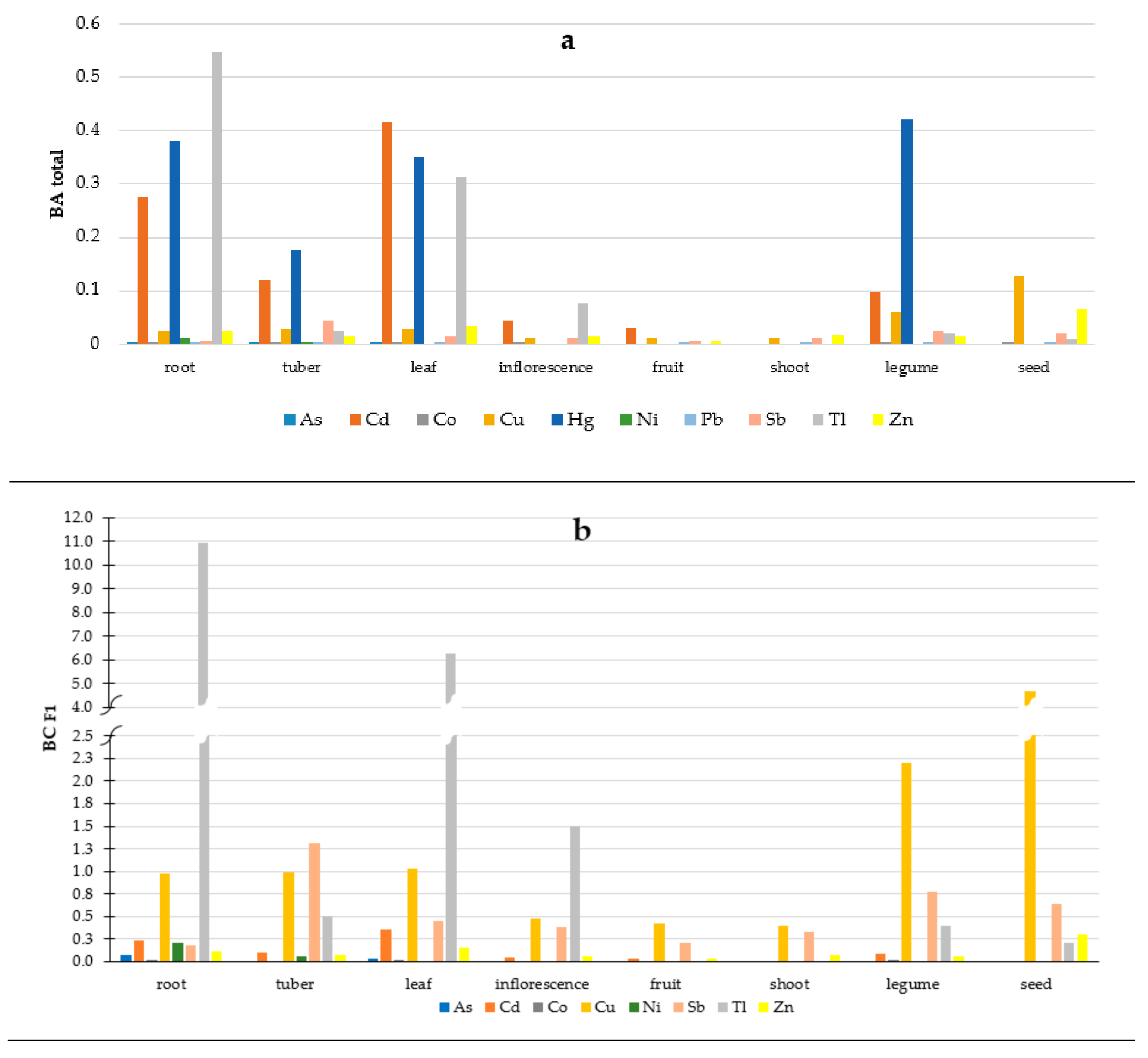
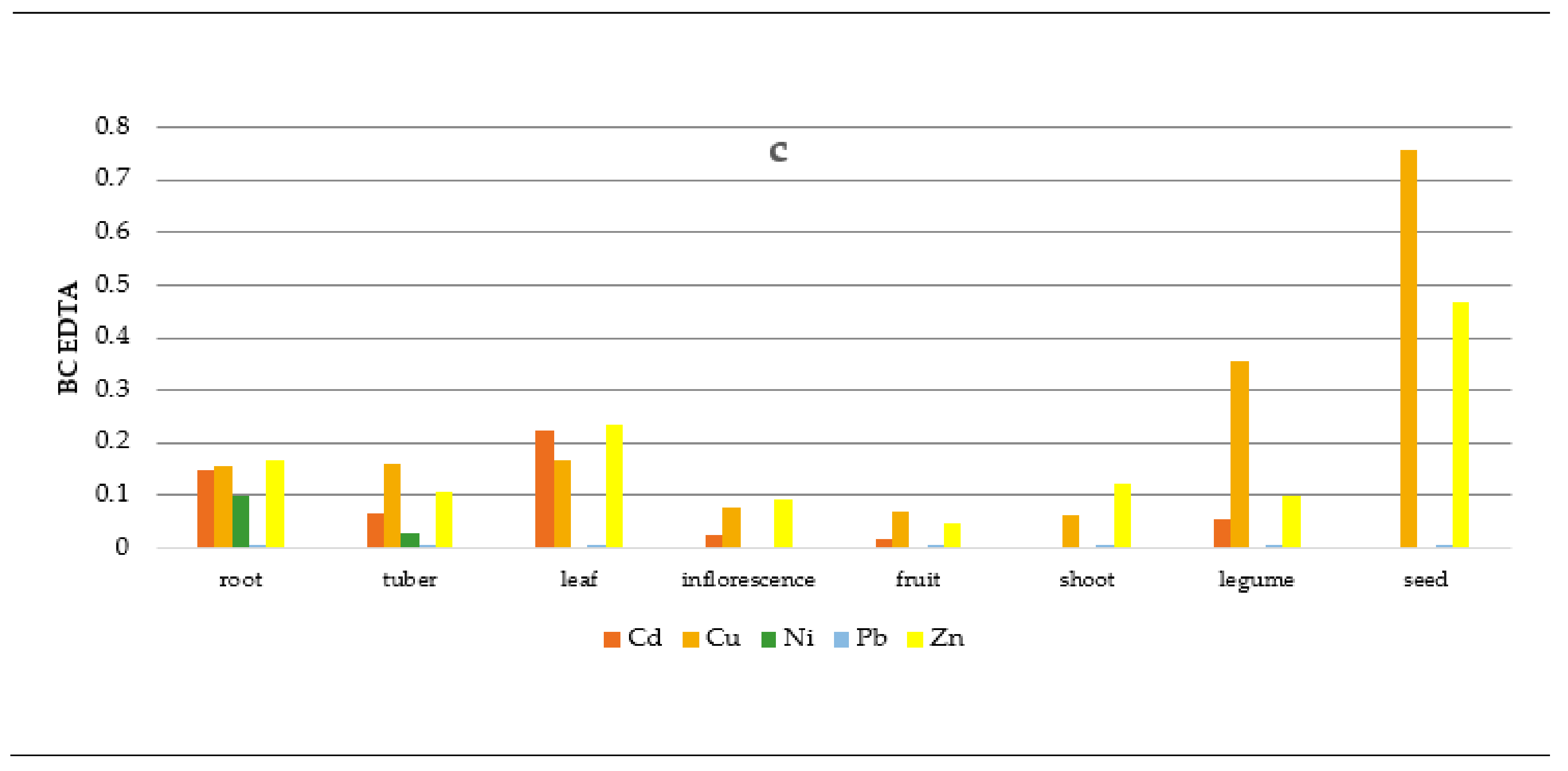

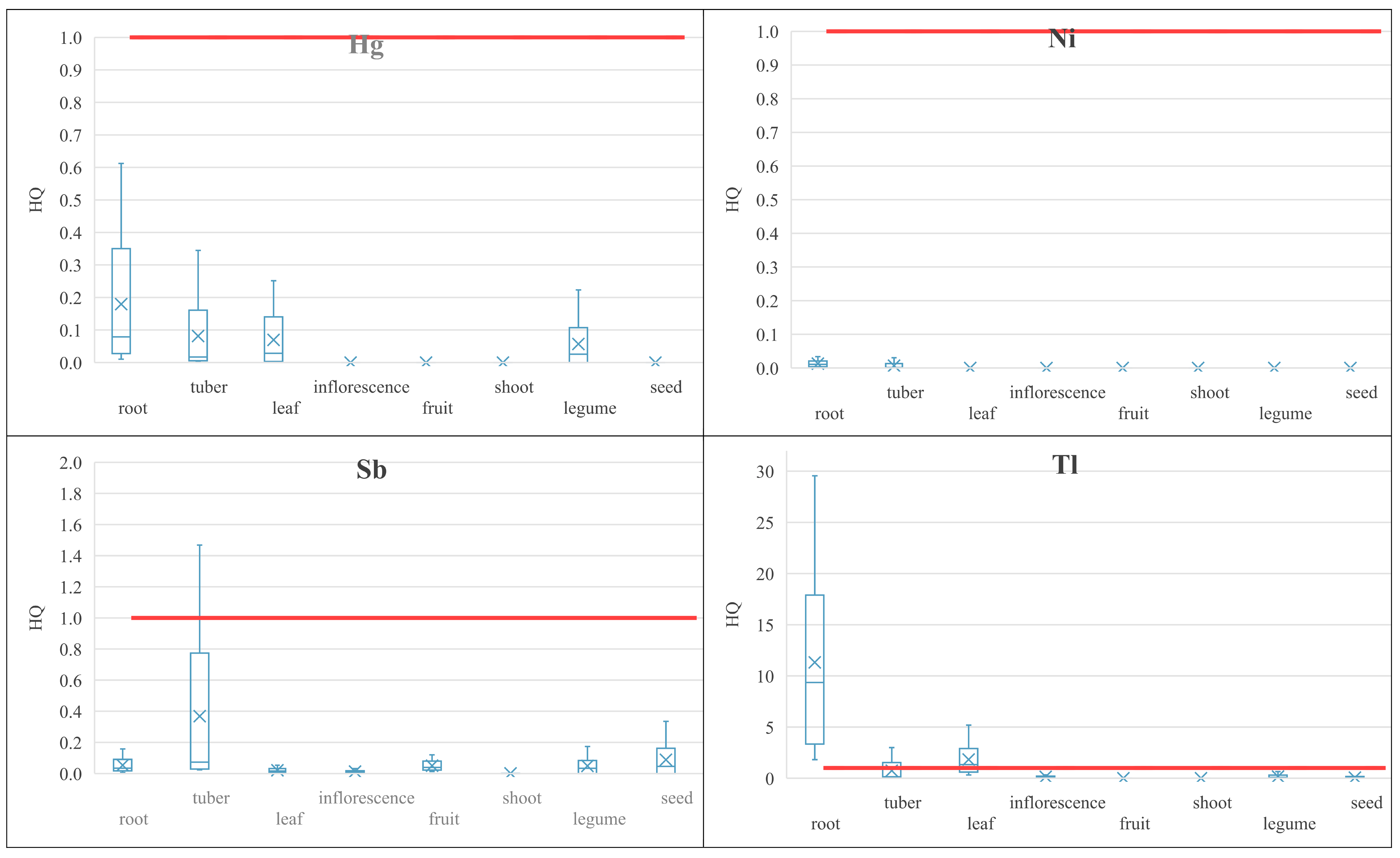

| No. | Common Name | Botanical Name | Number of Samples (n) | Edible Part |
|---|---|---|---|---|
| 1. | Beet root | Beta vulgaris | 11 | root |
| 2. | Carrot | Daucus carota | 7 | root |
| 3. | Celery root | Apium graveolens | 8 | root |
| 4. | Horseradish | Armoracia rusticana | 4 | root |
| 5. | Parsley root | Petroselinum hortense | 5 | root |
| 6. | Radish | Raphanus sativus var. sativus | 4 | root |
| 7. | Turnip | Brassica rapa | 2 | root |
| 8. | Garlic | Allium sativum | 5 | tuber |
| 9. | Kohlrabi | Brassica oleracea var. gongylodes | 8 | tuber |
| 10. | Onion | Allium cepa | 9 | tuber |
| 11. | Potato | Solanum tuberosum | 5 | tuber |
| 12. | Arugula | Eruca sativa | 3 | leaf |
| 13. | Beet tops | Beta vulgaris | 8 | leaf |
| 14. | Cabbage | Brassica oleracea var. capitata f. alba | 5 | leaf |
| 15. | Celery tops | Apium graveolens | 7 | leaf |
| 16. | Chives | Allium schoenoprasum | 9 | leaf |
| 17. | Kale | Brassica oleracea var. sabellica | 3 | leaf |
| 18. | Leek | Allium porrum | 6 | leaf |
| 19. | Lettuce | Lactuca sativa | 8 | leaf |
| 20. | Lovage | Levisticum officinale | 4 | leaf |
| 21. | Parsley tops | Petroselinum hortense | 8 | leaf |
| 22. | Rhubarb | Rheum rhaponticum | 8 | leaf |
| 23. | Spinach | Spinacia oleracea | 5 | leaf |
| 24. | Broccoli | Brassica oleracea | 5 | inflorescence |
| 25. | Cauliflower | Brassica oleracea var. botrytis | 5 | inflorescence |
| 26. | Zucchini | Cucurbita pepo convar. giromontiina Greb | 6 | fruit |
| 27. | Pumpkin | Cucurbita pepo | 4 | fruit |
| 28. | Cucumber | Cucumis sativus | 6 | fruit |
| 29. | Sweet pepper | Capsicum annuum | 4 | fruit |
| 30. | Tomato | Lycopersicon esculentum | 6 | fruit |
| 31. | Asparagus | Asparagus officinalis | 2 | shoot |
| 32. | Green bean | Phaseolus vulgaris | 7 | legume |
| 33. | Broad bean | Vicia faba | 5 | seed |
| 34. | Kidney bean | Phaseolus vulgaris | 4 | seed |
| 35. | Pea | Pisum sativum | 4 | seed |
| 36. | Pumpkin seeds | Cucurbita pepo | 6 | seed |
| IR (g ww./Person-Day) | |||
|---|---|---|---|
| Vegetable Part | Adult PL | Adult USEPA | Child USEPA |
| root | 23.3 | 77 | 38.4 |
| tuber | 289.5 | 12.6 | 3.45 |
| leaf | 15.6 | 7.1 | 8.1 |
| inflorescence | 5.75 | 6.3 | 3.3 |
| fruit | 45 | 84.7 | 32.7 |
| shoot | nd | 1.4 | 0.3 |
| legume | nd | 14 | 9.75 |
| seed | nd | 23.8 | 24.6 |
| PHE | Statistical Parameters mg/kg ww. | Root (n = 41) | Tuber (n = 27) | Leaf (n = 74) | Inflorescence (n = 10) | Fruit (n = 29) | Shoot (n = 2) | Legume (n = 7) | Seed (n = 19) |
|---|---|---|---|---|---|---|---|---|---|
| As | min | nd | nd | nd | ND | ND | ND | ND | ND |
| mean ± SD | 0.021 ± 0.004 | 0.002 ± 0.001 | 0.009 ± 0.006 | ||||||
| max | 0.056 | 0.009 | 0.028 | ||||||
| P95 | 0.048 | 0.007 | 0.023 | ||||||
| >LOD (%) | 27.2 | 12.7 | 25.6 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Cd | min | nd | nd | nd | nd | nd | ND | nd | ND |
| mean ± SD | 0.107 ± 0.012 | 0.046 ± 0.014 | 0.162 ± 0.016 | 0.017 ± 0.008 | 0.012 ± 0.005 | 0.038 ± 0.005 | |||
| max | 0.375 | 0.119 | 0.556 | 0.031 | 0.022 | 0.144 | |||
| P95 | 0.311 | 0.107 | 0.453 | 0.029 | 0.021 | 0.135 | |||
| >LOD (%) | 88.6 | 86.0 | 97.6 | 90.0 | 80.2 | 0.00 | 71.4 | 0.00 | |
| MAC | 0.10 | 0.10 | 0.20 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | |
| Co | min | nd | nd | nd | nd | ND | ND | nd | nd |
| mean ± SD | 0.005 ± 0.003 | 0.004 ± 0.003 | 0.007 ± 0.002 | 0.002 ± 0.001 | 0.006 ± 0.002 | 0.004 ± 0.002 | |||
| max | 0.029 | 0.012 | 0.017 | 0.005 | 0.027 | 0.022 | |||
| P95 | 0.019 | 0.010 | 0.015 | 0.004 | 0.024 | 0.017 | |||
| >LOD (%) | 15.0 | 30.0 | 46.0 | 30.0 | 0.00 | 0.00 | 57.1 | 36.7 | |
| Cu | min | nd | 0.126 | nd | 0.151 | 0.042 | 0.234 | 0.128 | 0.299 |
| mean ± SD | 0.698 ± 0.077 | 0.712 ± 0.064 | 0.744 ± 0.068 | 0.339 ± 0.059 | 0.303 ± 0.085 | 0.282 ± 0.014 | 1.582 ± 0.072 | 3.373 ± 0.163 | |
| max | 1.894 | 1.768 | 1.711 | 0.650 | 0.620 | 0.330 | 7.638 | 5.403 | |
| P95 | 1.630 | 1.462 | 1.383 | 0.591 | 0.574 | 0.282 | 5.692 | 5.264 | |
| >LOD (%) | 92.1 | 100.0 | 95.8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |
| Hg | min | nd | nd | nd | ND | ND | ND | nd | ND |
| mean ± SD | 0.019 ± 0.009 | 0.009 ± 0.007 | 0.018 ± 0.005 | 0.021 ±0.018 | |||||
| max | 0.101 | 0.029 | 0.163 | 0.147 | |||||
| P95 | 0.072 | 0.025 | 0.140 | 0.103 | |||||
| >LOD (%) | 36.4 | 31.7 | 44.7 | 0.00 | 0.00 | 0.00 | 14.3 | 0.00 | |
| MAC | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Ni | min | 0.073 | nd | ND | ND | ND | ND | ND | ND |
| mean ± SD | 0.130 ± 0.056 | 0.038 ± 0.022 | |||||||
| max | 0.299 | 0.186 | |||||||
| P95 | 0.264 | 0.149 | |||||||
| >LOD (%) | 4.20 | 10.5 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Pb | min | nd | nd | 0.001 | ND | nd | nd | nd | nd |
| mean ± SD | 0.063 ± 0.011 | 0.079 ± 0.010 | 0.094 ± 0.013 | 0.030 ± 0.008 | 0.036 ± 0.010 | 0.093 ± 0.006 | 0.019 ± 0.009 | ||
| max | 0.333 | 0.312 | 0.340 | 0.087 | 0.041 | 0.580 | 0.070 | ||
| P95 | 0.248 | 0.262 | 0.278 | 0.079 | 0.036 | 0.420 | 0.059 | ||
| >LOD (%) | 38.0 | 43.2 | 69.2 | 0.00 | 40.2 | 50.0 | 57.1 | 28.7 | |
| MAC | 0.10 | 0.10 | 0.30 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | |
| Sb | min | nd | nd | nd | nd | nd | nd | nd | nd |
| mean ± SD | 0.007 ± 0.004 | 0.053 ± 0.012 | 0.018 ± 0.004 | 0.015 ± 0.008 | 0.008 ± 0.002 | 0.013 ± 0.006 | 0.031 ± 0.004 | 0.026 ± 0.006 | |
| max | 0.071 | 0.163 | 0.086 | 0.071 | 0.083 | 0.002 | 0.116 | 0.097 | |
| P95 | 0.068 | 0.142 | 0.081 | 0.058 | 0.081 | 0.013 | 0.107 | 0.082 | |
| >LOD (%) | 38.8 | 44.5 | 55.5 | 40.0 | 31.6 | 50.0 | 42.9 | 30.0 | |
| Tl | min | nd | nd | nd | nd | ND | ND | nd | nd |
| mean ± SD | 0.055 ± 0.001 | 0.003 ± 0.001 | 0.031 ± 0.002 | 0.008 ± 0.002 | 0.002 ± 0.001 | 0.001 ± 0.000 | |||
| max | 0.452 | 0.008 | 0.115 | 0.017 | 0.014 | 0.002 | |||
| P95 | 0.408 | 0.007 | 0.096 | 0.015 | 0.010 | 0.001 | |||
| >LOD (%) | 43.1 | 38.5 | 53.7 | 60.0 | 0.00 | 0.00 | 28.6 | 12.5 | |
| Zn | min | 2.22 | 2.50 | 4.51 | 2.64 | 1.23 | 3.93 | 2.52 | 10.5 |
| mean ± SD | 6.72 ± 1.24 | 4.36 ± 0.91 | 9.46 ± 1.25 | 3.72 ± 1.25 | 1.85 ± 0.75 | 4.89 ± 1.08 | 3.94 ± 0.90 | 18.8 ± 1.29 | |
| max | 16.2 | 6.77 | 16.4 | 5.05 | 2.58 | 5.86 | 5.64 | 34.9 | |
| P95 | 14.2 | 6.36 | 15.3 | 4.88 | 2.46 | 4.89 | 5.46 | 32.0 | |
| >LOD (%) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| PHEs | Varimax Rotated | |||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |
| As | 0.716 | 0.112 | 0.586 | 0.041 |
| Cd | 0.646 | 0.106 | −0.055 | 0.512 |
| Co | 0.871 | 0.251 | −0.129 | 0.084 |
| Cu | 0.095 | 0.972 | 0.000 | 0.010 |
| Hg | 0.926 | 0.021 | 0.102 | 0.094 |
| Ni | −0.103 | 0.012 | 0.902 | −0.042 |
| Pb | 0.558 | 0.161 | 0.154 | 0.656 |
| Sb | 0.022 | 0.109 | −0.011 | 0.908 |
| Tl | 0.594 | 0.237 | 0.639 | 0.249 |
| Zn | 0.197 | 0.907 | 0.149 | 0.267 |
| Eigenvalues | 4.57 | 1.52 | 1.41 | 1.02 |
| Explained variance % | 32.7 | 19.5 | 16.4 | 16.7 |
| Cumulative variance % | 32.7 | 52.2 | 68.6 | 85.3 |
| Vegetable Intake Scenario | MOE (Mean Exposure) | MOE (P95 Exposure) |
|---|---|---|
| Adult PL | 3.1 | 0.9 |
| Adult USEPA | 7.7 | 2.2 |
| Child USEPA | 1.6 | 0.4 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruszecka-Kosowska, A. Potentially Harmful Element Concentrations in the Vegetables Cultivated on Arable Soils, with Human Health-Risk Implications. Int. J. Environ. Res. Public Health 2019, 16, 4053. https://doi.org/10.3390/ijerph16204053
Gruszecka-Kosowska A. Potentially Harmful Element Concentrations in the Vegetables Cultivated on Arable Soils, with Human Health-Risk Implications. International Journal of Environmental Research and Public Health. 2019; 16(20):4053. https://doi.org/10.3390/ijerph16204053
Chicago/Turabian StyleGruszecka-Kosowska, Agnieszka. 2019. "Potentially Harmful Element Concentrations in the Vegetables Cultivated on Arable Soils, with Human Health-Risk Implications" International Journal of Environmental Research and Public Health 16, no. 20: 4053. https://doi.org/10.3390/ijerph16204053
APA StyleGruszecka-Kosowska, A. (2019). Potentially Harmful Element Concentrations in the Vegetables Cultivated on Arable Soils, with Human Health-Risk Implications. International Journal of Environmental Research and Public Health, 16(20), 4053. https://doi.org/10.3390/ijerph16204053





