Tobacco Smoke Exposure, Urban and Environmental Factors as Respiratory Disease Predictors in Italian Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
- Questionnaire: Questions selected from the most extensive SIDRIA questionnaire [16], as described previously [17], were administered to each subject enrolled. This information was used to establish individual and clinical features (i.e., age, weight, height, Body Mass Index –BMI-, gender, residence, hobbies, therapies, and health conditions). Questions on tobacco smoke and urbanization included a parental and subjective evaluation of the exposure (absent, low, moderate, or high). The questionnaire was also structured to gain in-depth knowledge of personal lifestyle habits of the subjects and to gather information on the main asthma-like symptoms, such as asthma attacks, wheeze with breathlessness, current use of treatments for asthma, current hay fever/nasal allergies, waking with chest tightness, being woken by shortness of breath, and being woken by coughing [18].
- Spirometry measurements: These were expressed as maximal expiratory flow–volume curves to establish forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), maximal expiratory flows at peak 50%, 25%, and among 25–75% of FVC (PEF, FEF50, FEF25, FEF25-75) and the FEV1/FVC ratio. The instrument (CPFS/D, MGC Diagnostics Corporation, St Paul, MN, USA) was calibrated daily with a 3 L syringe. After a brief training, the measurements were carried out in accordance with the current ATS/ERS standards [19] and repeated until the volume variability did not exceed 150 mL for at least 2 times in order to comply with both within- and between-maneuver criteria [20].
- Respiratory mechanics: These were measured by FOT by means of a Resmon Pro FULL device (Restech, Milan, Italy). This method is noninvasive and employs small-amplitude pressure oscillations superimposed on the normal breathing, not requiring the performance of respiratory maneuvers [21]. A couple of measurements of at least 10 breaths each were obtained from each individual. The quality of breath was assessed through a specific algorithm contained inside the device and subsequent mathematical evaluation. Resistance and reactance obtained at a frequency of 5 Hz were used for the analysis.
- Morning urine spot: This test was performed to measure the following parameters:
- Cotinine. Cotinine measurements were carried out to objectively quantify the passive and active exposure to tobacco smoke. Cotinine levels were also regarded as a possible inductor of oxidative stress (OS) imbalance [22,23]. Urine samples were prepared for analysis as previously described [10,20,24]. Gas chromatography mass spectrometry (GC-MS) analysis was performed using an Agilent Technologies 6890 GC, interfaced to a 5973 MSD Inert Agilent mass spectrometer. The MS operated in electron impact and SIM mode. The limit of detection (LOD) and limit of quantification (LOQ) were 0.01 μg mL−1 and 0.02 μg mL−1, respectively. The coefficient of variation (CV), calculated to test repeatability, was below 5% for both Cotinine and the internal standard;
- 15-F2t-Isoprostane (15-F2t-IsoP). 15-F2t-IsoP was measured to quantify OS by the ELISA technique using a specific microplate kit (Oxford, MI, USA) according to the manufacturer’s instructions. To achieve better accuracy in the competitive ELISA method, each sample was diluted 1:4. The procedure is described in more detail elsewhere [25,26]; and
- Creatinine (Crea). Crea quantification was performed by the kinetic Jaffè procedure in order to normalize the excretion rate of Cotinine and 15-F2t-IsoP [20].
- Statistical analyses: They were all carried out using the Stata 14 Statistical Package (Stata Corp LP, Lakeway Drive, TX, USA). In univariate analysis, the variables in ordinal or interval scale were compared between gender and age classes through the non-parametric Kolmogorov–Smirnov 2 sample equality-of-distributions test and the Kruskal–Wallis equality-of-populations rank test. The frequency differences were tested with Pearson’s chi-squared test. Differences with a p < 0.05 were considered significant. To analyze the determinants of 15-F2t-IsoP, multiple linear regression analysis was performed using Box–Cox-transformed [27] 15-F2t-IsoP as the dependent variable. Height, age (6–10, 10–15, or >15-year groups), log Cotinine, and smoking exposure (recorded as yes or no) were used as predictive variables. In all models, variables were retained when they reached a level of 5% significance. To assess the effect of covariates on lung function parameters (measured through spirometry and FOT), we compared the findings of the spirometric parameters with the Global Lung Function Initiative (GLI) reference values [28], assuming as cut-off of “normal” values the lower 10% confidence limit of normality (LLN), as recommended by GLI authors. A sub-sample of asymptomatic subjects not exposed to tobacco smoke was selected from the whole group to calculate the reference values for FOT still missing in a well-stabilized form. This was achieved through multiple regression analysis of Box–Cox-transformed resistance and reactance calculated at a frequency of 5 Hz as dependent variables, selecting age, height, weight, and gender (female as reference value) as independent variables. The limits of normal test variability were computed following GLI recommendations. For spirometric values, the normal values were defined by comparing them with the lower limits of normality (LLN), while FOT and oscillatory resistances were compared with elastance and the upper limits of normality (ULN). A set of multiple logistic regression analyses were performed using the abnormality of findings as dependent variable, smoking, and traffic exposure as predictors, and age, gender, and BMI as confounders. A p value ≤ 0.05 (two-tailed) was considered significant in all tests. All variables that were not significant at the 5% level and not influencing other parameters were excluded.
2.2. Compliance with Ethical Standards
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rolland-Cachera, M.F.; Deheeger, M.; Maillot, M.; Bellisle, F. Early adiposity rebound: Causes and consequences for obesity in children and adults. Int. J. Obes. (Lond). 2006, 30 (Suppl. 4), S11–S17. [Google Scholar] [CrossRef]
- Hanley, B.; Dijane, J.; Fewtrell, M.; Grynberg, A.; Hummel, S.; Junien, C.; Koletzko, B.; Lewis, S.; Renz, H.; Symonds, M.; et al. Metabolic imprinting, programming and epigenetics—A review of present priorities and future opportunities. Br. J. Nutr. 2010, 104, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Antic, V.; Yang, Z.; Montani, J.-P. Propellers of growth trajectories to obesity and the metabolic syndrome. Int. J. Obes. 2006, 30, S1–S3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mbulo, L.; Palipudi, K.M.; Andes, L.; Morton, J.; Bashir, R.; Fouad, H.; Ramanandraibe, N.; Caixeta, R.; Dias, R.C.; Wijnhoven, T.M.A.; et al. Secondhand smoke exposure at home among one billion children in 21 countries: Findings from the Global Adult Tobacco Survey (GATS). Tob. Control 2016, 25, e95–e100. [Google Scholar] [CrossRef] [PubMed]
- Oberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet (London, England) 2011, 377, 139–146. [Google Scholar] [CrossRef]
- Raghuveer, G.; White, D.A.; Hayman, L.L.; Woo, J.G.; Villafane, J.; Celermajer, D.; Ward, K.D.; de Ferranti, S.D.; Zachariah, J. Cardiovascular Consequences of Childhood Secondhand Tobacco Smoke Exposure: Prevailing Evidence, Burden, and Racial and Socioeconomic Disparities: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e336–e359. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Romanazzi, V.; Bellisario, V.; Tassinari, R.; Trucco, G.; Urbino, A.; Cassardo, C.; Siniscalco, C.; Marchetti, P.; Marcon, A. Air pollution, aeroallergens and admissions to pediatric emergency room for respiratory reasons in Turin, northwestern Italy. BMC Public Health 2016, 16, 722. [Google Scholar] [CrossRef] [PubMed]
- Babin, S.M.; Burkom, H.S.; Holtry, R.S.; Tabernero, N.R.; Stokes, L.D.; Davies-Cole, J.O.; DeHaan, K.; Lee, D.H. Pediatric patient asthma-related emergency department visits and admissions in Washington, DC, from 2001–2004, and associations with air quality, socio-economic status and age group. Environ. Health 2007, 6, 9. [Google Scholar] [CrossRef]
- Wang, I.-J.; Tung, T.-H.; Tang, C.-S.; Zhao, Z.-H. Allergens, air pollutants, and childhood allergic diseases. Int. J. Hyg. Environ. Health 2016, 219, 66–71. [Google Scholar] [CrossRef]
- Bono, R.; Bellisario, V.; Romanazzi, V.; Pirro, V.; Piccioni, P.; Pazzi, M.; Bugiani, M.; Vincenti, M. Oxidative stress in adolescent passive smokers living in urban and rural environments. Int. J. Hyg. Environ. Health 2014, 217, 287–293. [Google Scholar] [CrossRef]
- Marcon, A.; Pesce, G.; Calciano, L.; Bellisario, V.; Dharmage, S.C.; Garcia-Aymerich, J.; Gislasson, T.; Heinrich, J.; Holm, M.; Janson, C.; et al. Trends in smoking initiation in Europe over 40 years: A retrospective cohort study. PLoS ONE 2018, 13, e0201881. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Golding, J.; Davey Smith, G.; Miller, L.L.; Pembrey, M. Prepubertal start of father’s smoking and increased body fat in his sons: Further characterisation of paternal transgenerational responses. Eur. J. Hum. Genet. 2014, 22, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdóttir, B.; Bertelsen, R.J.; Bråbäck, L.; Carsin, A.-E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.; Zuccaro, P.; Pacifici, R.; Gorini, G.; Colombo, P.; La Vecchia, C.; Gallus, S. Smoking in Italy in 2015–2016: Prevalence, trends, roll-your-own cigarettes, and attitudes towards incoming regulations. Tumori J. 2017, 103, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Charrier, L.; Berchialla, P.; Galeone, D.; Spizzichino, L.; Borraccino, A.; Lemma, P.; Dalmasso, P.; Cavallo, F. Smoking habits among Italian adolescents: What has changed in the last decade? Biomed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Migliore, E.; Berti, G.; Galassi, C.; Pearce, N.; Forastiere, F.; Calabrese, R.; Armenio, L.; Biggeri, A.; Bisanti, L.; Bugiani, M.; et al. Respiratory symptoms in children living near busy roads and their relationship to vehicular traffic: Results of an Italian multicenter study (SIDRIA 2). Environ. Heal. 2009, 8, 27. [Google Scholar] [CrossRef]
- Migliore, E.; Piccioni, P.; Garrone, G.; Ciccone, G.; Borraccino, A.; Bugiani, M. Changing prevalence of asthma in Turin school children between 1994 and 1999. Monaldi Arch Chest Dis. 2005, 63, 74–78. [Google Scholar] [CrossRef][Green Version]
- Jarvis, D.; Newson, R.; Janson, C.; Corsico, A.; Heinrich, J.; Anto, J.M.; Abramson, M.J.; Kirsten, A.-M.; Zock, J.P.; Bono, R.; et al. Prevalence of asthma-like symptoms with ageing. Thorax 2018, 73, 37–48. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Bono, R.; Tassinari, R.; Bellisario, V.; Gilli, G.; Pazzi, M.; Pirro, V.; Mengozzi, G.; Bugiani, M.; Piccioni, P. Urban air and tobacco smoke as conditions that increase the risk of oxidative stress and respiratory response in youth. Environ. Res. 2015, 137, 141–146. [Google Scholar] [CrossRef]
- Oostveen, E.; MacLeod, D.; Lorino, H.; Farré, R.; Hantos, Z.; Desager, K.; Marchal, F.; ERS Task Force on Respiratory Impedance Measurements. The forced oscillation technique in clinical practice: Methodology, recommendations and future developments. Eur. Respir. J. 2003, 22, 1026–1041. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Bellisario, V.; Tassinari, R.; Squillacioti, G.; Manetta, T.; Bugiani, M.; Migliore, E.; Piccioni, P. Bisphenol a, tobacco smoke, and age as predictors of oxidative stress in children and adolescents. Int. J. Environ. Res. Public Health 2019, 16, 2025. [Google Scholar] [CrossRef] [PubMed]
- Bono, R.; Romanazzi, V. Isoprostanes as biomarkers of disease and early biological effect. In General Methods in Biomarker Research and their Applications; Springer Nature: New York, NY, USA, 2015; pp. 383–404. [Google Scholar]
- Bono, R.; Munnia, A.; Romanazzi, V.; Bellisario, V.; Cellai, F.; Peluso, M.E.M. Formaldehyde-induced toxicity in the nasal epithelia of workers of a plastic laminate plant. Toxicol. Res. (Camb). 2016, 5. [Google Scholar] [CrossRef]
- Romanazzi, V.; Pirro, V.; Bellisario, V.; Mengozzi, G.; Peluso, M.; Pazzi, M.; Bugiani, M.; Verlato, G.; Bono, R. 15-F2t isoprostane as biomarker of oxidative stress induced by tobacco smoke and occupational exposure to formaldehyde in workers of plastic. Sci. Total Environ. Total Environ. 2013, 442, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bellisario, V.; Mengozzi, G.; Grignani, E.; Bugiani, M.; Sapino, A.; Bussolati, G.; Bono, R. Towards a formalin-free hospital. Levels of 15-F2t-isoprostane and malondialdehyde to monitor exposure to formaldehyde in nurses from operating theatres. Toxicol. Res. (Camb.) 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J.R. Stat. Soc. 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Hall, G.L.; Stanojevic, S.; Cole, T.J.; Stocks, J. Global Lungs Initiative Age- and height-based prediction bias in spirometry reference equations. Eur. Respir. J. 2012, 40, 190–197. [Google Scholar] [CrossRef]
- Fuertes, E.; Antó, J.M.; Bono, R.; Carsin, A.-E.; Dharmage, S.; Gislason, T.; Jarvis, D.; Johannessen, A.; Heinrich, J.; Leynaert, B.; et al. Long-term physical activity pattern and lung function in European adults. Eur. Respir. J. 2016. [Google Scholar] [CrossRef]
- Flexeder, C.; Zock, J.P.; Jarvis, D.; Verlato, G.; Olivieri, M.; Benke, G.; Ambranson, M.J.; Sigsgaard, T.; Svanes, C.; Torén, K.; et al. Second-hand smoke exposure in adulthood and lower respiratory health during 20 year follow up in the European Community Respiratory Health Survey. Respir. Res. 2019, 20, 33. [Google Scholar] [CrossRef]
- Hakim, I.A.; Harris, R.; Garland, L.; Cordova, C.A.; Mikhael, D.M.; Sherry Chow, H.-H. Gender difference in systemic oxidative stress and antioxidant capacity in current and former heavy smokers. Cancer Epidemiol. Biomarkers Prev. 2012, 21, 2193–2200. [Google Scholar] [CrossRef]
- Taylor, A.W.; Bruno, R.S.; Traber, M.G. Women and smokers have elevated urinary F(2)-isoprostane metabolites: A novel extraction and LC-MS methodology. Lipids 2008, 43, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, G.; Bellisario, V.; Grignani, E.; Mengozzi, G.; Bardaglio, G.; Dalmasso, P.; Bono, R. The asti study: The induction of oxidative stress in a population of children according to their body composition and passive tobacco smoking exposure. Int. J. Environ. Res. Public Health 2019, 16, 490. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P. Nicotine Chemistry, Metabolism, Kinetics and Biomarkers. In Nicotine Psychopharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 29–60. [Google Scholar]
- Tanner, J.-A.; Prasad, B.; Claw, K.G.; Stapleton, P.; Chaudhry, A.; Schuetz, E.G.; Thummel, K.E.; Tyndale, R.F. Predictors of variation in CYP2A6 mRNA, protein, and enzyme activity in a human liver bank: Influence of genetic and nongenetic factors. J. Pharmacol. Exp. Ther. 2017, 360, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Beydon, N. Pulmonary function testing in young children. Paediatr. Respir. Rev. 2009, 10, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, J.; Angbratt, M.; Valter, L.; Nordvall, M.; Timpka, T. History matters: Childhood weight trajectories as a basis for planning community-based obesity prevention to adolescents. Int. J. Obes. (Lond.) 2012, 36, 524–528. [Google Scholar] [CrossRef][Green Version]
- Marchetti, P.; Pesce, G.; Villani, S.; Antonicelli, L.; Ariano, R.; Attena, F.; Bono, R.; Bellisario, V.; Fois, A.; Gibelli, N.; et al. Pollen concentrations and prevalence of asthma and allergic rhinitis in Italy: Evidence from the GEIRD study. Sci. Total Environ. 2017, 584–585, 1093–1099. [Google Scholar] [CrossRef]
- Frazer, K.; Callinan, J.E.; McHugh, J.; van Baarsel, S.; Clarke, A.; Doherty, K.; Kelleher, C. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane database Syst. Rev. 2016, 2, CD005992. [Google Scholar] [CrossRef]
| Individual Characteristics | Total (n = 188) | Male (n = 103) | Female (n = 85) | pValue (KS/KW test) | |
| Age (years) Mean ± S.D. | 12.9 ± 3.8 | 12.9 + 3.9 | 12.9 + 3.6 | 0.719 | |
| Height (m) Mean ± S.D. | 1.6 ± 1.7 | 1.6 + 1.9 | 1.5 + 1.3 | 0.729 | |
| Weight (Kg) Mean ± S.D. | 50.1 ± 17.3 | 52.7 + 19 | 46.8 + 13.8 | 0.090 | |
| BMI Mean ± S.D. | 19.6 ± 3.8 | 19.9 + 4 | 19.1 + 3.4 | 0.229 | |
| BMI IOTF No. (%) | Underweight | 17 (9%) | 7 (6.8%) | 10 (11.6%) | |
| Normal weight | 132 (69.8%) | 76 (73.8%) | 56 (65.1%) | ||
| Overweight | 27 (14.3%) | 10 (9.7%) | 17 (19.8%) | ||
| Obese | 12 (6.9%) | 10 (9.7%) | 2 (3.5%) | ||
| Smoking habits No. (%) | No | 134 (70.9%) | 71 (68.9%) | 63 (73.2%) | |
| Passive | 41 (21.7%) | 20 (19.4%) | 21 (24.4%) | ||
| Active | 14 (7.4%) | 9 (11.7%) | 5 (5.8%) | ||
| Isoprostane (ng/mg Crea) Mean ± S.D. (Min–Max) | 4.5 ± 4.7 (0.2–38.8) | 4 ± 3.8 (0.8–17.7) | 5.1 ± 5.7 (0.2–38.8) | 0.06 | |
| Cotinine (ng/mg Crea) Mean ± S.D. (Min–Max) | 102 ± 196.9 (0.1–1730.9) | 92.6 ± 151.4 (0.1–742.5) | 115.5 ± 241 (0.1–1730.9) | 0.15 | |
| FVC Mean ± S.D. | 3.5 ± 1.5 | 3.8 ± 1.7 | 3.1 ± 1.2 | 0.00 | |
| FEV1 Mean ± S.D. | 3.1 ±1.3 | 3.4 ± 1.5 | 2.7 ± 0.9 | 0.00 | |
| FEF25 Mean ± S.D. | 5.4 ± 2.3 | 6 ± 2.7 | 4.8 ± 1.4 | 0.00 | |
| FEF50 Mean ± S.D. | 3.9 ± 1.7 | 4.3 ± 2.1 | 3.6 ± 1.1 | 0.00 | |
| FEF25–75 Mean ± S.D. | 3.5 ± 1.6 | 3.9 ±1.7 | 3.2 ± 1.1 | 0.00 | |
| FEV1/FVC Mean ± S.D. | 0.8 ± 0.1 | 0.9 ± 0.04 | 0.8 ± 0.08 | 0.011 | |
| R5 tot Mean ± S.D. | 4.2 ± 1.7 | 4.2 ± 1.9 | 4.2 ± 1.4 | 0.01 | |
| 6–10 years old (n = 74) | 11–15 years old (n = 53) | 15 + years old (n = 61) | pValue (KS/KW test) | ||
| Isoprostane (ng/mg Crea) Mean ± S.D. | 4.7 ± 5.3 | 3.8 ± 4.2 | 5.1 ± 4.5 | 0.00 | |
| Cotinine (ng/mg Crea) Mean ± S.D. | 74.6 ± 109.7 | 33.2 ± 111.6 | 196.7 ± 284.7 | 0.00 | |
| FVC Mean ± S.D. | 2.2 ± 0.4 | 3.7 ± 1.3 | 4.9 ± 1.3 | 0.00 | |
| FEV1 Mean ± S.D. | 1.9 ± 0.3 | 3.3 ± 1 | 4.3 ± 1.2 | 0.00 | |
| FEF25 Mean ± S.D. | 3.7 ± 0.6 | 5.7 ± 1.7 | 7.4 ± 2.4 | 0.00 | |
| FEF50 Mean ± S.D. | 2.7 ± 0.5 | 4.1 ± 1.2 | 5.4 ± 1.9 | 0.00 | |
| FEF25–75 Mean ± S.D. | 2.4 ± 0.5 | 3.7 ± 1.1 | 4.9 ± 1.8 | 0.00 | |
| FEV1/FVC Mean ± S.D. | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.00 | |
| R5 tot Mean ± S.D. | 5.7 ± 1.3 | 4 ± 1.3 | 2.8 ± 0.7 | 0.00 | |
| X5 tot Mean ± S.D. | −1.8 ± 0.8 | −1.2 ± 0.7 | 0.9 ± 0.3 | 0.54 | |
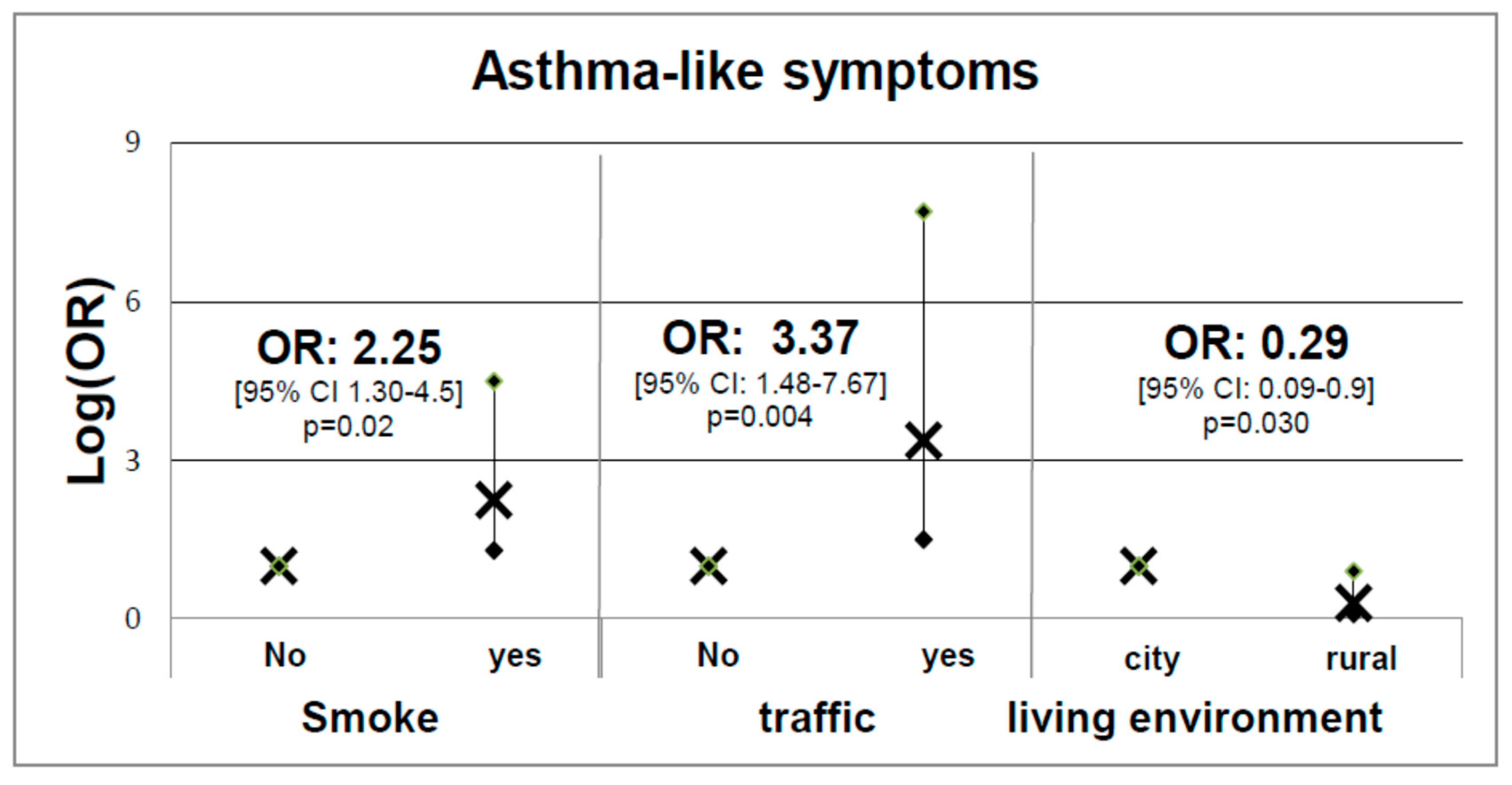
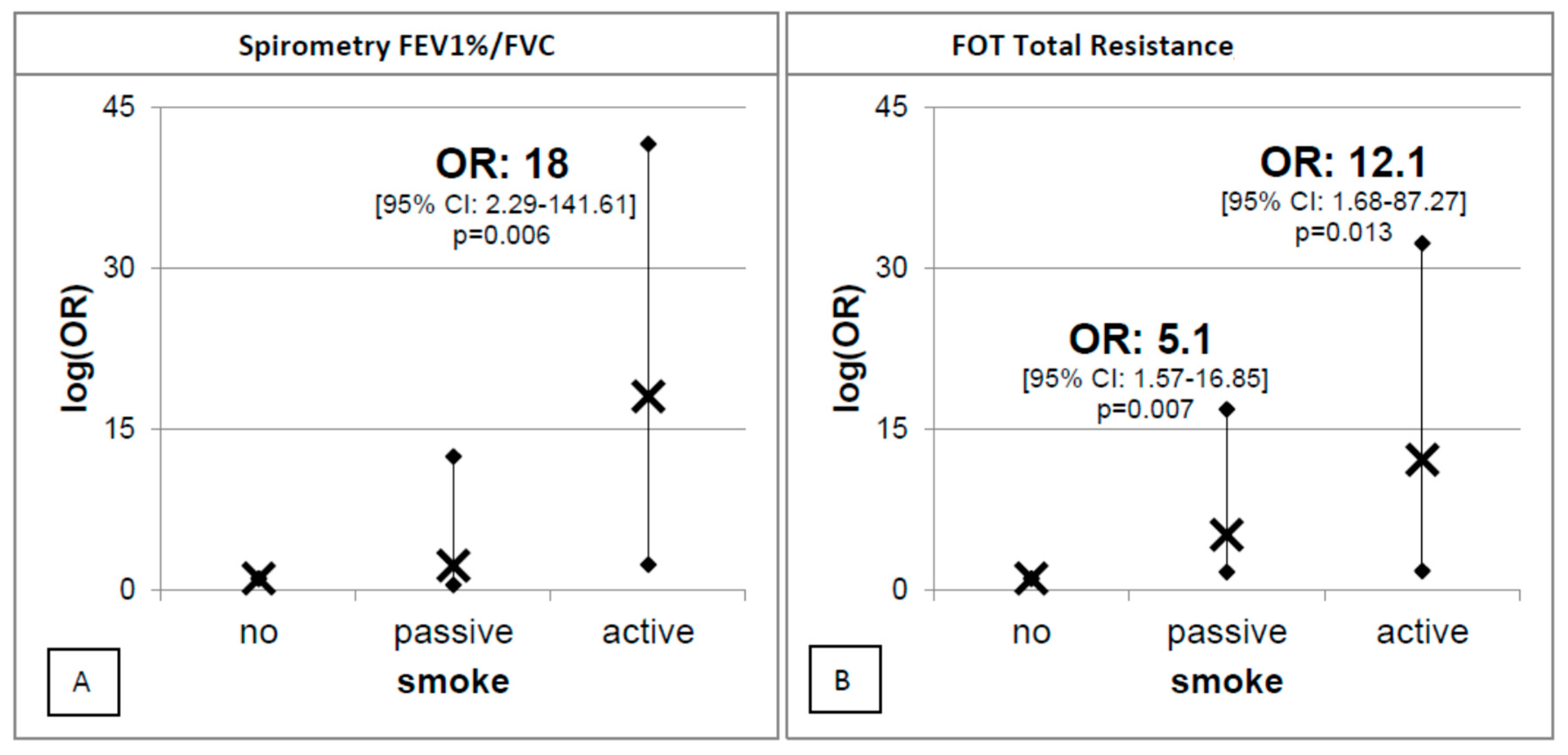
| Independent Variables | Predictive Margins (95% C.I.) | p | |
|---|---|---|---|
| Total sample | No | 1.04 (0.72–1.36) | 0.000 |
| Passive | 1.17 (1.01–1.34) | ||
| Active | 1.19 (0.97–1.41) | ||
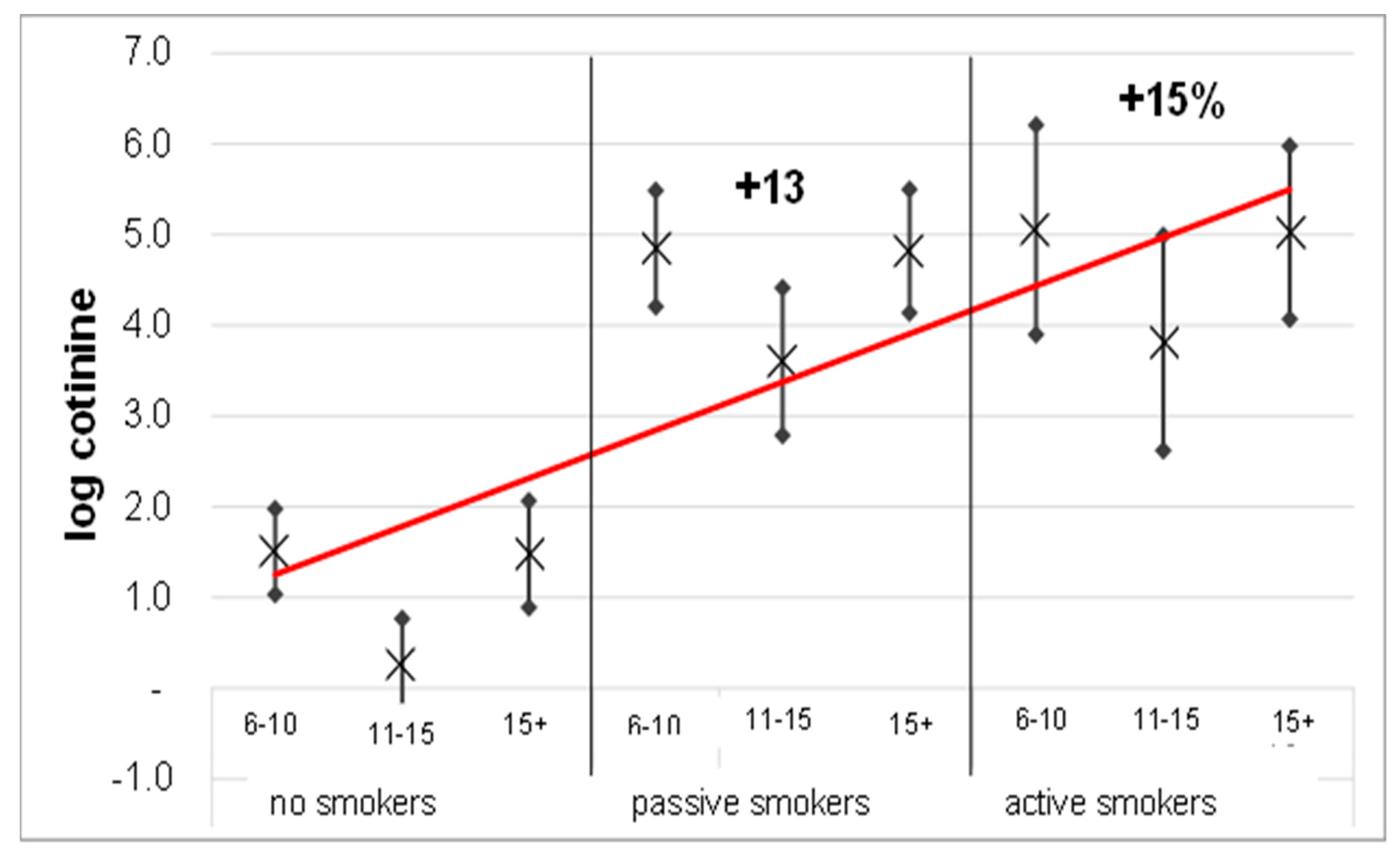
| Nosmokers | 6–10 years old | 1.5 (1–2) | 0.050 |
| 11–15 years old | 0.3 (−0.2–0.8) | ||
| 15 + years old | 1.5 (0.9–2.1) | ||
| Passive smokers | 6–10 years old | 4.8 (4.2–5.5) | |
| 11–15 years old | 3.6 (2.8–4.4) | ||
| 15 + years old | 4.8 (4.1–5.5) | ||
| Activesmokers | 6–10 years old | 5.1 (3.9–6.2) | |
| 11–15 years old | 3.8 (2.6–5) | ||
| 15 + years old | 5.0 (4.1–6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellisario, V.; Piccioni, P.; Bugiani, M.; Squillacioti, G.; Levra, S.; Gulotta, C.; Mengozzi, G.; Perboni, A.; Grignani, E.; Bono, R. Tobacco Smoke Exposure, Urban and Environmental Factors as Respiratory Disease Predictors in Italian Adolescents. Int. J. Environ. Res. Public Health 2019, 16, 4048. https://doi.org/10.3390/ijerph16204048
Bellisario V, Piccioni P, Bugiani M, Squillacioti G, Levra S, Gulotta C, Mengozzi G, Perboni A, Grignani E, Bono R. Tobacco Smoke Exposure, Urban and Environmental Factors as Respiratory Disease Predictors in Italian Adolescents. International Journal of Environmental Research and Public Health. 2019; 16(20):4048. https://doi.org/10.3390/ijerph16204048
Chicago/Turabian StyleBellisario, Valeria, Pavilio Piccioni, Massimiliano Bugiani, Giulia Squillacioti, Stefano Levra, Carlo Gulotta, Giulio Mengozzi, Alberto Perboni, Elena Grignani, and Roberto Bono. 2019. "Tobacco Smoke Exposure, Urban and Environmental Factors as Respiratory Disease Predictors in Italian Adolescents" International Journal of Environmental Research and Public Health 16, no. 20: 4048. https://doi.org/10.3390/ijerph16204048
APA StyleBellisario, V., Piccioni, P., Bugiani, M., Squillacioti, G., Levra, S., Gulotta, C., Mengozzi, G., Perboni, A., Grignani, E., & Bono, R. (2019). Tobacco Smoke Exposure, Urban and Environmental Factors as Respiratory Disease Predictors in Italian Adolescents. International Journal of Environmental Research and Public Health, 16(20), 4048. https://doi.org/10.3390/ijerph16204048






