Removal of High Concentrations of Ammonium from Groundwater in a Pilot-Scale System through Aeration at the Bottom Layer of a Chemical Catalytic Oxidation Filter
Abstract
1. Introduction
2. Materials and Methods
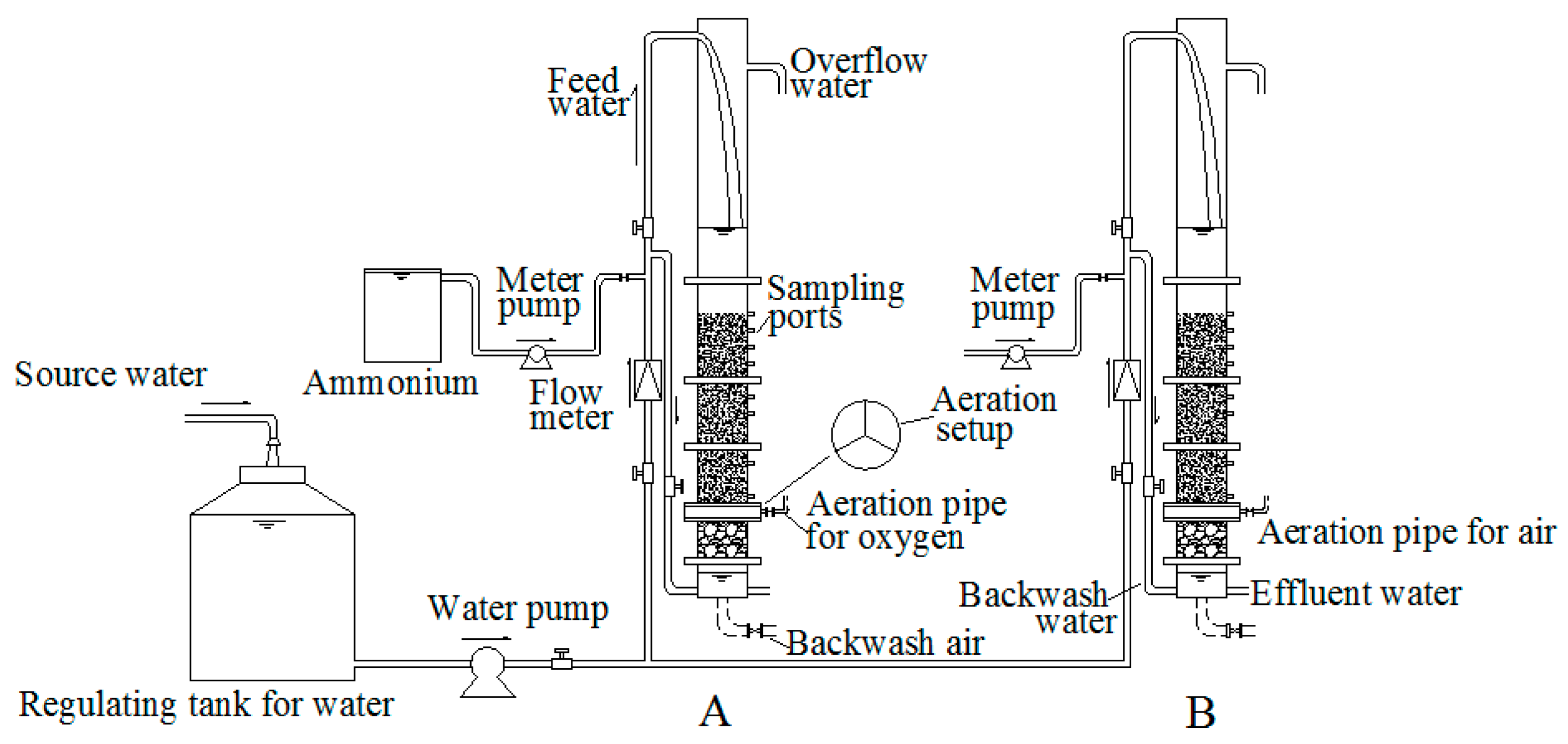
2.1. Pilot-Scale Filter System
2.2. Effects of Aeration Types
2.3. Effects of the Aeration Rates and Aeration Position
2.4. Effects of Filtration Rates
2.5. Mechanism of Ammonium Removal
2.6. Analytical Methods
3. Results and Discussion
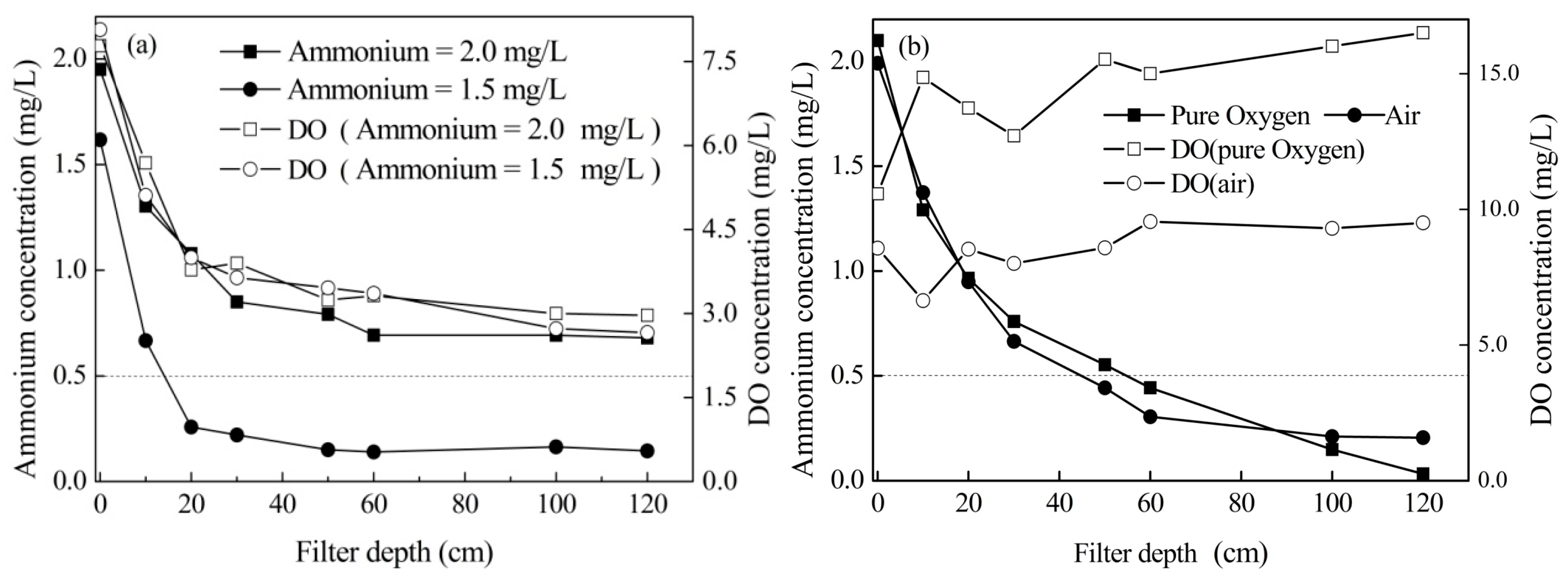
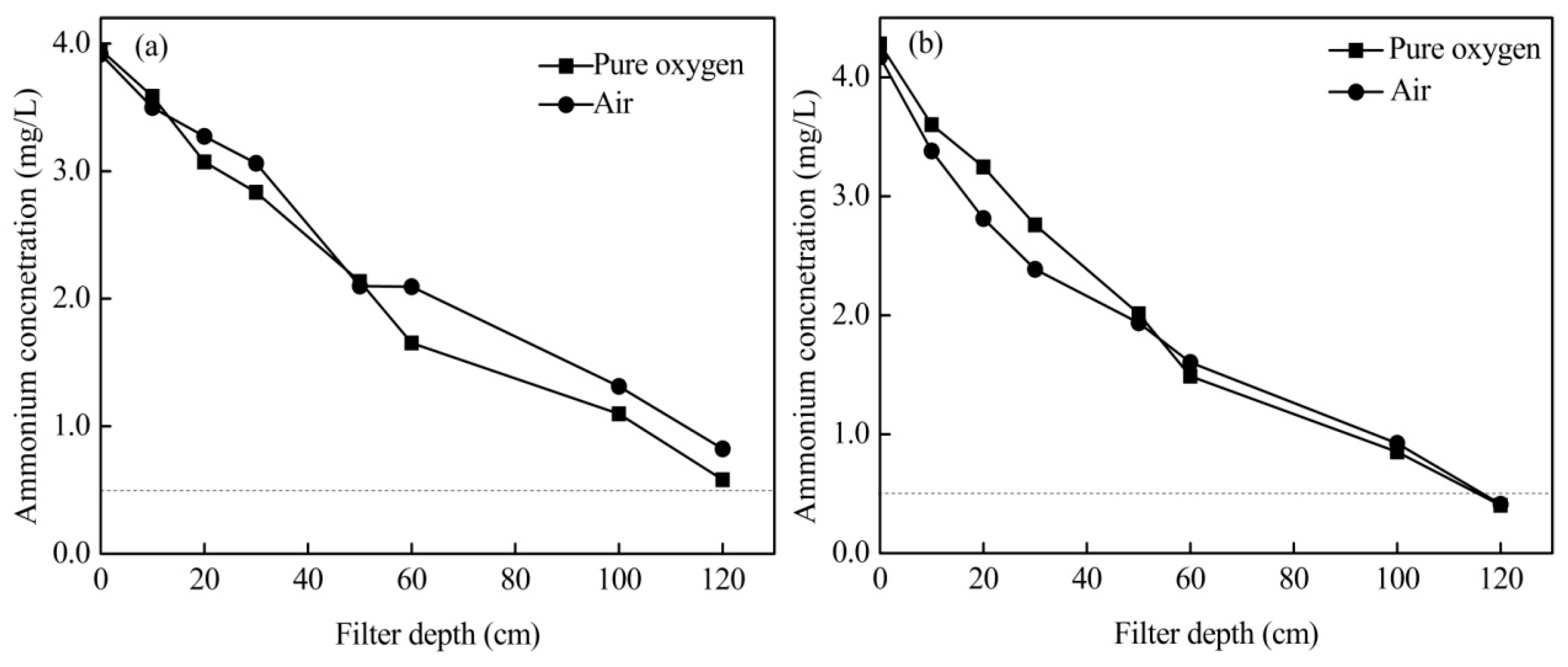
3.1. Effects of Aeration Types
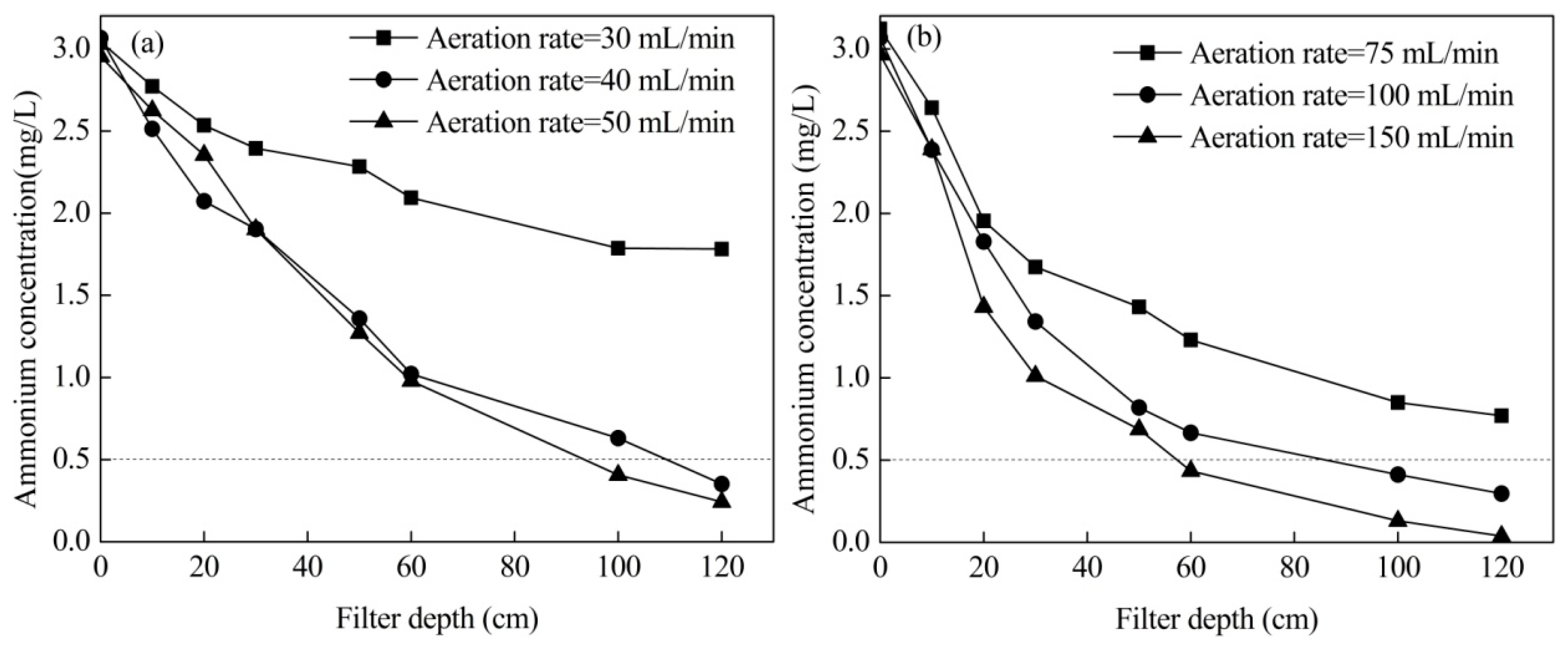
3.2. Effects of Aeration Rate
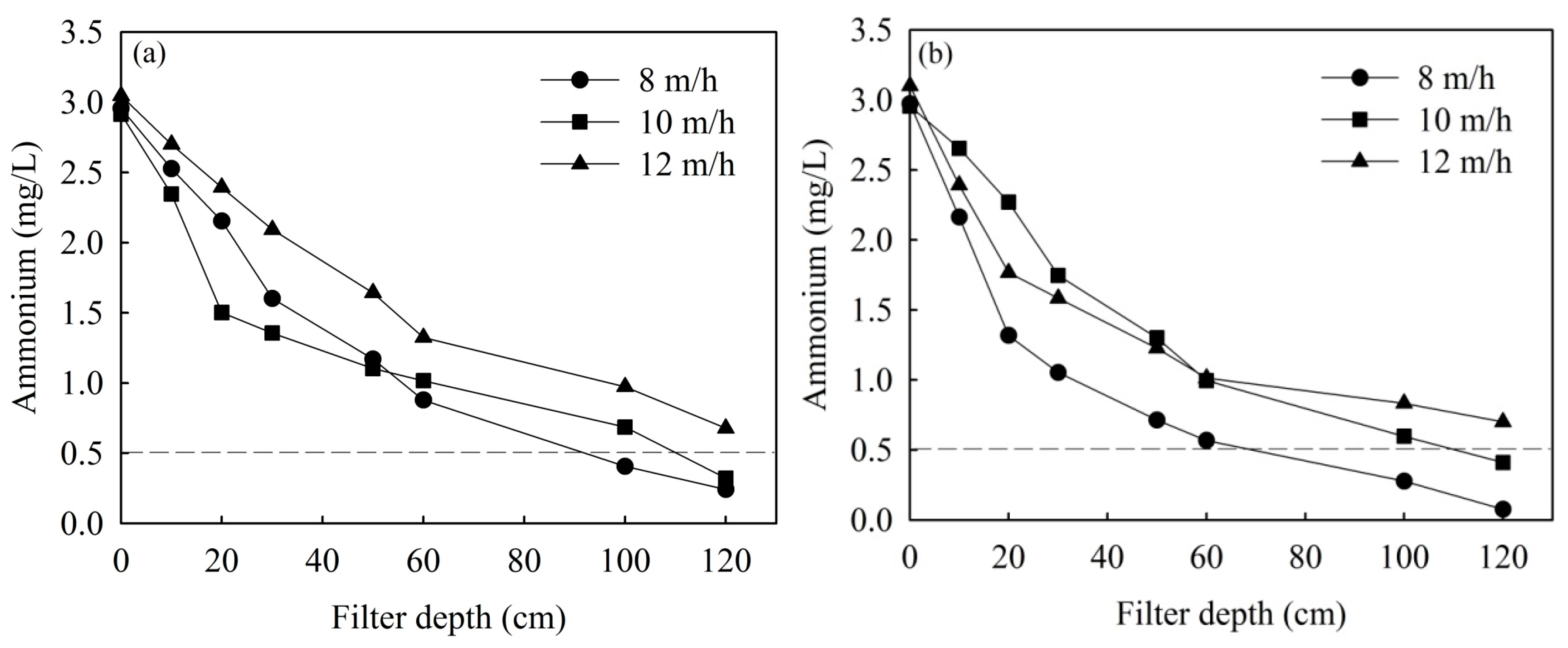
3.3. Effects of Filtration Velocity
3.4. Effects of Aeration on the Turbidity of the Effluent Water
3.5. Effects of Aeration Position
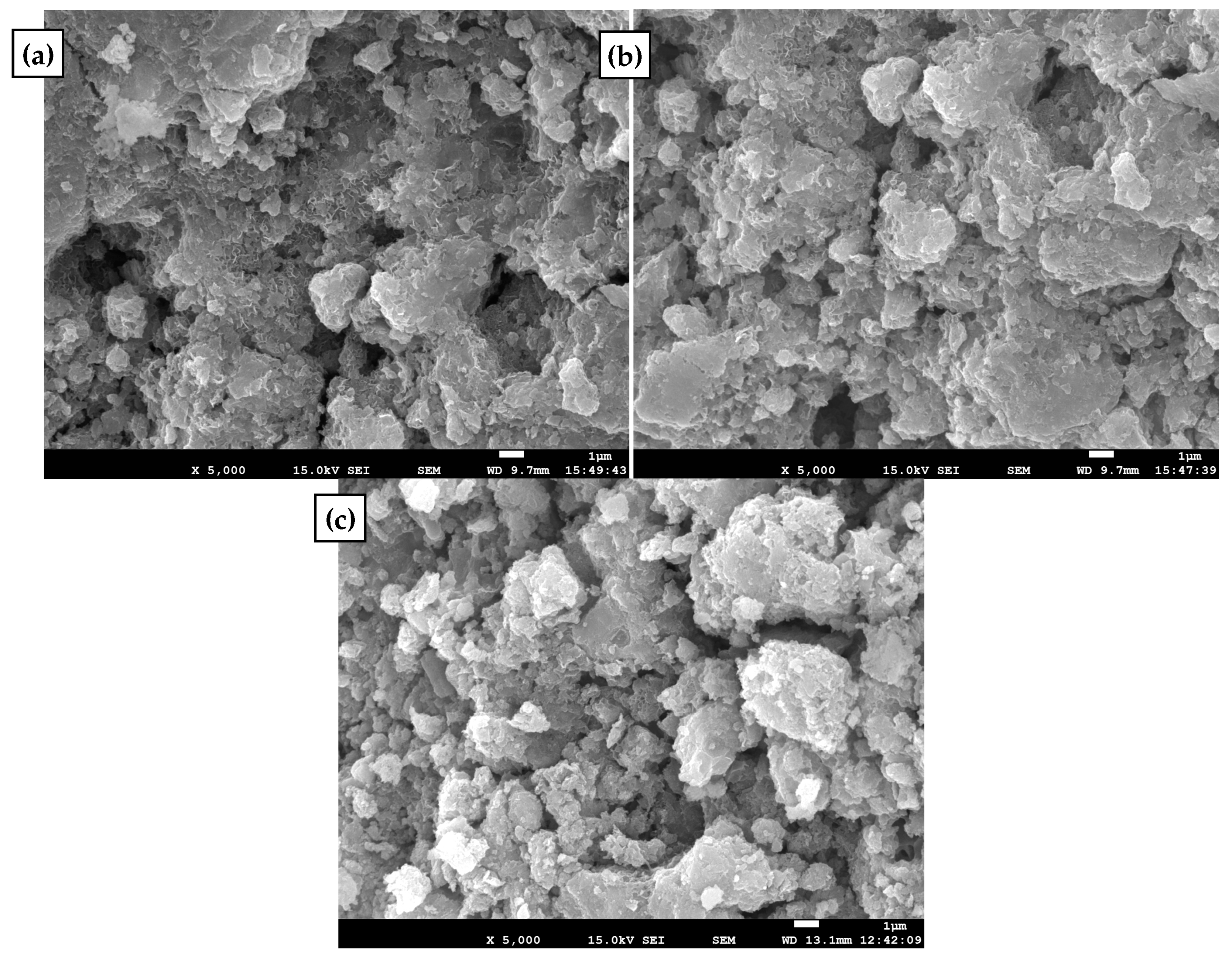
3.6. Ammonium Removal Mechanism
3.7. Comparison with Other Studies
4. Conclusions
- (1)
- The removal efficiency is mainly limited by the content of dissolved oxygen in treated water. By aeration with pure oxygen and compressed air, high concentrations of ammonium can be removed effectively, and the suggested operational flow rates were 100 mL/min compressed air and 40 mL/min pure oxygen.
- (2)
- Compared with aeration from the bottom, aeration at a position of 1/3 of the height of the filter bed can lower the required aeration rates and strengthened turbidity removal.
- (3)
- Pure oxygen and compressed air can provide adequate oxygen without changing the mechanism of ammonium removal. In this process, ammonium was removed mainly by chemical catalytic oxidation.
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ministry of Ecology and Environment of China. Bulletin on China’s Ecological Environment in 2018. Available online: http://www.mee.gov.cn/home/jrtt_1/201905/t20190529_704841.shtml (accessed on 29 May 2019).
- Kooij, D.V.D.; Hijnen, W.A.M.; Kruithof, J.C. The Effects of Ozonation, Biological Filtration and Distribution on the Concentration of Easily Assimilable Organic Carbon (AOC) in Drinking Water. Ozone Sci. Eng. 1989, 11, 297–311. [Google Scholar] [CrossRef]
- Rittmann, B.; Tang, Y.; Meyer, K.; Bellamy, W. Biological Processes. In Water Treatment Plant Design; American Water Works Association: Denver, CO, USA, 2012; Chapter 17. [Google Scholar]
- Choi, J.; Valentine, R.L. Formation of N-nitrosodimethylamine (NDMA) from reaction of monochloramine: A new disinfection by-product. Water Res. 2002, 36, 817–824. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, T.; Wen, G.; Cao, X. The simultaneous removal of ammonium and manganese from groundwater by iron-manganese co-oxide filter film: The role of chemical catalytic oxidation for ammonium removal. Chem. Eng. J. 2016, 308, 322–329. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, T.; Sun, Y.; Shi, X. Catalytic oxidation removal of ammonium from groundwater by manganese oxides filter: Performance and mechanisms. Chem. Eng. J. 2017, 322, 82–89. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, T.; Wen, G.; Chen, Y.; Cao, X.; Zhang, B.; Wang, B. Phosphate dosing to sustain the ammonium removal activity of an iron-manganese co-oxide filter film at pilot scale: Effects on chemical catalytic oxidation. Chem. Eng. J. 2018, 344, 1186–1194. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.; Tian, X.; Yin, Y. Seasonal variation of bacterial community in biological aerated filter for ammonia removal in drinking water treatment. Water Res. 2017, 123, 668–677. [Google Scholar] [CrossRef]
- Wagner, F.B.; Nielsen, P.B.; Boehansen, R.; Albrechtsen, H.J. Copper deficiency can limit nitrification in biological rapid sand filters for drinking water production. Water Res. 2016, 95, 280–288. [Google Scholar] [CrossRef]
- De Vet, W.W.J.M.; Van Loosdrecht, M.C.M.; Rietveld, L.C. Phosphorus limitation in nitrifying groundwater filters. Water Res. 2012, 46, 1061–1069. [Google Scholar] [CrossRef]
- Andersson, A.; Laurent, P.; Kihn, A.; Prévost, M.; Servais, P. Impact of temperature on nitrification in biological activated carbon (BAC) filters used for drinking water treatment. Water Res. 2001, 35, 2923–2934. [Google Scholar] [CrossRef]
- Scheibe, A.; Lins, U.; Imbihl, R. Kinetics of ammonia oxidation on stepped platinum surfaces. I. Experimental results. Surf. Sci. 2005, 577, 1–14. [Google Scholar] [CrossRef]
- Rebrov, E.V.; Croon, M.D.; Schouten, J.C. Development of the kinetic model of platinum catalyzed ammonia oxidation in a microreactor. Chem. Eng. J. 2002, 90, 61–76. [Google Scholar] [CrossRef]
- Liang, L.I.; Liu, Y. Ammonia removal in electrochemical oxidation: Mechanism and pseudo-kinetics. J. Hazard. Mater. 2009, 161, 1010–1016. [Google Scholar]
- Huang, T.-L.; Cao, X.; Zhang, Q.; Su, Z.-M.; Zheng, N. Catalytic oxidation of high-concentration ammonia in groundwater by a naturally formed co-oxide filter film. Desalin. Water Treat. 2014, 52, 1615–1623. [Google Scholar] [CrossRef]
- Li, D.; Cao, R.; Yang, H.; Wang, Y.; Zeng, H.; Zhang, J. Removal mechanism of ammonia nitrogen in bio-purification process for high iron and manganese removal from low temperature groundwater. China Environ. Sci. 2017, 37, 2623–2632. (In Chinese) [Google Scholar]
- Feng, S.; Xie, S.; Zhang, X.; Yang, Z.; Wei, D.; Liao, X.; Liu, Y.; Chen, C. Ammonium removal pathways and microbial community in GAC-sand dual media filter in drinking water treatment. J. Environ. Sci. 2012, 24, 1587–1593. [Google Scholar] [CrossRef]
- Yu, X.; Qi, Z.; Zhang, X.; Yu, P.; Liu, B.; Zhang, L.; Fu, L. Nitrogen loss and oxygen paradox in full-scale biofiltration for drinking water treatment. Water Res. 2007, 41, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.O.; Boe-Hansen, R.; Musovic, S.; Smets, B.; Albrechtsen, H.J.; Binning, P. Effects of dynamic operating conditions on nitrification in biological rapid sand filters for drinking water treatment. Water Res. 2014, 64, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Pearce, P.; Williams, S. A Nitrification Model for Mineral-Media Trickling Filters. Water Environ. J. 2010, 13, 84–92. [Google Scholar] [CrossRef]
- Pearce, P.; Edwards, W. A design model for nitrification on structured cross flow plastic media trickling filters. Water Environ. J. 2011, 25, 257–265. [Google Scholar] [CrossRef]
- SEPA. Analytical Methods of Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- Dong, W.Y.; Wang, H.J.; Li, W.G.; Ying, W.C.; Gan, G.H.; Yang, Y. Effect of DO on simultaneous removal of carbon and nitrogen by a membrane aeration/filtration combined bioreactor. J. Membr. Sci. 2009, 344, 219–224. [Google Scholar] [CrossRef]
- De Vet, W.W.J.M.; Rietveld, L.C.; Van Loosdrecht, M.C.M. Influence of iron on nitrification in full-scale drinking water trickling filters. J. Water Supply. Res. Technol.-AQUA 2009, 58, 247–256. [Google Scholar] [CrossRef]
- Tekerlekopoulou, A.G.; Vayenas, D.V. Ammonia, iron and manganese removal from potable water using trickling filters. Desalination 2007, 210, 225–235. [Google Scholar] [CrossRef]
- Akker, B.V.D.; Holmes, M.; Pearce, P.; Cromar, N.J.; Fallowfield, H.J. Structure of nitrifying biofilms in a high-rate trickling filter designed for potable water pre-treatment. Water Res. 2011, 45, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhao, Z.W.; Gao, W.; Cui, F.Y. Study on the factors affecting simultaneous removal of ammonia and manganese by pilot-scale biological aerated filter (BAF) for drinking water pre-treatment. Bioresour. Technol. 2013, 145, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Vayenas, D.V.; Lyberatos, G. On the design of nitrifying trickling filters for potable water treatment. Water Res. 1995, 29, 1079–1084. [Google Scholar] [CrossRef]
- Tembal, T.; Marki, M.; RibiI, N.A.; BriKi, F.; Sipos, L. Removal of ammonia, iron and manganese from groundwaters of northern Croatia—Pilot plant studies. Process Biochem. 2005, 40, 327–335. [Google Scholar] [CrossRef]
- Hasan, H.A.; Abdullah, S.R.S.; Kamarudin, S.K.; Kofli, N.T. Response surface methodology for optimization of simultaneous COD, NH4+-N and Mn2+ removal from drinking water by biological aerated filter. Desalination 2011, 275, 50–61. [Google Scholar] [CrossRef]
- Kors, L.J.; Moorman, J.H.N.; Wind, A.P.M.; Hoek, J.P.V.D. Nitrification and low temperature in a raw water reservoir and rapid sand filters. Water Sci. Technol. 1998, 37, 169–176. [Google Scholar] [CrossRef]
- Hasan, H.A.; Abdullah, S.R.S.; Kamarudin, S.K.; Kofli, N.T. On–off control of aeration time in the simultaneous removal of ammonia and manganese using a biological aerated filter system. Process Saf. Environ. Prot. 2013, 91, 415–422. [Google Scholar] [CrossRef]
- Boller, M.; Gujer, W. Nitrification in tertiary trickling filters followed by deep-bed filters. Water Res. 1986, 20, 1363–1373. [Google Scholar] [CrossRef]

| Parameters | Units | Values | Standards for Drinking Water Quality China (GB5749-2006) |
|---|---|---|---|
| NH4+-N | (mg/L) | 0.70–1.67 | 0.5 |
| NO3--N | (mg/L) | 0.0–0.3 | 10 |
| Water temperature | (°C) | 17.0–22.0 | / |
| pH | / | 7.50–8.5 | 6.50–8.5 |
| Turbidity | (NTU) | 0.80–1.6 | 1 |
| DO | (mg/L) | 2.00–3.2 | / |
| CODMn | (mg/L) | 1.14–1.47 | 3 |
| Iron | (mg/L) | 0.70–1.1 | 0.3 |
| Manganese | (mg/L) | 1.10–1.56 | 0.1 |
| Elements | wt% | ||
|---|---|---|---|
| Original | Aeration with Pure Oxygen | Aeration with Compressed Air | |
| O | 14.51 | 13.54 | 13.24 |
| Al | 1.12 | 0.72 | 0.72 |
| Ca | 5.21 | 6.14 | 4.92 |
| Mn | 74 | 76.4 | 77.01 |
| Fe | 5.16 | 3.2 | 4.11 |
| Treatment System | Type of Water | Filter Media Types | DO in Effluent (mg/L) | Aeration Types | Temperature (°C) | Ammonium in Influent (mg/L) | Removal Efficiency (%) |
|---|---|---|---|---|---|---|---|
| Pilot-scale filters [This study] | Ground water for drinking water | MeOx-coated on quartz sand | 7.45~8.8 | Aeration from the bottom and the 1/3 of the filter bed | 17~22 | 4.2 | 95.23 |
| Pilot plant with at a pressure of 2 bars [30] | Ground water for drinking water | Quartz sand | 16–17 | Aeration at a pressure of 2 bars | 14.6 | 2.62 | 100 |
| Pilot-scale trickling filters [26] | Surface water for Potable water | Gravel | 7~8 | Natural ventilation | 20 | 2 | 77.3~100 |
| Sequence Batch BAF system [31] | Surface water for Drinking water | Poly-propylene | 4.68 | Aeration form the bottom | / | 9.8 | 98.4 |
| Of the filter | |||||||
| Continuous flow full Scale BAF [9] | Surface water for Drinking water | Lava particles | >7 | Aeration form the bottom of the filter | 8.6~10.8 | 2.90 ± 0.96 | 77.52~92.62 |
| GAC-sand dual-media biofilters [19] | Surface Water for drinking water | GAC and sand | 1.4~7.2 | By aeration tank | / | 0.74~5.3 | 39.77~99.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, R.; Yang, Y.; Huang, T.; Wen, G. Removal of High Concentrations of Ammonium from Groundwater in a Pilot-Scale System through Aeration at the Bottom Layer of a Chemical Catalytic Oxidation Filter. Int. J. Environ. Res. Public Health 2019, 16, 3989. https://doi.org/10.3390/ijerph16203989
Zhang W, Zhang R, Yang Y, Huang T, Wen G. Removal of High Concentrations of Ammonium from Groundwater in a Pilot-Scale System through Aeration at the Bottom Layer of a Chemical Catalytic Oxidation Filter. International Journal of Environmental Research and Public Health. 2019; 16(20):3989. https://doi.org/10.3390/ijerph16203989
Chicago/Turabian StyleZhang, Wushou, Ruifeng Zhang, Yanfeng Yang, Tinglin Huang, and Gang Wen. 2019. "Removal of High Concentrations of Ammonium from Groundwater in a Pilot-Scale System through Aeration at the Bottom Layer of a Chemical Catalytic Oxidation Filter" International Journal of Environmental Research and Public Health 16, no. 20: 3989. https://doi.org/10.3390/ijerph16203989
APA StyleZhang, W., Zhang, R., Yang, Y., Huang, T., & Wen, G. (2019). Removal of High Concentrations of Ammonium from Groundwater in a Pilot-Scale System through Aeration at the Bottom Layer of a Chemical Catalytic Oxidation Filter. International Journal of Environmental Research and Public Health, 16(20), 3989. https://doi.org/10.3390/ijerph16203989





