Effects of Hydrogen Peroxide and Sodium Hypochlorite Aging on Properties and Performance of Polyethersulfone Ultrafiltration Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Agents
2.2. Membranes and Accelerated Aging Procedure
2.3. Evaluation of Membrane Performance
2.4. Characterization of Membrane Properties
3. Results and Discussion
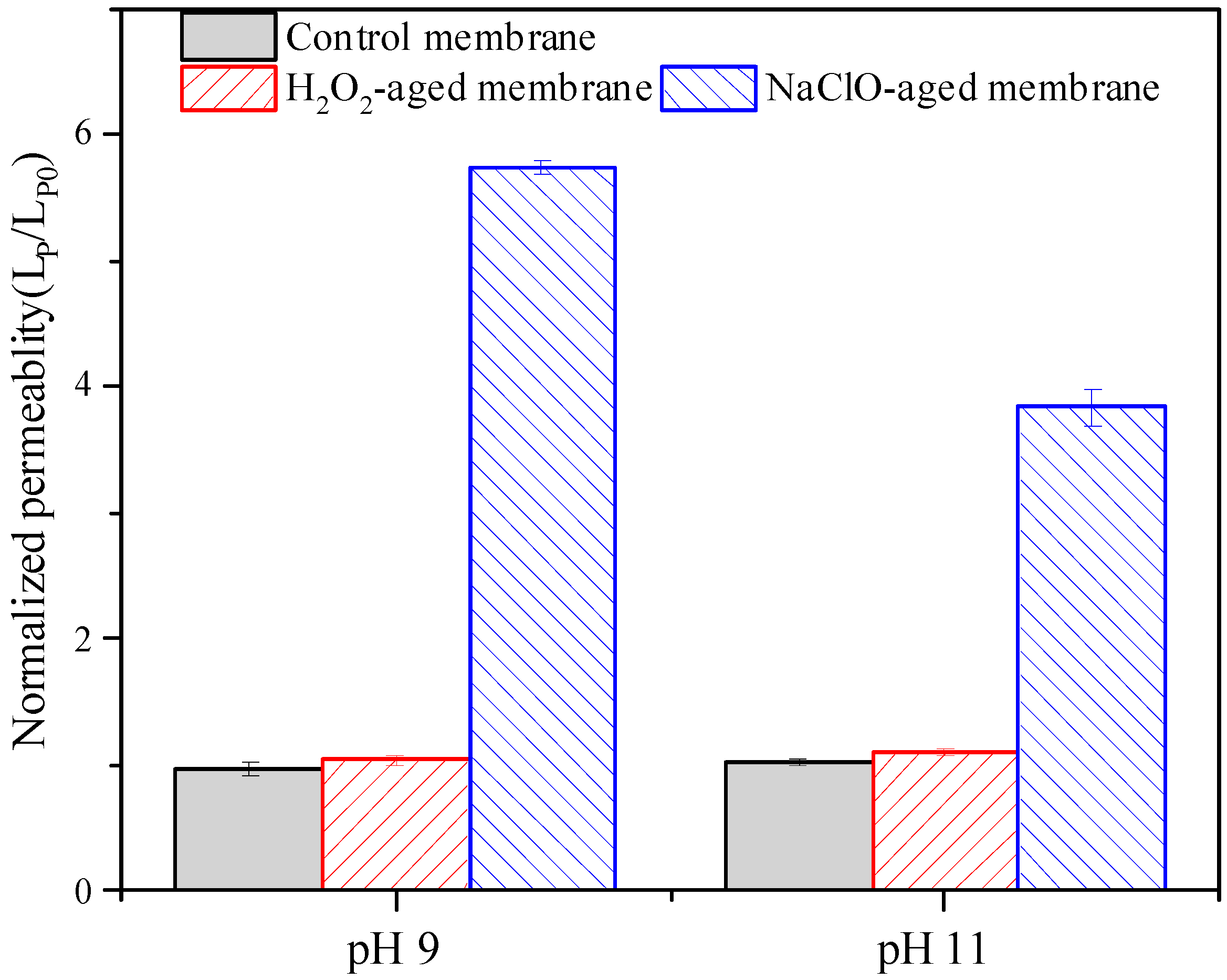
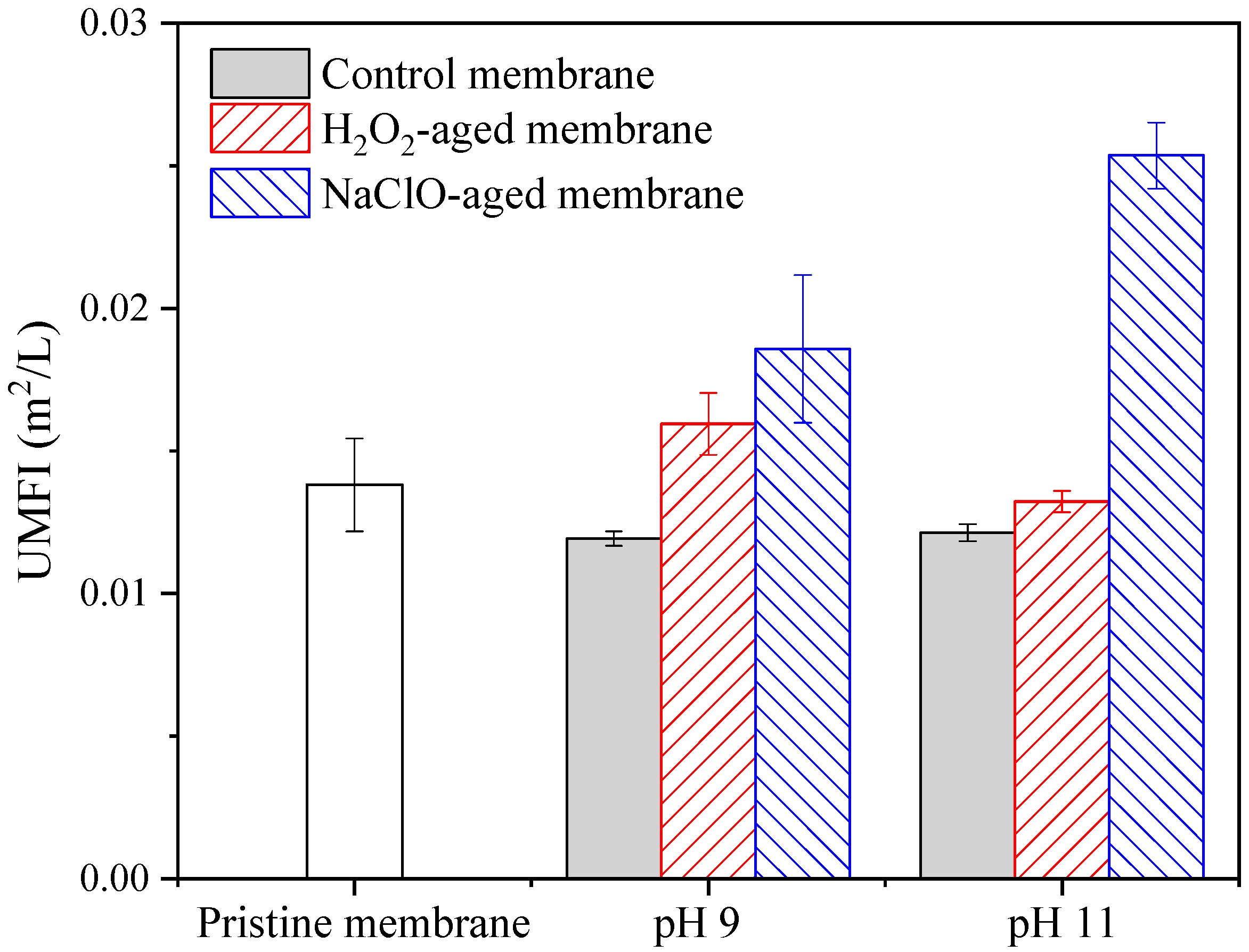
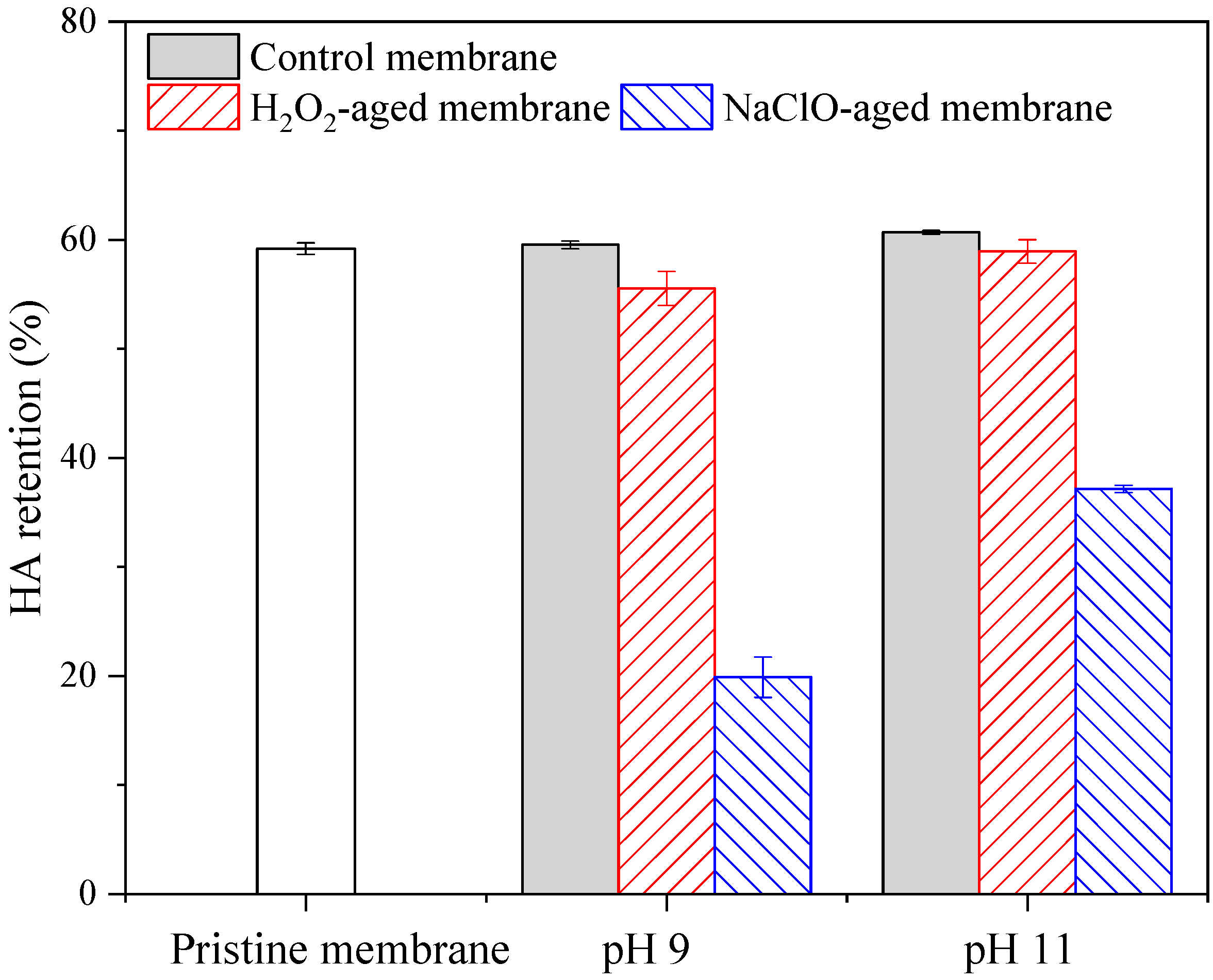
3.1. Filtration Performance of Pristine and Aged Membranes
3.2. Chemical Composition of Pristine and Aged Membranes
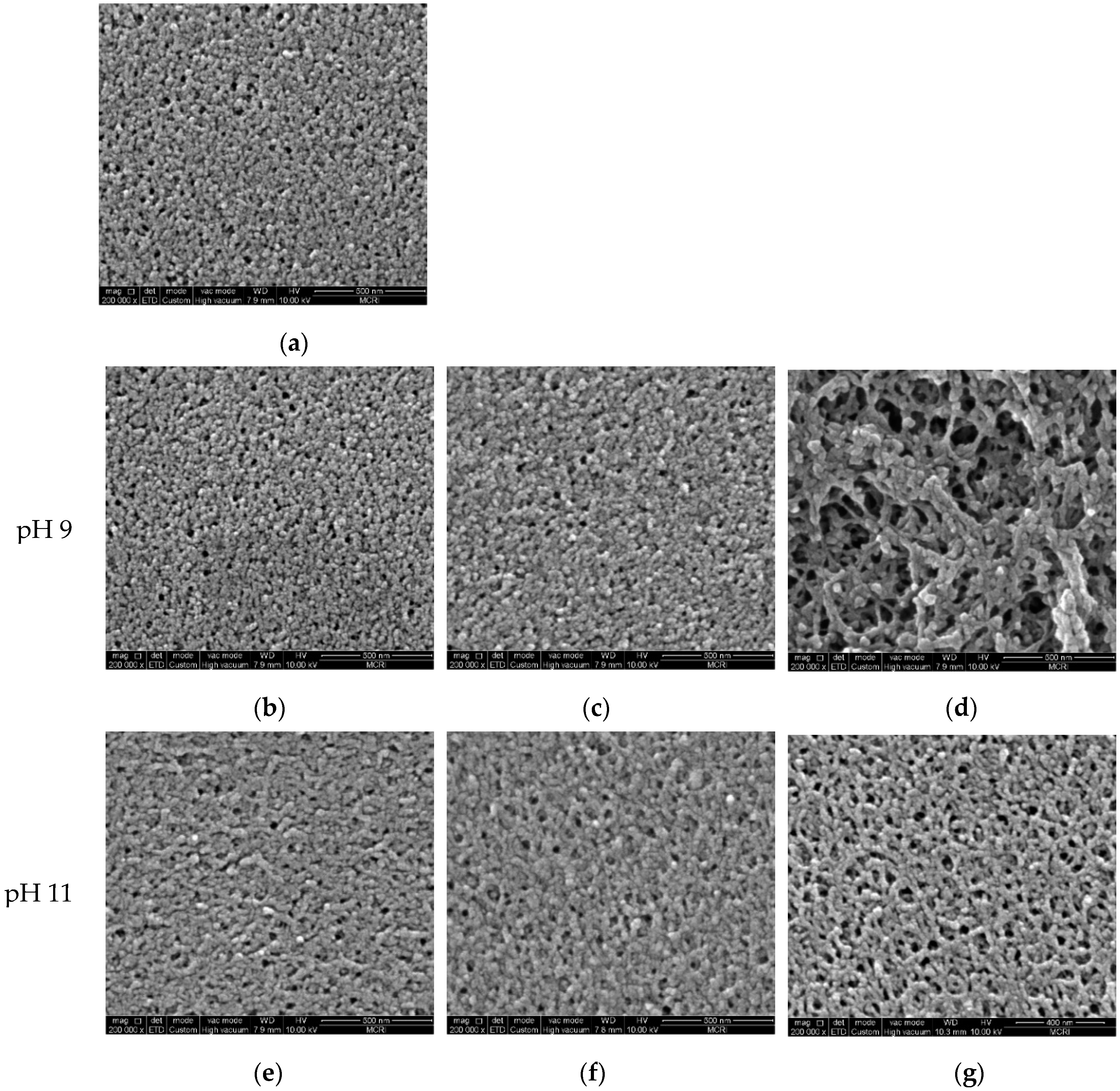
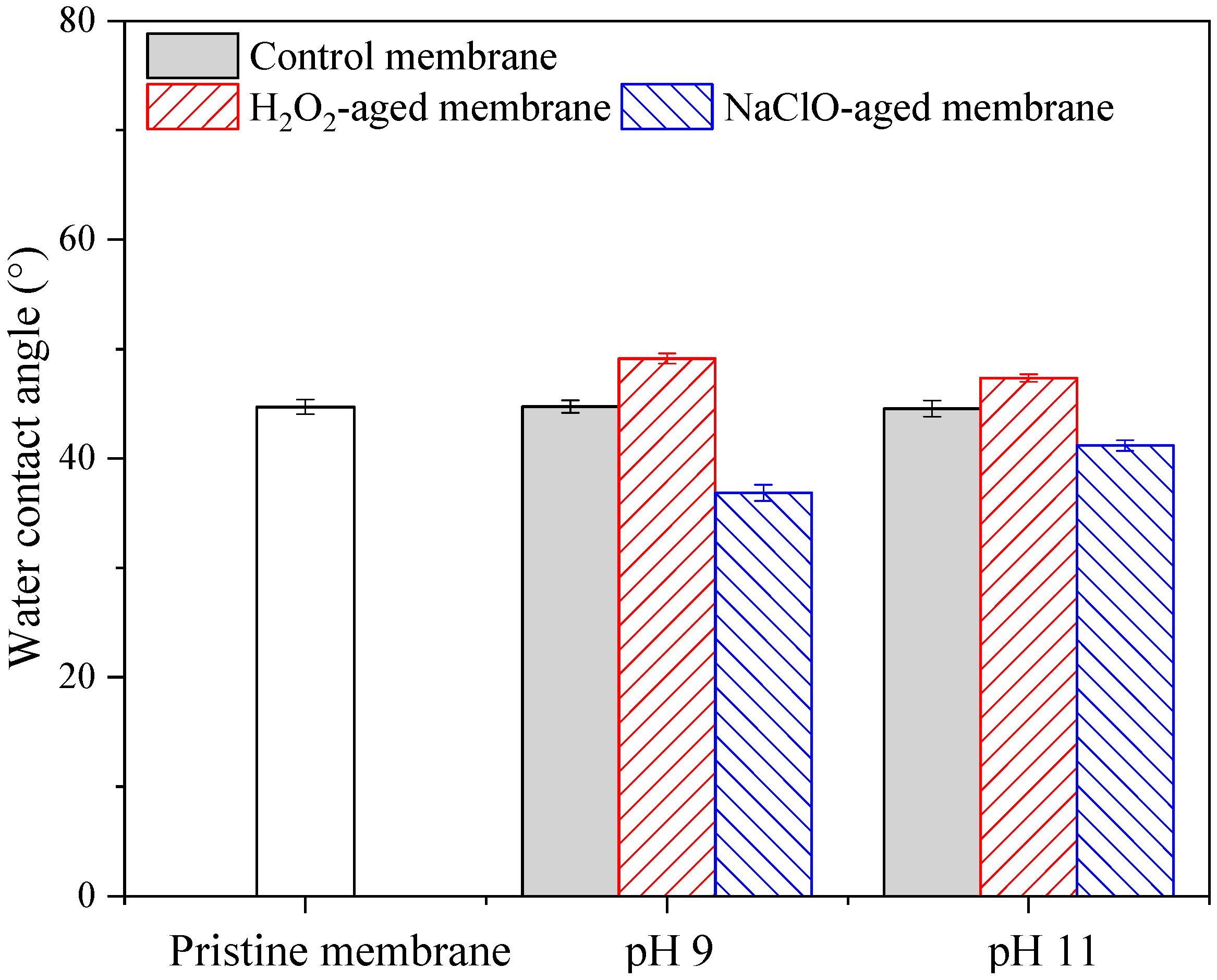
3.3. Morphology and Surface Properties of Pristine and Aged Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, X.; Zhou, W.; Li, P.; Ren, Z.; Wu, D.; Luo, C.; Tang, X.; Wang, J.; Liang, H. Improving ultrafiltration membrane performance with pre-deposited carbon nanotubes/nanofibers layers for drinking water treatment. Chemosphere 2019, 234, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Narbaitz, R.M. Hollow fiber ultrafiltration of Ottawa River water: Floatation versus sedimentation pre-treatment. Chem. Eng. J. 2016, 288, 228–237. [Google Scholar] [CrossRef]
- Shao, S.; Fu, W.; Li, X.; Shi, D.; Jiang, Y.; Li, J.; Gong, T.; Li, X. Membrane fouling by the aggregations formed from oppositely charged organic foulants. Water Res. 2019, 159, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wen, G.; Li, S.; Chang, H.; Shao, S.; Huang, T.; Li, G.; Liang, H. Effect of pre-oxidation on low pressure membrane (LPM) for water and wastewater treatment: A review. Chemosphere 2019, 231, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gesan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Rabuni, M.F.; Nik Sulaiman, N.M.; Aroua, M.K.; Hashim, N.A. Effects of Alkaline Environments at Mild Conditions on the Stability of PVDF Membrane: An Experimental Study. Ind. Eng. Chem. Res. 2013, 52, 15874–15882. [Google Scholar] [CrossRef]
- Alresheedi, M.T.; Basu, O.D.; Barbeau, B. Chemical cleaning of ceramic ultrafiltration membranes - Ozone versus conventional cleaning chemicals. Chemosphere 2019, 226, 668–677. [Google Scholar] [CrossRef]
- Shao, S.; Wang, Y.; Shi, D.; Zhang, X.; Tang, C.Y.; Liu, Z.; Li, J. Biofouling in ultrafiltration process for drinking water treatment and its control by chlorinated-water and pure water backwashing. Sci Total Environ 2018, 644, 306–314. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Halogenated organics generated during online chemical cleaning of MBR: An emerging threat to water supply and public health. Sci. Total Environ. 2019, 656, 547–549. [Google Scholar] [CrossRef]
- Robinson, S.; Abdullah, S.Z.; Bérubé, P.; Le-Clech, P. Ageing of membranes for water treatment: Linking changes to performance. J. Membr. Sci. 2016, 503, 177–187. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Velizarov, S.; Van der Bruggen, B. Stability of polyethersulfone membranes to oxidative agents: A review. Polym. Degrad. Stabil. 2018, 157, 15–33. [Google Scholar] [CrossRef]
- Kourde-Hanafi, Y.; Loulergue, P.; Szymczyk, A.; Van der Bruggen, B.; Nachtnebel, M.; Rabiller-Baudry, M.; Audic, J.-L.; Pölt, P.; Baddari, K. Influence of PVP content on degradation of PES/PVP membranes: Insights from characterization of membranes with controlled composition. J. Membr. Sci. 2017, 533, 261–269. [Google Scholar] [CrossRef]
- Hanafi, Y.; Loulergue, P.; Ababou-Girard, S.; Meriadec, C.; Rabiller-Baudry, M.; Baddari, K.; Szymczyk, A. Electrokinetic analysis of PES/PVP membranes aged by sodium hypochlorite solutions at different pH. J. Membr. Sci. 2016, 501, 24–32. [Google Scholar] [CrossRef]
- Prulho, R.; Therias, S.; Rivaton, A.; Gardette, J.-L. Ageing of polyethersulfone/polyvinylpyrrolidone blends in contact with bleach water. Polym. Degrad. Stabil. 2013, 98, 1164–1172. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, G.; Xiong, Y.; Zhou, M.; Zhang, S.; Tang, C.Y.; Meng, F. Unveiling the Susceptibility of Functional Groups of Poly(ether sulfone)/Polyvinylpyrrolidone Membranes to NaOCl: A Two-Dimensional Correlation Spectroscopic Study. Environ. Sci. Technol. 2017, 51, 14342–14351. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, B.; Prulho, R.; Rivaton, A.; Thérias, S.; Gardette, J.-L.; Gaudichet-Maurin, E.; Causserand, C. Multi-scale analysis of hypochlorite induced PES/PVP ultrafiltration membranes degradation. J. Membr. Sci. 2013, 447, 287–296. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Huang, T.; Dong, C.; Li, J.; Zhao, B.; Zhang, S. Chemical Cleaning of Ultrafiltration Membrane Fouled by Humic Substances: Comparison between Hydrogen Peroxide and Sodium Hypochlorite. Int. J. Environ. Res. Public Health 2019, 16, 2568. [Google Scholar] [CrossRef]
- Zou, J.; Cai, H.; Wang, D.; Xiao, J.; Zhou, Z.; Yuan, B. Spectrophotometric determination of trace hydrogen peroxide via the oxidative coloration of DPD using a Fenton system. Chemosphere 2019, 224, 646–652. [Google Scholar] [CrossRef]
- Wang, X.; Hu, T.; Wang, Z.; Li, X.; Ren, Y. Permeability recovery of fouled forward osmosis membranes by chemical cleaning during a long-term operation of anaerobic osmotic membrane bioreactors treating low-strength wastewater. Water Res. 2017, 123, 505–512. [Google Scholar] [CrossRef]
- Strugholtz, S.; Sundaramoorthy, K.; Panglisch, S.; Lerch, A.; Brügger, A.; Gimbel, R. Evaluation of the performance of different chemicals for cleaning capillary membranes. Desalination 2005, 179, 191–202. [Google Scholar] [CrossRef]
- Ling, R.; Yu, L.; Pham, T.P.T.; Shao, J.; Chen, J.P.; Reinhard, M. The tolerance of a thin-film composite polyamide reverse osmosis membrane to hydrogen peroxide exposure. J. Membr. Sci. 2017, 524, 529–536. [Google Scholar] [CrossRef]
- Yu, L.; Ling, R.; Chen, J.P.; Reinhard, M. Quantitative assessment of the iron-catalyzed degradation of a polyamide nanofiltration membrane by hydrogen peroxide. J. Membr. Sci. 2019, 588. [Google Scholar] [CrossRef]
- Yu, W.; Graham, N.; Liu, T. Prevention of UF membrane fouling in drinking water treatment by addition of H2O2 during membrane backwashing. Water Res. 2019, 149, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, X.; Cao, G. Impacts of sodium hydroxide and sodium hypochlorite aging on polyvinylidene fluoride membranes fabricated with different methods. J. Environ. Sci. 2018, 67, 294–308. [Google Scholar] [CrossRef]
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. H2O2 determination by the I3− method and by KMnO4 titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Yadav, K.; Morison, K.; Staiger, M.P. Effects of hypochlorite treatment on the surface morphology and mechanical properties of polyethersulfone ultrafiltration membranes. Polym. Degrad. Stabil. 2009, 94, 1955–1961. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: A review. Sep. Purif. Technol. 2010, 71, 137–143. [Google Scholar] [CrossRef]
- Wang, X.; Ma, J.; Wang, Z.; Chen, H.; Liu, M.; Wu, Z. Reinvestigation of membrane cleaning mechanisms using NaOCl: Role of reagent diffusion. J. Membr. Sci. 2018, 550, 278–285. [Google Scholar] [CrossRef]
- Li, K.; Liang, H.; Qu, F.; Shao, S.; Yu, H.; Han, Z.-s.; Du, X.; Li, G. Control of natural organic matter fouling of ultrafiltration membrane by adsorption pretreatment: Comparison of mesoporous adsorbent resin and powdered activated carbon. J. Membr. Sci. 2014, 471, 94–102. [Google Scholar] [CrossRef]
- Huang, H.; Young, T.A.; Jacangelo, J.G. Unified Membrane Fouling Index for Low Pressure Membrane Filtration of Natural Waters: Principles and Methodology. Environ. Sci. Technol. 2008, 42, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Malczewska, B.; Zak, A. Structural Changes and Operational Deterioration of the Uf Polyethersulfone (Pes) Membrane Due to Chemical Cleaning. Sci. Rep. 2019, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Arkhangelsky, E.; Kuzmenko, D.; Gitis, V. Impact of chemical cleaning on properties and functioning of polyethersulfone membranes. J. Membr. Sci. 2007, 305, 176–184. [Google Scholar] [CrossRef]
- Causserand, C.; Pellegrin, B.; Rouch, J.-C. Effects of sodium hypochlorite exposure mode on PES/PVP ultrafiltration membrane degradation. Water Res. 2015, 85, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; da Silva, C.A.; Fogo, F.C.; Pineda, E.A.G.; Hechenleitner, A.A.W. Thermal and FTIR study of polyvinylpyrrolidone/lignin blends. J. Therm. Anal. Calorim. 2005, 79, 367–370. [Google Scholar] [CrossRef]
- Hajibabania, S.; Antony, A.; Leslie, G.; Le-Clech, P. Relative impact of fouling and cleaning on PVDF membrane hydraulic performances. Sep. Purif. Technol. 2012, 90, 204–212. [Google Scholar] [CrossRef]
- Rabiller-Baudry, M.; Bouzin, A.; Hallery, C.; Girard, J.; Leperoux, C. Evidencing the chemical degradation of a hydrophilised PES ultrafiltration membrane despite protein fouling. Sep. Purif. Technol. 2015, 147, 62–81. [Google Scholar] [CrossRef]
- Touffet, A.; Baron, J.; Welte, B.; Joyeux, M.; Teychene, B.; Gallard, H. Impact of pretreatment conditions and chemical ageing on ultrafiltration membrane performances. Diagnostic of a coagulation/adsorption/filtration process. J. Membr. Sci. 2015, 489, 284–291. [Google Scholar] [CrossRef]
- Wienk, I.; Meuleman, E.; Borneman, Z.; Boomgaard, T.; Smolders, C. Chemical treatment of membranes of a polymer blend: Mechanism of the reaction of hypochlorite with poly (vinyl pyrrolidone). J. Polym. Sci. Pol. Chem. 1995, 33, 49–54. [Google Scholar] [CrossRef]
- Hanafi, Y.; Szymczyk, A.; Rabiller-Baudry, M.; Baddari, K. Degradation of Poly(Ether Sulfone)/Polyvinylpyrrolidone Membranes by Sodium Hypochlorite: Insight from Advanced Electrokinetic Characterizations. Environ. Sci. Technol. 2014, 48, 13419–13426. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Gao, F.; Chen, Y.; Zhang, H. A comparison study: The different impacts of sodium hypochlorite on PVDF and PSF ultrafiltration (UF) membranes. Water Res. 2017, 109, 227–236. [Google Scholar] [CrossRef] [PubMed]


| Element. | Pristine | pH 9 | pH 11 | ||||
|---|---|---|---|---|---|---|---|
| Control | H2O2-Aged | NaClO-Aged | Control | H2O2-Aged | NaClO-Aged | ||
| C | 73.72 | 74.47 | 73.66 | 71.15 | 74.57 | 74.51 | 72.49 |
| N | 4.14 | 4.57 | 3.27 | 2.40 | 4.30 | 3.80 | 2.59 |
| O | 17.65 | 16.65 | 17.71 | 18.88 | 16.35 | 16.65 | 18.51 |
| S | 4.50 | 4.31 | 5.35 | 6.26 | 4.79 | 5.03 | 5.72 |
| Cl | - | - | - | 1.30 | - | - | 0.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, S.; Su, Q.; Wen, G.; Huang, T. Effects of Hydrogen Peroxide and Sodium Hypochlorite Aging on Properties and Performance of Polyethersulfone Ultrafiltration Membrane. Int. J. Environ. Res. Public Health 2019, 16, 3972. https://doi.org/10.3390/ijerph16203972
Li K, Li S, Su Q, Wen G, Huang T. Effects of Hydrogen Peroxide and Sodium Hypochlorite Aging on Properties and Performance of Polyethersulfone Ultrafiltration Membrane. International Journal of Environmental Research and Public Health. 2019; 16(20):3972. https://doi.org/10.3390/ijerph16203972
Chicago/Turabian StyleLi, Kai, Shu Li, Qian Su, Gang Wen, and Tinglin Huang. 2019. "Effects of Hydrogen Peroxide and Sodium Hypochlorite Aging on Properties and Performance of Polyethersulfone Ultrafiltration Membrane" International Journal of Environmental Research and Public Health 16, no. 20: 3972. https://doi.org/10.3390/ijerph16203972
APA StyleLi, K., Li, S., Su, Q., Wen, G., & Huang, T. (2019). Effects of Hydrogen Peroxide and Sodium Hypochlorite Aging on Properties and Performance of Polyethersulfone Ultrafiltration Membrane. International Journal of Environmental Research and Public Health, 16(20), 3972. https://doi.org/10.3390/ijerph16203972






