A Comparative Analysis of Risk Perception and Coping Behaviors among Chinese Poultry Farmers Regarding Human and Poultry Infection with Avian Influenza
Abstract
1. Introduction
2. Methods
2.1. Study Location and Sampling
2.2. Ethics, Consent, and Permission
2.3. Study Instrument
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Familiarity (poultry infection) | Familiarity (1–5 gradually increase) | ||||
| 1 Familiarity of occurrence reason to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 2 Familiarity of route of transmission and infectivity to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 3 Familiarity of cure rate to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 4 Familiarity of prevention measure to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 5 Familiarity of death rate to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 6 Familiarity of overall feelings about poultry infection | □1 | □2 | □3 | □4 | □5 |
| Controllability (poultry infection) | Controllability (1–5 gradually increase) | ||||
| 1 Controllability of poultry infection | □1 | □2 | □3 | □4 | □5 |
| 2 Controllability of route of transmission and infectivity to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 3 Controllability of cure rate to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 4 Controllability of prevention measure to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 5 Controllability of death rate to poultry infection | □1 | □2 | □3 | □4 | □5 |
| 6 Controllability of overall feelings about poultry infection | □1 | □2 | □3 | □4 | □5 |
| Familiarity (human infection) | Familiarity (1–5 gradual increase) | ||||
| 1 Familiarity of occurrence reason to human infection | □1 | □2 | □3 | □4 | □5 |
| 2 Familiarity of route of transmission and infectivity to human infection | □1 | □2 | □3 | □4 | □5 |
| 3 Familiarity of cure rate to human infection | □1 | □2 | □3 | □4 | □5 |
| 4 Familiarity of prevention measure to human infection | □1 | □2 | □3 | □4 | □5 |
| 5 Familiarity of death rate to human infection | □1 | □2 | □3 | □4 | □5 |
| 6 Familiarity of overall feelings about human infection | □1 | □2 | □3 | □4 | □5 |
| Controllability (human infection) | Controllability (1–5 gradual increase) | ||||
| 1 Controllability of human infection occurrence | □1 | □2 | □3 | □4 | □5 |
| 2 Controllability of route of transmission and infectivity to human infection | □1 | □2 | □3 | □4 | □5 |
| 3 Controllability of cure rate to human infection | □1 | □2 | □3 | □4 | □5 |
| 4 Controllability of prevention measure to human infection | □1 | □2 | □3 | □4 | □5 |
| 5 Controllability of death rate to human infection | □1 | □2 | □3 | □4 | □5 |
| 6 Controllability of overall feelings about human infection | □1 | □2 | □3 | □4 | □5 |
| Preventive Measures | Never Adopted | Sometimes Adopted | Often Adopted | Usually Adopted | Adopted All the Time |
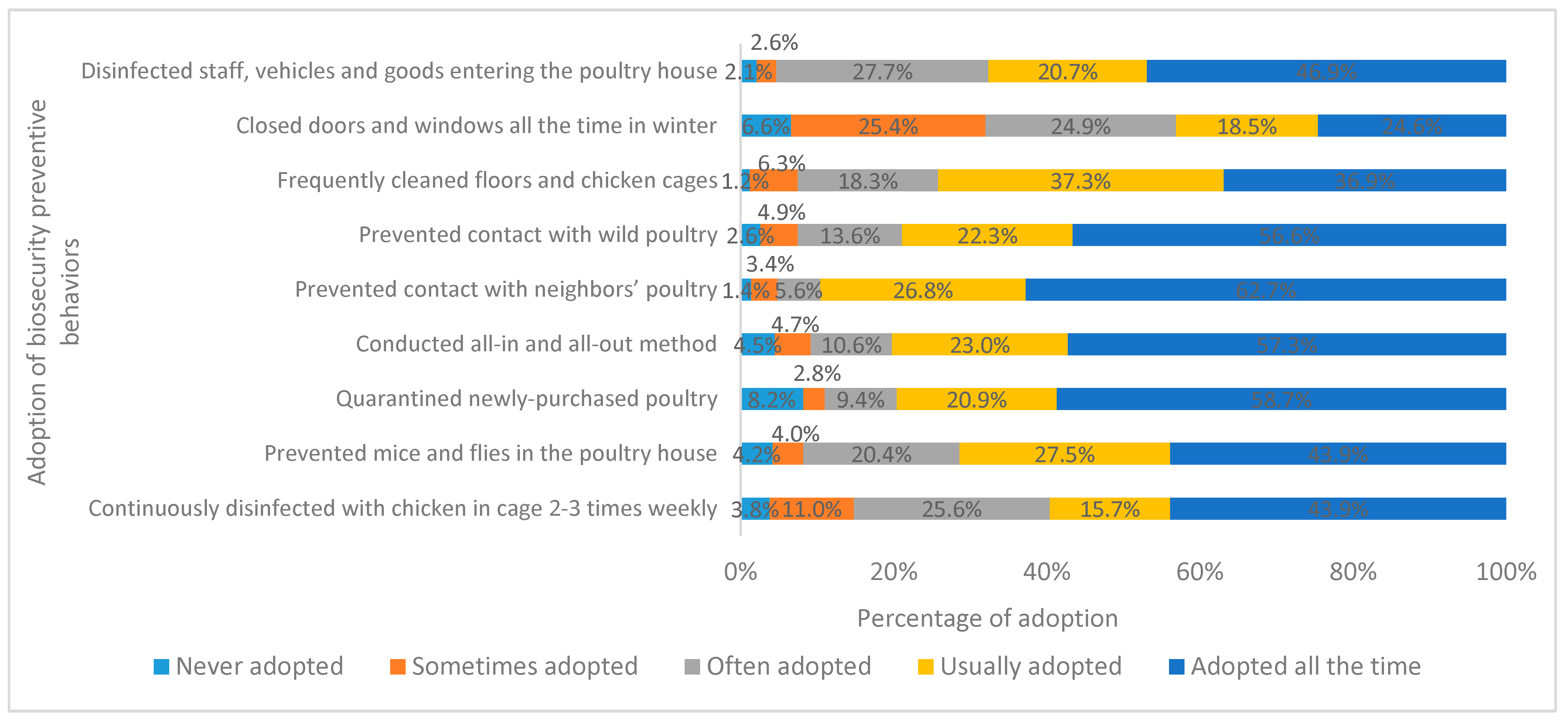
| 1 Closed doors and windows all the time in winter | □ | □ | □ | □ | □ |
| 2 Prevented mice and flies in the poultry house | □ | □ | □ | □ | □ |
| 3 Conducted all-in and all-out method | □ | □ | □ | □ | □ |
| 4 Prevented contact with neighbors’ poultry | □ | □ | □ | □ | □ |
| 5 Prevented contact with wild poultry | □ | □ | □ | □ | □ |
| 6 Frequently cleaned floors and chicken cages | □ | □ | □ | □ | □ |
| 7 Disinfected staff, vehicles, and goods entering the poultry house | □ | □ | □ | □ | □ |
| 8 Quarantined newly-purchased poultry | □ | □ | □ | □ | □ |
| 9 Continuously disinfected with chicken in cage 2–3 times weekly | □ | □ | □ | □ | □ |
| Preventive Measures | Never Adopted | Sometimes Adopted | Often Adopted | Usually Adopted | Adopted All the Time |
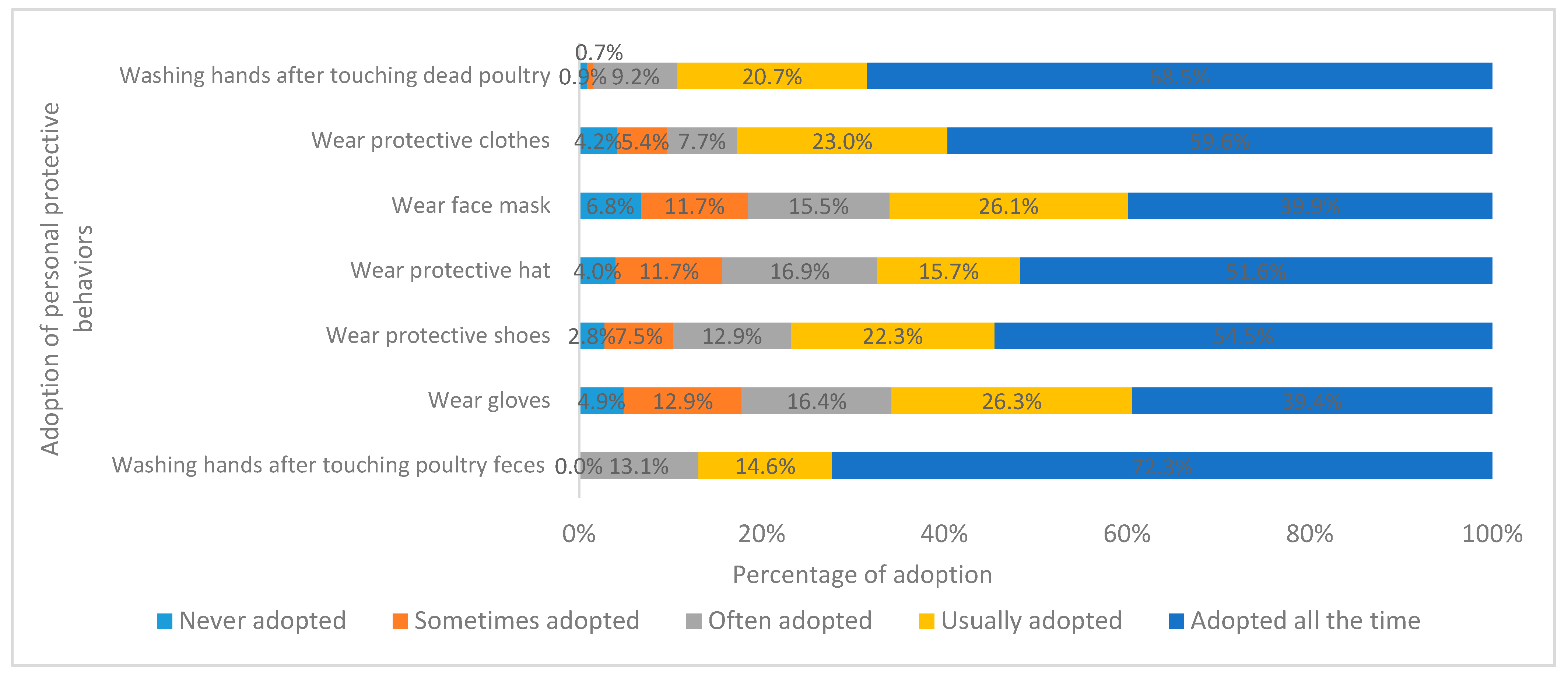
| 1 Wear gloves | □ | □ | □ | □ | □ |
| 2 Wear protective clothes | □ | □ | □ | □ | □ |
| 3 Wear face mask | □ | □ | □ | □ | □ |
| 4 Wear protective hat | □ | □ | □ | □ | □ |
| 5 Wear protective shoes | □ | □ | □ | □ | □ |
| 6 Washing hands after touching dead poultry | □ | □ | □ | □ | □ |
| 7 Washing hands after touching poultry feces | □ | □ | □ | □ | □ |
References
- OIE. Avian Influenza “at a Glance”. Available online: http://www.oie.int/en/animal-health-in-the-world/web-portal-on-avian-influenza/#oe_mainContent (accessed on 3 May 2018).
- OIE. Update on Avian Influenza in Animals (Types H5 and H7). Available online: http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza (accessed on 31 March 2018).
- WHO. Human Infection with Avian Influenza A (H7N9) Virus—China: Update. Available online: https://www.who.int/csr/don/05-september-2018-ah7n9-china/en/ (accessed on 5 September 2018).
- Yun, X.; Song, Y. China Animal Industry Yearbook 2017; China Agriculture Press Publishing: Beijing, China, 2017; pp. 203–204. [Google Scholar]
- Yang, P.; Ma, C.; Cui, S.; Zhang, D.; Shi, W.; Pan, Y.; Sun, Y.; Lu, G.; Peng, X.; Zhao, J.; et al. Avian influenza A(H7N9) and (H5N1) infections among poultry and swine workers and the general population in Beijing, China, 2013–2015. Sci. Rep. 2016, 6, 33877. [Google Scholar] [CrossRef] [PubMed]
- Van Kerkhove, M.D. Brief literature review for the WHO global influenza research agenda—highly pathogenic avian influenza H5N1 risk in humans. Influenza Other Respir. Viruses 2013, 7 (Suppl. 2), 26–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, L.; Zhou, M.; Chen, Z.; Li, F.; Wu, H.; Xiang, N.; Chen, E.; Tang, F.; Wang, D.; et al. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N. Engl. J. Med. 2014, 370, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zeng, D.; Wang, J. Factors affecting Chinese broiler farmers’ main preventive practices in response to highly pathogenic avian influenza. Prev. Vet. Med. 2016, 134, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Tiongco, M.; Narrod, C.; Scott, R.; Kobayashi, M.; Omiti, J. Understanding Knowledge, Attitude, Perceptions, and Practices for HPAI Risks and Management Options Among Kenyan Poultry Producers. In Health and Animal Agriculture in Developing Countries; Zilberman, D., Otte, J., Roland-Holst, D., Pfeiffer, D., Eds.; Springer: New York, NY, USA, 2012; pp. 281–304. [Google Scholar]
- Cui, B.; Liu, Z.P. Determinants of Knowledge and Biosecurity Preventive Behaviors for Highly Pathogenic Avian Influenza Risk Among Chinese Poultry Farmers. Avian Dis. 2016, 60, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Neupane, D.; Khanal, V.; Ghimire, K.; Aro, A.R.; Leppin, A. Knowledge, attitudes and practices related to avian influenza among poultry workers in Nepal: A cross sectional study. BMC Infect. Dis. 2012, 12, 76. [Google Scholar] [CrossRef]
- Ma, X.; Liao, Q.; Yuan, J.; Liu, Y.; Liu, Y.; Chen, J.; Liu, J.; Cai, W.; Cowling, B.J.; Di, B. Knowledge, attitudes and practices relating to influenza A(H7N9) risk among live poultry traders in Guangzhou City, China. BMC Infect. Dis. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Cui, B.; Liao, Q.Y.; Lam, W.W.T.; Liu, Z.P.; Fielding, R. Avian influenza A/H7N9 risk perception, information trust and adoption of protective behaviours among poultry farmers in Jiangsu Province, China. BMC Public Health 2017, 17, 463. [Google Scholar] [CrossRef]
- Martin, V.; Pfeiffer, D.U.; Zhou, X.; Xiao, X.; Prosser, D.J.; Guo, F.; Gilbert, M. Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China. PLoS Pathog. 2011, 7, e1001308. [Google Scholar] [CrossRef]
- Li, X.-L.; Liu, K.; Yao, H.-W.; Sun, Y.; Chen, W.-J.; Sun, R.-X.; de Vlas, S.J.; Fang, L.-Q.; Cao, W.-C. Highly Pathogenic Avian Influenza H5N1 in Mainland China. Int. J. Environ. Res. Public Health 2015, 12, 5026–5045. [Google Scholar] [CrossRef]
- Kuchipudi, S.V.; Tellabati, M.; Sebastian, S.; Londt, B.Z.; Jansen, C.; Vervelde, L.; Brookes, S.M.; Brown, I.H.; Dunham, S.P.; Chang, K.C. Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses. Vet. Res. 2014, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Slovic, P. Perception of risk. Science 1987, 236, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity measures for backyard poultry in developing countries: A systematic review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Metras, R.; Stevens, K.B.; Abdu, P.; Okike, I.; Randolph, T.; Grace, D.; Pfeiffer, D.U.; Costard, S. Identification of potential risk factors associated with highly pathogenic avian influenza subtype H5N1 outbreak occurrence in Lagos and Kano States, Nigeria, during the 2006–2007 epidemics. Transbound. Emerg. Dis. 2013, 60, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.; Bethmont, A.; Chughtai, A.A.; Gardner, L.; Sarkar, S.; Hassan, S.; Seale, H.; MacIntyre, C.R. A Systematic Review of the Comparative Epidemiology of Avian and Human Influenza A H5N1 and H7N9-Lessons and Unanswered Questions. Transbound. Emerg. Dis. 2016, 63, 602–620. [Google Scholar] [CrossRef] [PubMed]
- Goldizen, F.C. From SARS to Avian Influenza: The Role of International Factors in China’s Approach to Infectious Disease Control. Ann. Glob. Health 2016, 82, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Vong, S.; O’Leary, M.; Feng, Z. Early response to the emergence of influenza A(H7N9) virus in humans in China: The central role of prompt information sharing and public communication. Bull. World Health Organ. 2014, 92, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xie, R.; Yang, C.; Frost, M. Perceptions on the risk communication strategy during the 2013 avian influenza A/H7N9 outbreak in humans in China: A focus group study. Western Pac. Surveill. Response 2016, 7, 21–28. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, W.; Yang, S.; Wu, N.; Gao, H.; Sheng, J.; Yao, H.; Wo, J.; Fang, Q.; Cui, D.; et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: Clinical analysis and characterisation of viral genome. Lancet 2013, 381, 1916–1925. [Google Scholar] [CrossRef]
- Song, P.; Xia, J.; Gao, J.; Xu, L.; Huang, Y.; Yao, L.; Tang, W. Measures to combat H7N9 virus infection in China: Live poultry purchasing habits, poultry handling, and living conditions increase the risk of exposure to contaminated environments. Biosci. Trends 2013, 7, 168–171. [Google Scholar] [CrossRef][Green Version]
- Lee, S.S.; Wong, N.S.; Leung, C.C. Exposure to avian influenza H7N9 in farms and wet markets. Lancet 2013, 381, 1815. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.T.; Cowling, B.J.; Liao, Q.; Fang, V.J.; Zhou, S.; Wu, P.; Zhou, H.; Lau, E.H.Y.; Guo, D.; et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: An ecological study. Lancet 2014, 383, 541–548. [Google Scholar] [CrossRef]
- Cui, B.; Liu, Z.P. Factors Associated with Knowledge and Personal Protective Behavior Related to Avian Influenza A (H7N9) Risk among Chinese Poultry Farmers. Popul Health Manag 2016, 19, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.W. A protection motivation theory of fear appeals and attitude change. J. Psychol. 1975, 91, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, I.M. Historical origins of the health belief model. Health Educ. Monogr. 1974, 2, 328–335. [Google Scholar] [CrossRef]
- Jaeger, C.; Renn, O.; Rosa, E.; Webler, T. Risk and Rational Action; Earthscan: London, UK, 2002; pp. 73–113. [Google Scholar]
| Characteristics | N | % |
|---|---|---|
| Gender | ||
| Female | 99 | 23.2 |
| Male | 327 | 76.8 |
| Age | ||
| ≤35 years | 11 | 2.6 |
| 36–45 years | 112 | 26.3 |
| 46–55 years | 180 | 42.3 |
| ≥56 years | 123 | 28.9 |
| Education | ||
| Primary or below | 129 | 30.3 |
| Junior high school | 213 | 50.0 |
| Senior high school | 77 | 18.1 |
| Three-year college | 5 | 1.2 |
| Undergraduate college or above | 2 | 0.5 |
| Years raising poultry | ||
| ≤5 years | 128 | 30.0 |
| 6–10 years | 151 | 35.4 |
| 11–15 years | 69 | 16.2 |
| 16–20 years | 37 | 8.7 |
| 21–25 years | 21 | 4.9 |
| 26–30 years | 18 | 4.2 |
| ≥31 years | 2 | 0.5 |
| Experience with previous infection | ||
| Previous A/H5N1 chicken infection | 107 | 25.1 |
| No previous A/H5N1 chicken infection | 319 | 74.9 |
| Farming scale | ||
| ≤300 chickens | 38 | 8.9 |
| 301–1000 chickens | 54 | 12.7 |
| 1001–10,000 chickens | 241 | 56.6 |
| ≥10,001 chickens | 93 | 21.8 |
| Paired Sample (Poultry Infection—Human Infection) | Paired Differences | P Value | |
|---|---|---|---|
| Mean | Standard Deviation | ||
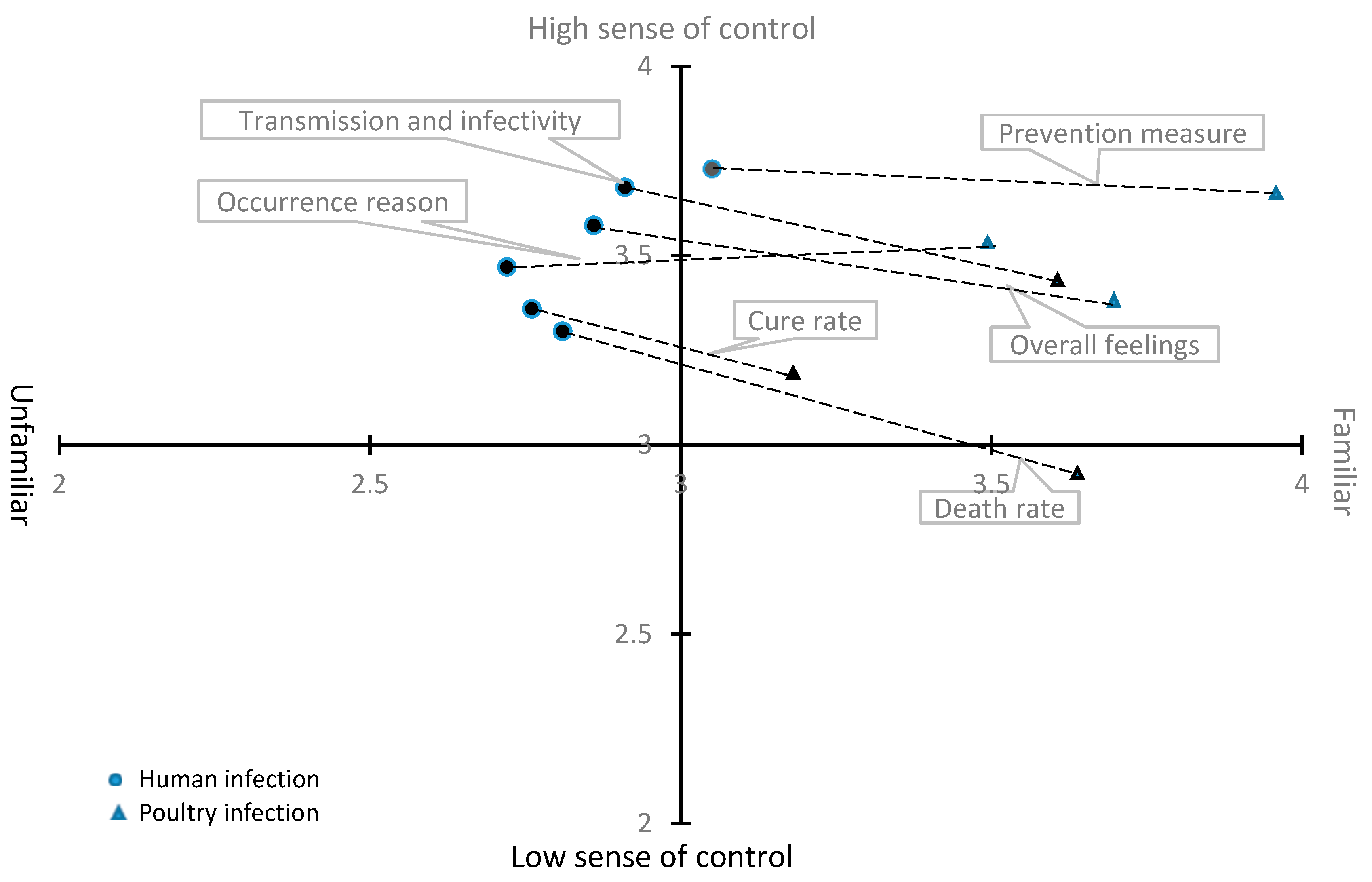
| Risk perception of poultry infection—Risk perception of human infection | 0.514 | 0.818 | 0.000 |
| Familiarity of occurrence reason to poultry infection—Familiarity of occurrence reason to human infection | 0.754 | 1.308 | 0.000 |
| Familiarity of route of transmission and infectivity to poultry infection—Familiarity of route of transmission and infectivity to human infection | 0.676 | 1.249 | 0.000 |
| Familiarity of cure rate to poultry infection—Familiarity of cure rate to human infection | 0.415 | 1.450 | 0.000 |
| Familiarity of prevention measure to poultry infection—Familiarity of prevention measure to human infection | 0.859 | 1.429 | 0.000 |
| Familiarity of death rate to poultry infection—Familiarity of death rate to human infection | 0.793 | 1.397 | 0.000 |
| Familiarity of overall feelings about poultry infection—Familiarity of overall feelings about human infection | 0.800 | 1.152 | 0.000 |
| Controllability of poultry infection—Controllability of human infection occurrence | 0.082 | 1.367 | 0.215 |
| Controllability of route of transmission and infectivity to poultry infection—Controllability of route of transmission and infectivity to human infection | −0.223 | 1.358 | 0.001 |
| Controllability of cure rate to poultry infection—Controllability of cure rate to human infection | −0.136 | 1.446 | 0.053 |
| Controllability of prevention measure to poultry infection—Controllability of prevention measure to human infection | −0.054 | 1.294 | 0.390 |
| Controllability of death rate to poultry infection—Controllability of death rate to human infection | −0.322 | 1.497 | 0.000 |
| Controllability of overall feelings about poultry infection—Controllability of overall feelings about human infection | −0.178 | 1.286 | 0.004 |
| Predictor Variables | Biosecurity Preventive Behaviors | Personal Protective Behaviors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | 95% Confidence Interval | VIF | β | SE | 95% Confidence Interval | VIF | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||||
| Gender (Male) | 0.001 | 0.101 | −0.197 | 0.201 | 1.103 | −0.122** | 0.111 | −0.507 | −0.072 | 1.103 |
| Age | 0.011 | 0.058 | −0.101 | 0.129 | 1.339 | −0.220** | 0.064 | −0.398 | −0.147 | 1.346 |
| Education level | −0.009 | 0.059 | −0.127 | 0.104 | 1.175 | 0.090 | 0.064 | −0.006 | 0.246 | 1.176 |
| Years of experience raising chickens | 0.111 * | 0.033 | 0.016 | 0.145 | 1.237 | 0.277 ** | 0.036 | 0.130 | 0.271 | 1.225 |
| Poultry infection experience | −0.258 ** | 0.108 | −0.806 | −0.383 | 1.310 | −0.262 ** | 0.112 | −0.824 | −0.382 | 1.200 |
| Farming operation size | 0.520 ** | 0.053 | 0.520 | 0.727 | 1.153 | 0.138 ** | 0.058 | 0.052 | 0.279 | 1.176 |
| Risk perception of poultry infection | 0.125 ** | 0.046 | 0.031 | 0.213 | 1.326 | - | - | - | - | - |
| Risk perception of human infection | - | - | - | - | - | 0.110 * | 0.055 | 0.013 | 0.230 | 1.257 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, B.; Wang, F.; Wang, L.D.-L.; Pan, C.; Ke, J.; Tian, Y. A Comparative Analysis of Risk Perception and Coping Behaviors among Chinese Poultry Farmers Regarding Human and Poultry Infection with Avian Influenza. Int. J. Environ. Res. Public Health 2019, 16, 3832. https://doi.org/10.3390/ijerph16203832
Cui B, Wang F, Wang LD-L, Pan C, Ke J, Tian Y. A Comparative Analysis of Risk Perception and Coping Behaviors among Chinese Poultry Farmers Regarding Human and Poultry Infection with Avian Influenza. International Journal of Environmental Research and Public Health. 2019; 16(20):3832. https://doi.org/10.3390/ijerph16203832
Chicago/Turabian StyleCui, Bin, Feifei Wang, Linda Dong-Ling Wang, Chengyun Pan, Jun Ke, and Yi Tian. 2019. "A Comparative Analysis of Risk Perception and Coping Behaviors among Chinese Poultry Farmers Regarding Human and Poultry Infection with Avian Influenza" International Journal of Environmental Research and Public Health 16, no. 20: 3832. https://doi.org/10.3390/ijerph16203832
APA StyleCui, B., Wang, F., Wang, L. D.-L., Pan, C., Ke, J., & Tian, Y. (2019). A Comparative Analysis of Risk Perception and Coping Behaviors among Chinese Poultry Farmers Regarding Human and Poultry Infection with Avian Influenza. International Journal of Environmental Research and Public Health, 16(20), 3832. https://doi.org/10.3390/ijerph16203832





