Assessment of Respiratory Health Symptoms and Asthma in Children near a Drying Saline Lake
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Centers of Disease Control and Prevention. National Current Asthma Prevalence. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 6 October 2019).
- Bhan, N.; Kawachi, I.; Glymour, M.M.; Subramanian, S.V. Time Trends in Racial and Ethnic Disparities in Asthma Prevalence in the United States From the Behavioral Risk Factor Surveillance System (BRFSS) Study (1999–2011). Am. J. Public Health 2015, 105, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Akinbami, L.J.; Simon, A.E.; Rossen, L.M. Changing Trends in Asthma Prevalence Among Children. Pediatrics 2016, 137, e20152354. [Google Scholar] [CrossRef] [PubMed]
- Leong, A.B.; Ramsey, C.D.; Celedon, J.C. The challenge of asthma in minority populations. Clin. Rev. Allergy Immunol. 2012, 43, 156–183. [Google Scholar] [CrossRef] [PubMed]
- Moorman, J.E.; Zahran, H.; Truman, B.I.; Molla, M.T.; Centers for Disease Control and Prevention. Current asthma prevalence—United States, 2006–2008. MMWR Surveill. 2011, 60, 84–86. [Google Scholar]
- Carr, T.F.; Beamer, P.I.; Rothers, J.; Stern, D.A.; Gerald, L.B.; Rosales, C.B.; Van Horne, Y.O.; Pivniouk, O.N.; Vercelli, D.; Halonen, M.; et al. Prevalence of Asthma in School Children on the Arizona-Sonora Border. J. Allergy Clin. Immunol. Pract. 2017, 5, 114–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krieger, N.; Chen, J.T.; Waterman, P.D.; Rehkopf, D.H.; Subramanian, S.V. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: A comparison of area-based socioeconomic measures--the public health disparities geocoding project. Am. J. Public Health 2003, 93, 1655–1671. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, M.; Smorodinsky, S.; English, P.; Copan, L. BASTA Border Asthma & Allergies Study: Final Report; Impact Assessment, Inc.: Richmond, VA, USA; San Diego, CA, USA, 2009; p. 81. [Google Scholar]
- Abuduwaili, J.; Liu, D.; Wu, G. Saline dust storms and their ecological impacts in arid regions. J. Arid Land 2010, 2, 144–150. [Google Scholar] [CrossRef]
- Crighton, E.J.; Elliott, S.J.; Van der Meer, J.; Small, I.; Upshur, R. Impacts of an environmental disaster on psychosocial health and well-being in Karakalpakstan. Soc. Sci. Med. 2003, 56, 551–567. [Google Scholar] [CrossRef]
- Kunii, O.; Hashizume, M.; Chiba, M.; Sasaki, S.; Shimoda, T.; Caypil, W.; Dauletbaev, D. Respiratory symptoms and pulmonary function among school-age children in the Aral Sea region. Arch. Environ. Health 2003, 58, 676–682. [Google Scholar] [CrossRef]
- Bennion, P.; Hubbard, R.; O’Hara, S.; Wiggs, G.; Wegerdt, J.; Lewis, S.; Small, I.; van der Meer, J.; Upshur, R. The impact of airborne dust on respiratory health in children living in the Aral Sea region. Int. J. Epidemiol. 2007, 36, 1103–1110. [Google Scholar] [CrossRef]
- Johnston, J.E.; Razafy, M.; Lugo, H.; Olmedo, L.; Farzan, S.F. The disappearing Salton Sea: A critical reflection on the emerging environmental threat of disappearing saline lakes and potential impacts on children’s health. Sci. Total Environ. 2019, 663, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Carvlin, G.; Austin, E.; Shirai, J.; Bejarano, E.; Lugo, H.; Olmedo, L.; Calderas, A.; Jerrett, M.; King, G.; et al. Next-Generation Community Air Quality Sensors for Identifying Air Pollution Episodes. Int. J. Environ. Res. Public Health 2019, 16, 3268. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Bejarano, E.; Carvlin, G.; Fellows, K.; King, G.; Lugo, H.; Jerrett, M.; Meltzer, D.; Northcross, A.; Olmedo, L.; et al. Combining Community Engagement and Scientific Approaches in Next-Generation Monitor Siting: The Case of the Imperial County Community Air Network. Int. J. Environ. Res. Public Health 2018, 15, 523. [Google Scholar] [CrossRef] [PubMed]
- Kemper, L. On the Frontier: Medi-Cal Brings Mangaged Care to California’s Rural Communities; California Health Care Foundation: Oakland, CA, USA, 2015. [Google Scholar]
- California Office of Statewide Health Planning and Development. Medical Service Study Area. Available online: https://www.arcgis.com/home/item.html?id=5c13665b10854480aa985d76cfcb9c5d (accessed on 18 September 2019).
- ISAAC. International Study of Asthma and Allergies in Childhood Manual. Available online: http://isaac.auckland.ac.nz/ (accessed on 10 October 2019).
- Salam, M.T.; Li, Y.-F.; Langholz, B.; Gilliland, F.D. Early-life environmental risk factors for asthma: Findings from the Children’s Health Study. Environ. Health Perspect. 2004, 112, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Millstein, J.; Gilliland, F.; Berhane, K.; Gauderman, W.J.; McConnell, R.; Avol, E.; Rappaport, E.B.; Peters, J.M. Effects of ambient air pollutants on asthma medication use and wheezing among fourth-grade school children from 12 southern California communities enrolled in the children’s health study. Arch. Environ. Health 2004, 59, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chakraborty, R.K. Asthma Medications. In Treasure Island. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531455/ (accessed on 10 October 2019).
- California Department of Public Health. California Breathing County Asthma Data Tool. Available online: https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/Pages/CaliforniaBreathingCountyAsthmaProfiles.aspx (accessed on 6 October 2019).
- Centers of Disease Control and Prevention. Most Recent National Asthma Data. Available online: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm (accessed on 6 October 2019).
- Grineski, S.E.; Collins, T.W.; Chavez-Payan, P.; Jimenez, A.M.; Clark-Reyna, S.; Gaines, M.; Kim, Y.-A. Social disparities in children’s respiratory health in El Paso, Texas. Int. J. Environ. Res. Public Health 2014, 11, 2941–2957. [Google Scholar] [CrossRef]
- Ramos, I.N.; Davis, L.B.; He, Q.; May, M.; Ramos, K.S. Environmental risk factors of disease in the Cameron Park Colonia, a Hispanic community along the Texas-Mexico border. J. Immigr. Minor. Health 2008, 10, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Stephen, G.A.; McRill, C.; Mack, M.D.; O’Rourke, M.K.; Flood, T.J.; Lebowitz, M.D. Assessment of respiratory symptoms and asthma prevalence in a U.S.-Mexico border region. Arch. Environ. Health 2003, 58, 156–162. [Google Scholar] [CrossRef]
- Sinclair, R.; Russell, C.; Kray, G.; Vesper, S. Asthma Risk Associated with Indoor Mold Contamination in Hispanic Communities in Eastern Coachella Valley, California. J. Environ. Public Health 2018, 2018, 9350370. [Google Scholar] [CrossRef]
- van Gent, R.; van Essen, L.E.; Rovers, M.M.; Kimpen, J.L.; van der Ent, C.K.; de Meer, G. Quality of life in children with undiagnosed and diagnosed asthma. Eur. J. Pediatr. 2007, 166, 843–848. [Google Scholar] [CrossRef]
- Yeatts, K.; Shy, C.; Sotir, M.; Music, S.; Herget, C. Health Consequences for Children With Undiagnosed Asthma-like SymptomsHealth Consequences for Children With Undiagnosed Asthma-like Symptoms. JAMA Pediatr. 2003, 157, 540–544. [Google Scholar]
- Aaron, S.D.; Boulet, L.P.; Reddel, H.K.; Gershon, A.S. Underdiagnosis and Overdiagnosis of Asthma. Am. J. Respir. Crit. Care Med. 2018, 198, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Sianez, M.; Highfield, L.; Collins, T.; Grineski, S. Burden of Illness, Primary Care Use, and Medication Utilization among US-Mexico Border Children with Wheezing. J. Racial Ethn. Health Disparities 2019, 6, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Siersted, H.C.; Boldsen, J.; Hansen, H.S.; Mostgaard, G.; Hyldebrandt, N. Population based study of risk factors for underdiagnosis of asthma in adolescence: Odense schoolchild study. BMJ 1998, 316, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Leith Sly, J.; Carpenter, D.O. Special vulnerability of children to environmental exposures. Rev. Environ. Health 2012, 27, 151–157. [Google Scholar] [PubMed]
- Toskala, E.; Kennedy, D.W. Asthma risk factors. Int. Forum Allergy Rhinol. 2015, 5 (Suppl. 1), S11–S16. [Google Scholar] [CrossRef]
- Castro-Rodriguez, J.A.; Forno, E.; Rodriguez-Martinez, C.E.; Celedon, J.C. Risk and Protective Factors for Childhood Asthma: What Is the Evidence? J. Allergy Clin. Immunol. Pract. 2016, 4, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, P. Maternal Smoking during Pregnancy and Its Effect on Childhood Asthma. Am. J. Respir. Crit. Care Med. 2012, 186, 941–942. [Google Scholar] [CrossRef]
- Neuman, Å.; Hohmann, C.; Orsini, N.; Pershagen, G.; Eller, E.; Kjaer, H.F.; Gehring, U.; Granell, R.; Henderson, J.; Heinrich, J.; et al. Maternal Smoking in Pregnancy and Asthma in Preschool Children. Am. J. Respir. Crit. Care Med. 2012, 186, 1037–1043. [Google Scholar] [CrossRef]
- Zacharasiewicz, A. Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Res. 2016, 2, 00042–2016. [Google Scholar] [CrossRef]
- Neophytou, A.M.; Oh, S.S.; White, M.J.; Mak, A.C.Y.; Hu, D.; Huntsman, S.; Eng, C.; Serebrisky, D.; Borrell, L.N.; Farber, H.J.; et al. Secondhand smoke exposure and asthma outcomes among African-American and Latino children with asthma. Thorax 2018, 73, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Continente, X.; Arechavala, T.; Fernandez, E.; Perez-Rios, M.; Schiaffino, A.; Soriano, J.B.; Carreras, G.; Lopez-Nicolas, A.; Gorini, G.; Lopez, M.J. Burden of respiratory disease attributable to secondhand smoke exposure at home in children in Spain (2015). Prev. Med. 2019, 123, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.D.; Lipsett, M.J.; Mann, J.K.; Wiener, M.B.; Selner, J. Indoor air pollution and asthma. Results from a panel study. Am. J. Respir. Crit. Care Med. 1994, 149, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Jhun, I.; Phipatanakul, W. Early exposure to dogs and farm animals reduces risk of childhood asthma. Evid. Based Med. 2016, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Lodrup Carlsen, K.C.; Roll, S.; Carlsen, K.H.; Mowinckel, P.; Wijga, A.H.; Brunekreef, B.; Torrent, M.; Roberts, G.; Arshad, S.H.; Kull, I.; et al. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS ONE 2012, 7, e43214. [Google Scholar] [CrossRef] [PubMed]
- Collin, S.M.; Granell, R.; Westgarth, C.; Murray, J.; Paul, E.; Sterne, J.A.C.; John Henderson, A. Pet ownership is associated with increased risk of non-atopic asthma and reduced risk of atopy in childhood: Findings from a UK birth cohort. Clin. Exp. Allergy 2015, 45, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Hyun, K. Hazard: The Future of the Salton Sea with No Restoration Project; Pacific Institute: Oakland, CA, USA, 2006; pp. 1–60. [Google Scholar]
- Reid, J.S.; Flocchini, R.G.; Cahill, T.A.; Ruth, R.S.; Salgado, D.P. Local meteorological, transport, and source aerosol characteristics of late autumn Owens Lake (dry) dust storms. Atmos. Environ. 1994, 28, 1699–1706. [Google Scholar] [CrossRef]
- Chalupa, D.C.; Morrow, P.E.; Oberdorster, G.; Utell, M.J.; Frampton, M.W. Ultrafine particle deposition in subjects with asthma. Environ. Health Perspect 2004, 112, 879–882. [Google Scholar] [CrossRef]
- Ostro, B.; Roth, L.; Malig, B.; Marty, M. The effects of fine particle components on respiratory hospital admissions in children. Environ. Health Perspect. 2009, 117, 475–480. [Google Scholar] [CrossRef]
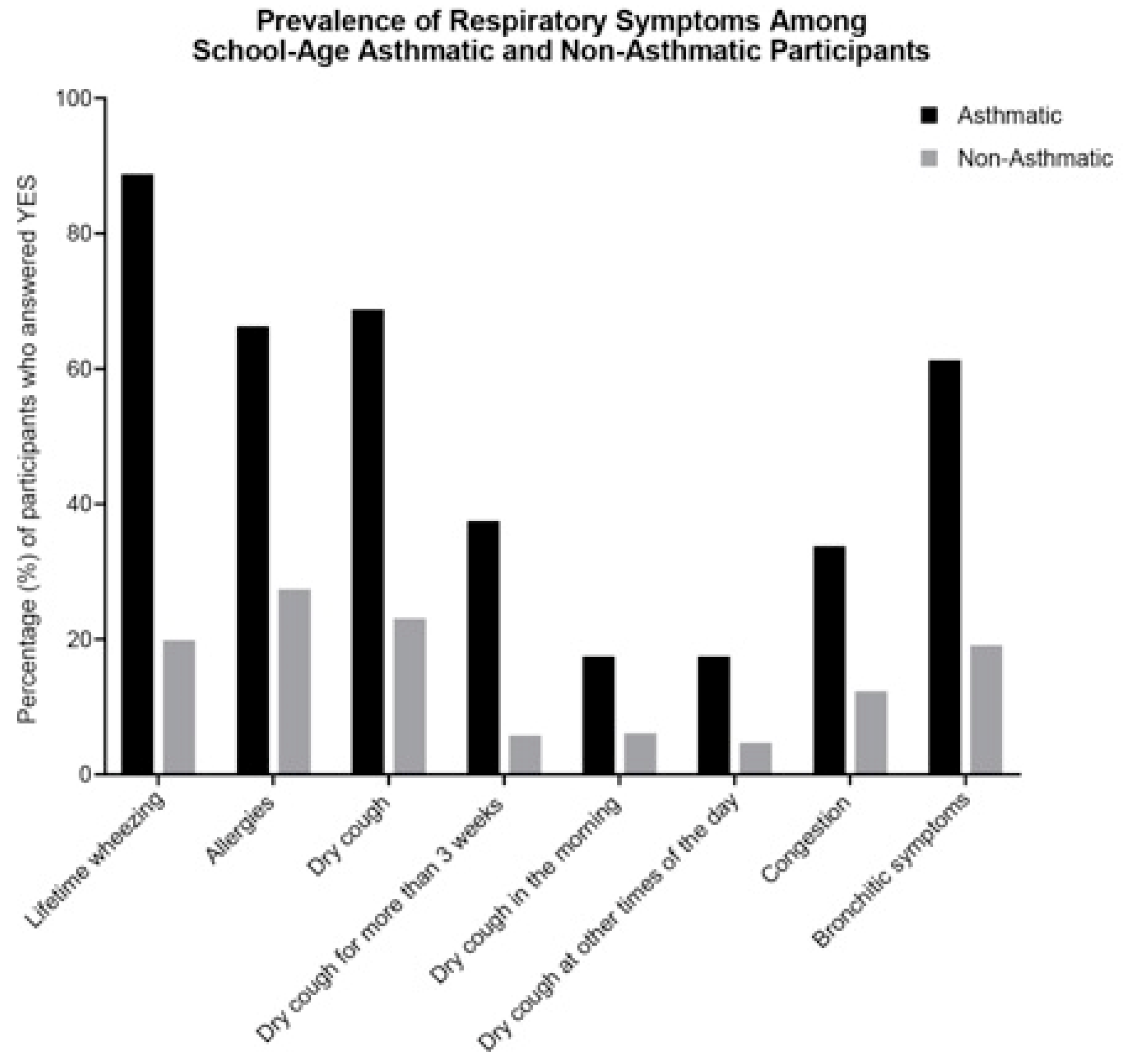
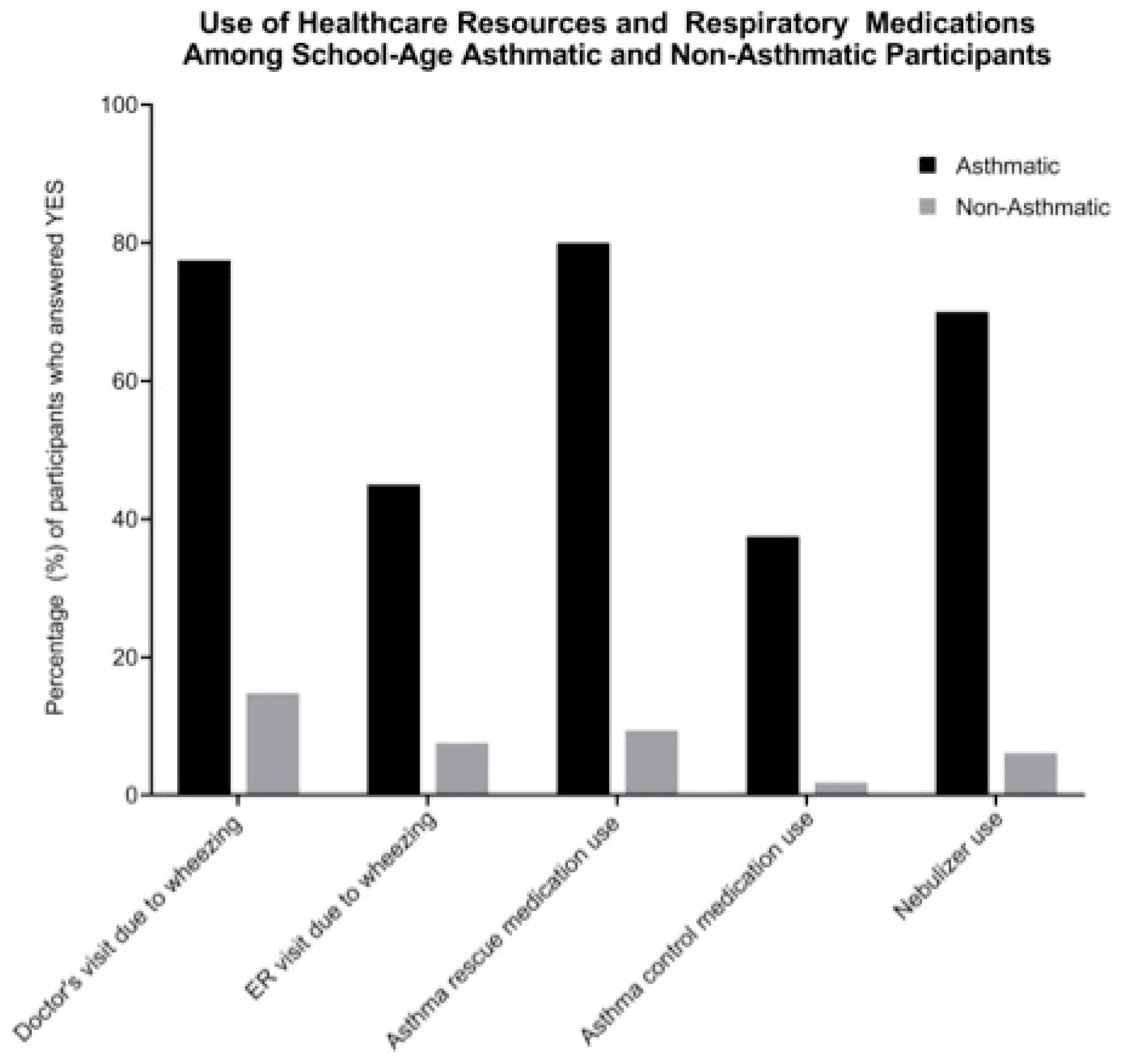
| Demographic Characteristics | All N = 357 (%) | Asthmatics N = 80 (%) | Nonasthmatics N = 277 (%) | p-Value a |
|---|---|---|---|---|
| Language of survey | ||||
| English | 229 (64.2) | 59 (73.8) | 170 (61.4) | 0.04 |
| Spanish | 128 (35.8) | 21 (26.3) | 107 (38.6) | |
| Sex | ||||
| Female | 196 (54.9) | 41 (51.3) | 155 (56.0) | 0.46 |
| Male | 161 (45.1) | 39 (48.8) | 122 (44.0) | |
| Race/Ethnicity | ||||
| Latino | 333 (93.3) | 74 (92.5) | 259 (93.5) | 0.38 |
| White | 18 (5.0) | 5 (6.3) | 13 (4.7) | |
| Black | 3 (0.8) | 0 (0.0) | 3 (1.1) | |
| Native American | 1 (0.3) | 1 (1.3) | 0 (0.0) | |
| Other | 2 (0.6) | 0 (0.0) | 2 (0.7) | |
| Age | ||||
| 5–6 years | 32 (9.0) | 9 (11.3) | 23 (8.3) | 0.69 |
| 7 years | 131 (36.7) | 28 (35.0) | 103 (37.2) | |
| 8 years | 148 (41.5) | 35 (43.8) | 113 (40.8) | |
| 9–10 years | 46 (12.9) | 8 (10.0) | 38 (13.7) | |
| Health Insurance | ||||
| Public | 251 (70.3) | 51 (63.8) | 200 (72.2) | 0.01 |
| Private | 72 (20.2) | 25 (31.3) | 47 (17.0) | |
| None | 34 (9.5) | 4 (5.0) | 30 (10.8) | |
| Total Household Income | ||||
| Less than $15,000 | 107 (30.0) | 22 (27.5) | 85 (30.7) | 0.01 |
| $15,000 to $29,999 | 71 (19.9) | 15 (18.8) | 56 (20.2) | |
| $30,000 to $49,999 | 54 (15.1) | 8 (10.0) | 46 (16.6) | |
| More than $50,000 | 51 (14.3) | 21 (26.3) | 30 (10.8) | |
| Don’t know | 74 (20.7) | 14 (17.5) | 60 (21.7) | |
| Parent/caregiver education b | ||||
| Less than 12th grade | 90 (25.2) | 13 (16.3) | 77 (27.8) | 0.08 |
| Completed 12th grade | 90 (25.2) | 23 (28.8) | 67 (24.2) | |
| Some college or technical school | 122 (34.2) | 30 (37.5) | 92 (33.2) | |
| 4 years of college or more | 35 (9.8) | 12 (15.0) | 23 (8.3) | |
| Missing | 20 (5.6) | 2 (2.5) | 18 (6.5) | |
| Two-adult household | ||||
| Yes | 202 (56.6) | 46 (57.5) | 156 (56.3) | 0.50 |
| No | 142 (39.8) | 34 (42.5) | 108 (39.0) | |
| Missing | 13 (3.6) | 0 (0.0) | 13 (4.7) |
| Health Characteristics | All N = 357 (%) | Asthmatics N = 80 (%) | Nonasthmatics N = 277 (%) | p-Value a |
|---|---|---|---|---|
| Biological mother has asthma | ||||
| Yes | 44 (12.3) | 20 (25.1) | 24 (8.7) | <0.001 |
| No | 304 (85.2) | 57 (71.3) | 247 (89.2) | |
| Missing | 9 (2.5) | 3 (3.8) | 6 (2.2) | |
| Biological mother smoked while pregnant with child | ||||
| Yes | 16 (4.5) | 5 (6.3) | 11 (4.0) | 0.36 |
| No | 333 (93.3) | 72 (90.0) | 261 (94.2) | |
| Missing | 8 (2.2) | 3 (3.8) | 5 (1.8) | |
| Lower respiratory infection during infancy | ||||
| Yes | 48 (13.5) | 26 (32.5) | 22 (7.9) | <0.001 |
| No | 309 (86.6) | 54 (67.5) | 255 (92.1) | |
| Upper respiratory infection during infancy | ||||
| Yes | 21 (5.9) | 13 (16.3) | 8 (2.9) | <0.001 |
| No | 336 (94.1) | 67 (83.8) | 269 (97.1) | |
| Asthma during infancy | ||||
| Yes | 51 (14.3) | 47 (58.8) | 4 (1.4) | <0.001 |
| No | 306 (85.7) | 33 (41.3) | 273 (98.6) | |
| Age of asthma diagnosis b | ||||
| Under 2 years old | -- | 28 (35.0) | -- | -- |
| 2–4 years old | -- | 34 (42.5) | -- | -- |
| 5 years old and above | -- | 18 (22.5) | -- | -- |
| Characteristics | Minimally Adjusted a OR (95% CI) | p-Value | Fully Adjusted b OR (95% CI) | p-Value |
|---|---|---|---|---|
| Participant demographics | ||||
| Household Income c | 1.05 (0.89–1.24) | 0.53 | 1.05 (0.88–1.25) | 0.62 |
| Health insurance | ||||
| Public | Ref. | -- | Ref. | -- |
| Private | 2.05 (1.15–3.65) | 0.02 | 1.68 (0.89–3.15) | 0.11 |
| None | 0.53 (0.18–1.59) | 0.26 | 0.49 (0.16–1.55) | 0.23 |
| Household environmental smoke | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 4.24 (1.37–13.07) | 0.01 | 4.00 (1.21–13.23) | 0.02 |
| Biological mother smoked while pregnant | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 1.66 (0.55–4.94) | 0.37 | 2.23 (0.59–8.30) | 0.23 |
| Biological mother has asthma | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 3.79 (1.94–7.39) | <0.001 | 2.92 (1.44–5.93) | 0.003 |
| Play sports at least twice a week | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 1.43 (0.85–2.42) | 0.17 | 1.62 (0.93–2.83) | 0.09 |
| Housing characteristics | ||||
| Housing type | ||||
| Home | Ref. | -- | Ref. | -- |
| Apartment | 0.97 (0.56–1.68) | 0.97 | 1.00 (0.55–1.80) | 0.99 |
| Mobile home or trailer | 0.86 (0.35–2.10) | 0.74 | 1.73 (0.62–4.82) | 0.29 |
| Gas cooking stove | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 0.59 (0.31–1.12) | 0.11 | 0.65 (0.32–1.34) | 0.25 |
| Length of daily gas stove use | ||||
| Less than 30 min | Ref. | -- | Ref. | -- |
| Less than 1 h | 1.45 (0.77–2.70) | 0.25 | 1.42 (0.74–2.75) | 0.29 |
| More than 1 h | 3.17 (1.31–7.65) | 0.01 | 2.58 (0.97–6.82) | 0.06 |
| Home heater | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 2.46 (1.06–5.68) | 0.04 | 2.08 (0.87–4.94) | 0.10 |
| Water damage in home | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 2.23 (1.00–4.98) | 0.05 | 1.73 (0.74–4.02) | 0.21 |
| Mold in home | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 1.37 (0.69–2.73) | 0.37 | 1.21 (0.59–2.48) | 0.60 |
| Musty odor in home | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 2.56 (0.85–7.74) | 0.10 | 2.14 (0.67–6.88) | 0.20 |
| Carpet in home | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 1.32 (0.78–2.25) | 0.30 | 1.53 (0.86–2.72) | 0.15 |
| Child has lived in same house for whole life | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 1.32 (0.77–2.30) | 0.31 | 1.29 (0.71–2.35) | 0.41 |
| Pet ownership | ||||
| None | Ref. | -- | Ref. | -- |
| Furry pet | 0.48 (0.26–0.91) | 0.02 | 0.42 (0.21–0.82) | 0.01 |
| Other pets | 1.17 (0.48–2.83) | 0.74 | 0.87 (0.34–2.24) | 0.77 |
| Regular contact with farm animals | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 1.21 (0.57–2.57) | 0.62 | 1.52 (0.67–3.45) | 0.32 |
| Problem with rodents in the home | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 0.90 (0.36–2.20) | 0.81 | 0.84 (0.33–2.13) | 0.72 |
| Problem with insects in the home | ||||
| No | Ref. | -- | Ref. | -- |
| Yes | 1.19 (0.66–2.16) | 0.57 | 0.97 (0.51–1.82) | 0.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farzan, S.F.; Razafy, M.; Eckel, S.P.; Olmedo, L.; Bejarano, E.; Johnston, J.E. Assessment of Respiratory Health Symptoms and Asthma in Children near a Drying Saline Lake. Int. J. Environ. Res. Public Health 2019, 16, 3828. https://doi.org/10.3390/ijerph16203828
Farzan SF, Razafy M, Eckel SP, Olmedo L, Bejarano E, Johnston JE. Assessment of Respiratory Health Symptoms and Asthma in Children near a Drying Saline Lake. International Journal of Environmental Research and Public Health. 2019; 16(20):3828. https://doi.org/10.3390/ijerph16203828
Chicago/Turabian StyleFarzan, Shohreh F., Mitiasoa Razafy, Sandrah P. Eckel, Luis Olmedo, Esther Bejarano, and Jill E. Johnston. 2019. "Assessment of Respiratory Health Symptoms and Asthma in Children near a Drying Saline Lake" International Journal of Environmental Research and Public Health 16, no. 20: 3828. https://doi.org/10.3390/ijerph16203828
APA StyleFarzan, S. F., Razafy, M., Eckel, S. P., Olmedo, L., Bejarano, E., & Johnston, J. E. (2019). Assessment of Respiratory Health Symptoms and Asthma in Children near a Drying Saline Lake. International Journal of Environmental Research and Public Health, 16(20), 3828. https://doi.org/10.3390/ijerph16203828





