Thyroid Cancer Incidence Rates in North Dakota are Associated with Land and Water Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Statistical Analysis
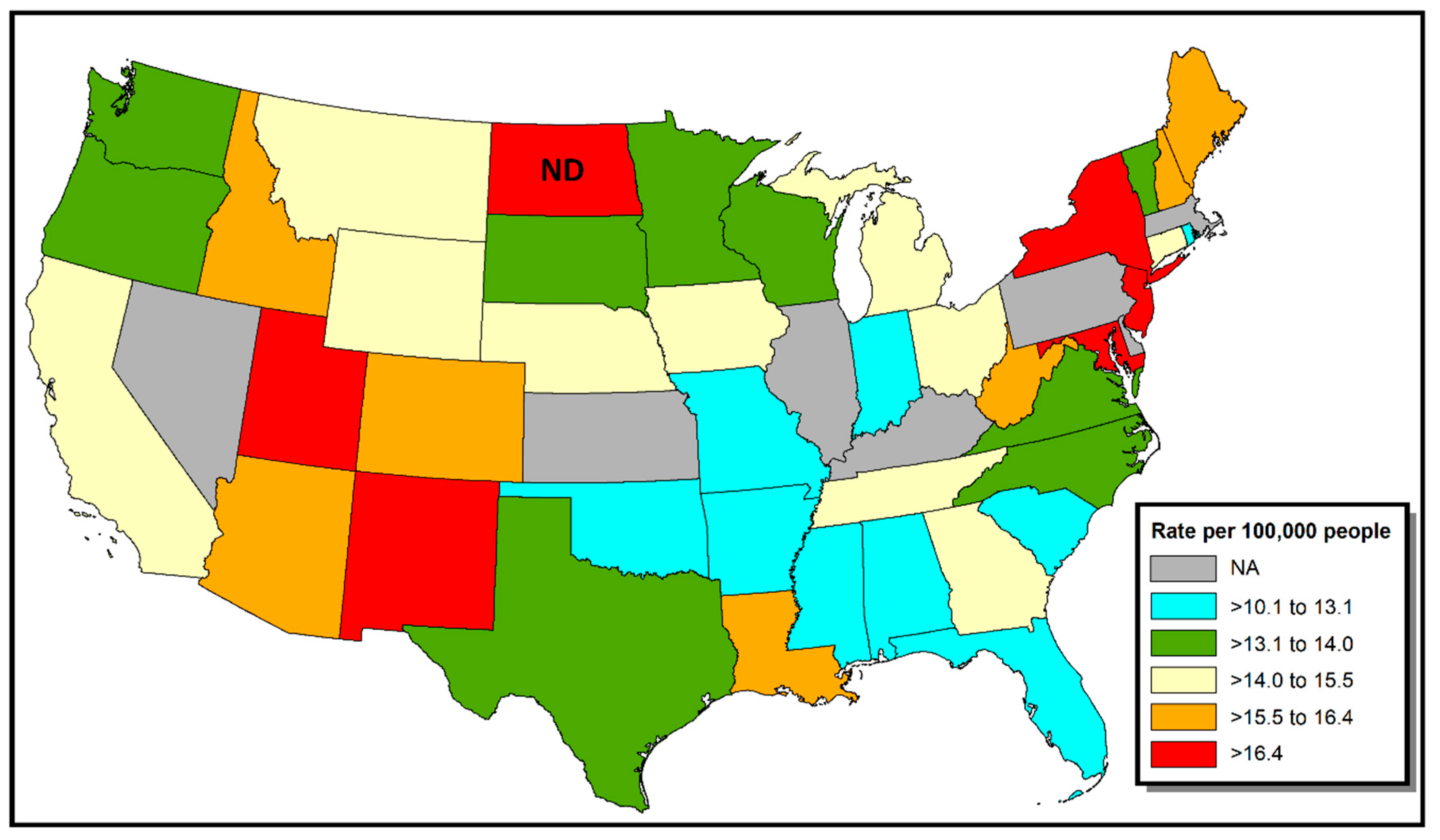
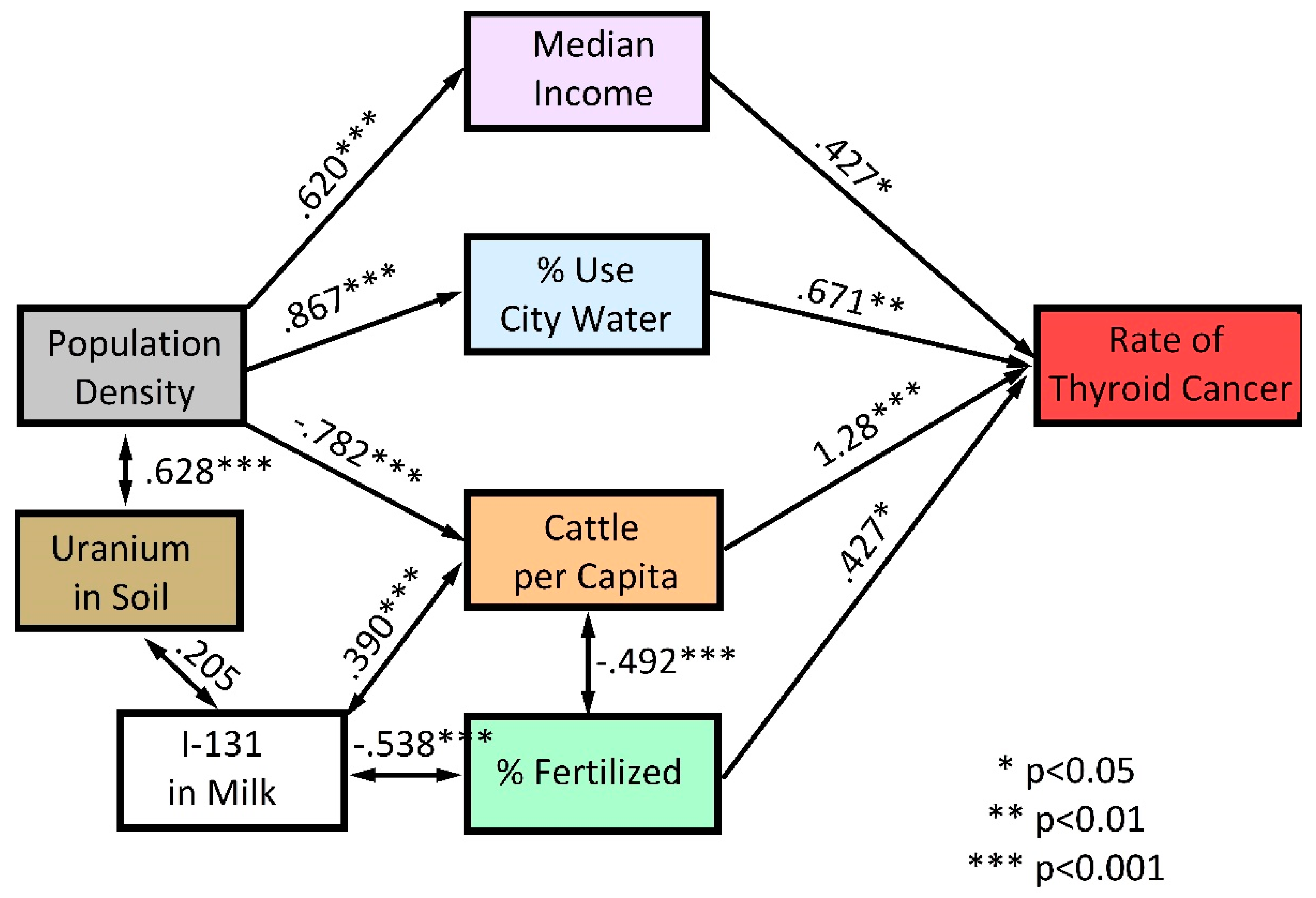
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Davies, L.; Welch, G. Current thyroid cancer trends in the United States. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 317–322. [Google Scholar] [CrossRef]
- Vigneri, R.; Maladrino, P.; Vigneri, P. The changing epidemiology of thyroid cancer: Why is incidence increasing? Curr. Opin. Oncol. 2015, 27, 1–7. [Google Scholar] [CrossRef]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Wartofsky, L. Increasing world incidence of thyroid cancer: Increased detection or higher radiation exposure? Hormones 2010, 9, 103–108. [Google Scholar] [CrossRef]
- Hundahl, S.A. Perspective: National Cancer Institute Summary Report about estimated exposures and thyroid doses received from Iodine 131 in fallout after Nevada atmospheric nuclear bomb tests. CA Cancer J. Clin. 1998, 48, 285–298. [Google Scholar] [CrossRef]
- Kerber, R.A.; Till, J.E.; Lyon, J.L.; Thomas, D.C.; Preston-Martin, S.; Ralliston, M.L.; Lloyd, R.D.; Stevens, W. A cohort study of thyroid disease in relation to fallout from nuclear weapons testing. JAMA 1993, 17, 2076–2082. [Google Scholar] [CrossRef]
- Lyon, J.L.; Alder, S.C.; Stone, M.B.; Scholl, A.; Reading, J.C.; Holubkov, R.; Hegmann, K.T.; Anspaugh, L.; Hoffman, F.O.; Thomas, B.; et al. Thyroid disease associated with exposure to the Nevada Nuclear Weapons Test Site Radiation. A Reevaluation based on corrected dosimetry and examination data. Epidemiology 2006, 17, 604–614. [Google Scholar] [CrossRef]
- Maladrino, P.; Scollo, C.; Mearturano, I.; Russo, M.; Tavarelli, M.; Attard, M.; Richiusa, P.; Violi, M.A.; Dardanoni, G.; Vigneri, R.; et al. Descriptive epidemiology of human thyroid cancer: Experience from a regional registry and the “volcanic factor”. Front. Endocrinol. 2013, 4, 65. [Google Scholar]
- State Cancer Profiles. Available online: https://statecancerprofiles.cancer.gov/map/ms.ap.noimage.php (accessed on 9 October 2019).
- Health Resources & Services Administration. Health Professional Shortage Areas (HPSAs). Available online: https://bhw.hrsa.gov/shortage-designation/hpsas (accessed on 6 September 2019).
- States by Percentage of Farmland. Available online: www.stuffaboutstates.com (accessed on 9 October 2019).
- About the North Dakota Statewide Cancer Registry. Available online: https://ndcancer.org/aboutmain.html (accessed on 9 October 2019).
- U.S. Census. 2010. Available online: https://www.census.gov/2010census/ (accessed on 9 October 2019).
- U.S. Census of Agriculture. 2012. Available online: https://www.agcensus.usda.gov/Publications/2012/ (accessed on 9 October 2019).
- County-Level Estimates of Nitrogen and Phosphorus from Commercial Fertilizer for the Coterminous U.S., 1987–2006. Available online: https://water.usgs.gov/GIS/metadata/usgswrd/XML/sir2012-5207_county_fertilizer.xml (accessed on 9 October 2019).
- Water Use for the Nation. Available online: http://waterdata.usgs.gov/nwis (accessed on 9 October 2019).
- Aeromagnetic and Aeroradiometric Data for the Conterminous U.S. and Alaska, National Uranium Resource Evaluation (NURE), U.S. Department of Energy. Available online: https://pubs.usgs.gov/of/2009/1129/NURE.html (accessed on 9 October 2019).
- Oancea, S.C.; Rundquist, B.C.; Simon, I.; Swartz, S.; Zheng, Y.; Zhou, X.; Sens, M.A.; Schwartz, G.G. County level incidence rates of chronic lymphocytic leukemia are associated with residential radon levels. Future Oncol. 2017, 21, 1873–1881. [Google Scholar] [CrossRef]
- State and County Exposure Levels to I-131. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/stateandcountyexposure (accessed on 9 October 2019).
- Schwartz, G.G.; Klug, M.G.; Rundquist, B.C. An exploration of colorectal cancer incidence rates in North Dakota, USA, via structural equation modeling. Int. J. Colorectal Dis. 2019. [Google Scholar] [CrossRef]
- Schumaker, R.E.; Lomax, R.G. A Beginner’s Guide to Structural Equation Modeling, 2nd ed.; Lawrence Erlbaum: Mahwah, NJ, USA, 2004. [Google Scholar]
- Goyal, N.; Camacho, C.; Mangano, J.; Goldenberg, D. Evaluating for a geospatial relationship between radon levels and thyroid cancer in Pennsylvania. Laryngoscope 2015, 125, E45–E49. [Google Scholar] [CrossRef]
- Oakland, C.; Meliker, J.R. County-level radon and incidence of female thyroid cancer in Iowa, New Jersey and Wisconsin, USA. Toxics 2018, 16, 17. [Google Scholar] [CrossRef]
- McDow, A.D.; Zahnd, W.E.; Angelos, P.; Mellinger, J.D.; Ganai, S. Impact of rurality on national trends in thyroid cancer incidence and long-term survival. J. Rural Health 2019. [Google Scholar] [CrossRef]
- Center for Rural Health, University of North Dakota School of Medicine & Health Sciences. Availability of Primary Care Physicians in North Dakota. Available online: https://ruralhealth.und.edu/assets/622-2136/availability-of-nd-primary-care-physicians.pdf (accessed on 27 September 2019).
- America’s Health Rankings. Available online: https://www.americashealthrankings.org/explore/annual/measure/PCP/state/ALL (accessed on 27 September 2019).
- Clayman, G. Thyroid Cancer: Diagnosis, Treatment and Prognosis. Available online: www.endocrineweb.com/conditions/thyroid-cancer/thyroid-cancer (accessed on 27 September 2019).
- Udelsman, R.; Zhang, Y. The epidemic of thyroid cancer in the United States: The role of endocrinologists and ultrasounds. Thyroid 2014, 24, 472–479. [Google Scholar] [CrossRef]
- Lu, H.; Holt, J.B.; Cheng, Y.J.; Zhang, X.; Onufrak, S.; Croft, J.B. Population-based geographic access to endocrinologists in the United States, 2012. BMC Health Serv. Res. 2015, 15, 541. [Google Scholar] [CrossRef]
- Zeng, F.; Lerro, C.; Lavoué, J.; Huang, H.; Semiatycki, J.; Zhao, N.; Ma, S.; Deziel, N.C.; Friesen, M.C.; Udelsman, R.; et al. Occupational exposure to pesticides and other biocides and risk of thyroid cancer. Occup. Environ. Med. 2017, 74, 502–510. [Google Scholar] [CrossRef]
- National Cancer Institute. Get the Facts about Exposure to I-131 Radiation. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/i-131 (accessed on 1 May 2019).
- Xie, L.; Mo, M.; Jia, H.-X.; Liang, F.; Yuan, J.; Zhu, J. Association between dietary nitrate and nitrite intake and site-specific cancer risk: Evidence from observational studies. Oncotarget 2016, 7, 56915–56932. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de KoK, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Ward, M.H. Too much of a good thing? Nitrate from nitrogen fertilizers and cancer. Rev. Environ. Health 2009, 24, 357–363. [Google Scholar] [CrossRef]
- Nolan, B.T.; Hitt, K.J. Vulnerability of shallow groundwater and drinking-water wells to nitrate in the United States. Environ. Sci. Technol. 2006, 40, 7834–7840. [Google Scholar] [CrossRef] [PubMed]
- Wones, R.G.; Deck, C.C.; Stadler, B.; Roark, S.; Hogg, E.; Frohman, L.A. Effects of drinking water monochloramine on lipid and thyroid metabolism in healthy men. Environ. Health Perspect. 1993, 99, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Wones, R.G.; Deck, C.C.; Stadler, B.; Roark, S.; Hogg, E.; Frohman, L.A. Lack of effect of drinking water chlorine on lipid and thyroid metabolism in healthy humans. Environ. Health Perspect. 1993, 99, 375–381. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Fluoride in Drinking Water. A Scientific Review of EPA’s Standards; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Malin, A.J.; Riddell, J.; McCague, H.; Till, C. Fluoride exposure and thyroid function among adults living in Canada: Effect modification by iodine status. Environ. Int. 2018, 121, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chahar, P.; Kajjari, S.; Rahman, F.; Bansal, D.K.; Kapadia, J.M. Fluoride, thyroid hormone derangements and its correlation with tooth eruption pattern among the pediatric population from endemic and non-endemic fluorosis areas. J. Contemp. Dent. Pract. 2018, 19, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.J.; Zhang, C.; Liang, L.; Wang, S.Y.; Zheng, S.C.; Zhang, Q.; Jiang, C.X.; Zhong, Q.; Huang, F. Fasting serum glucose, thyroid-stimulating hormone, and thyroid hormones and risk of papillary thyroid cancer: A case-control study. Head Neck 2019. [Google Scholar] [CrossRef] [PubMed]
- Fiore, E.; Vitti, P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J. Clin. Endocrinol. Metab. 2012, 97, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Nieto, H.; Boelaert, K. Thyroid-stimulating hormone in thyroid cancer: Does it matter? Endocr. Relat. Cancer 2016, 23, T109–T121. [Google Scholar] [CrossRef]
- Bailey, W.; Barker, L.; Durham, K.; Maas, W. Populations receiving optimally fluoridated public drinking water—United States—1992, 2006. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 737–741. [Google Scholar]
- Kheradpisheh, Z.; Mirzaei, M.; Mahvi, M.; Moktari, M.; Azizi, R.; Fallahzadeh, H.; Ehrampoush, M.H. Impact of drinking water fluoride on human thyroid hormones: A case-control study. Sci. Rep. 2018, 8, 2674. [Google Scholar] [CrossRef]
- Pellegritis, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Hutton, S.S. Guidelines for Preparation of State Water Use Estimates For 2005; U.S. Department of the Interior: Washington, DC, USA; U.S. Geological Survey: Reston, VA, USA, 2007.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwartz, G.G.; Klug, M.G. Thyroid Cancer Incidence Rates in North Dakota are Associated with Land and Water Use. Int. J. Environ. Res. Public Health 2019, 16, 3805. https://doi.org/10.3390/ijerph16203805
Schwartz GG, Klug MG. Thyroid Cancer Incidence Rates in North Dakota are Associated with Land and Water Use. International Journal of Environmental Research and Public Health. 2019; 16(20):3805. https://doi.org/10.3390/ijerph16203805
Chicago/Turabian StyleSchwartz, Gary G., and Marilyn G. Klug. 2019. "Thyroid Cancer Incidence Rates in North Dakota are Associated with Land and Water Use" International Journal of Environmental Research and Public Health 16, no. 20: 3805. https://doi.org/10.3390/ijerph16203805
APA StyleSchwartz, G. G., & Klug, M. G. (2019). Thyroid Cancer Incidence Rates in North Dakota are Associated with Land and Water Use. International Journal of Environmental Research and Public Health, 16(20), 3805. https://doi.org/10.3390/ijerph16203805




