Airborne Rodent Allergen Levels in Dutch Households: A Pilot Study
Abstract
1. Introduction
2. Materials & Methods
2.1. Study Design
2.2. Recruitment of Study Participants
2.3. Patient and Public Involvement
2.4. Questionnaire
2.5. Collection of Airborne Dust Samples
2.6. Allergen Extraction
2.7. Measurement of Allergen Levels
2.8. Statistical Analysis
3. Results
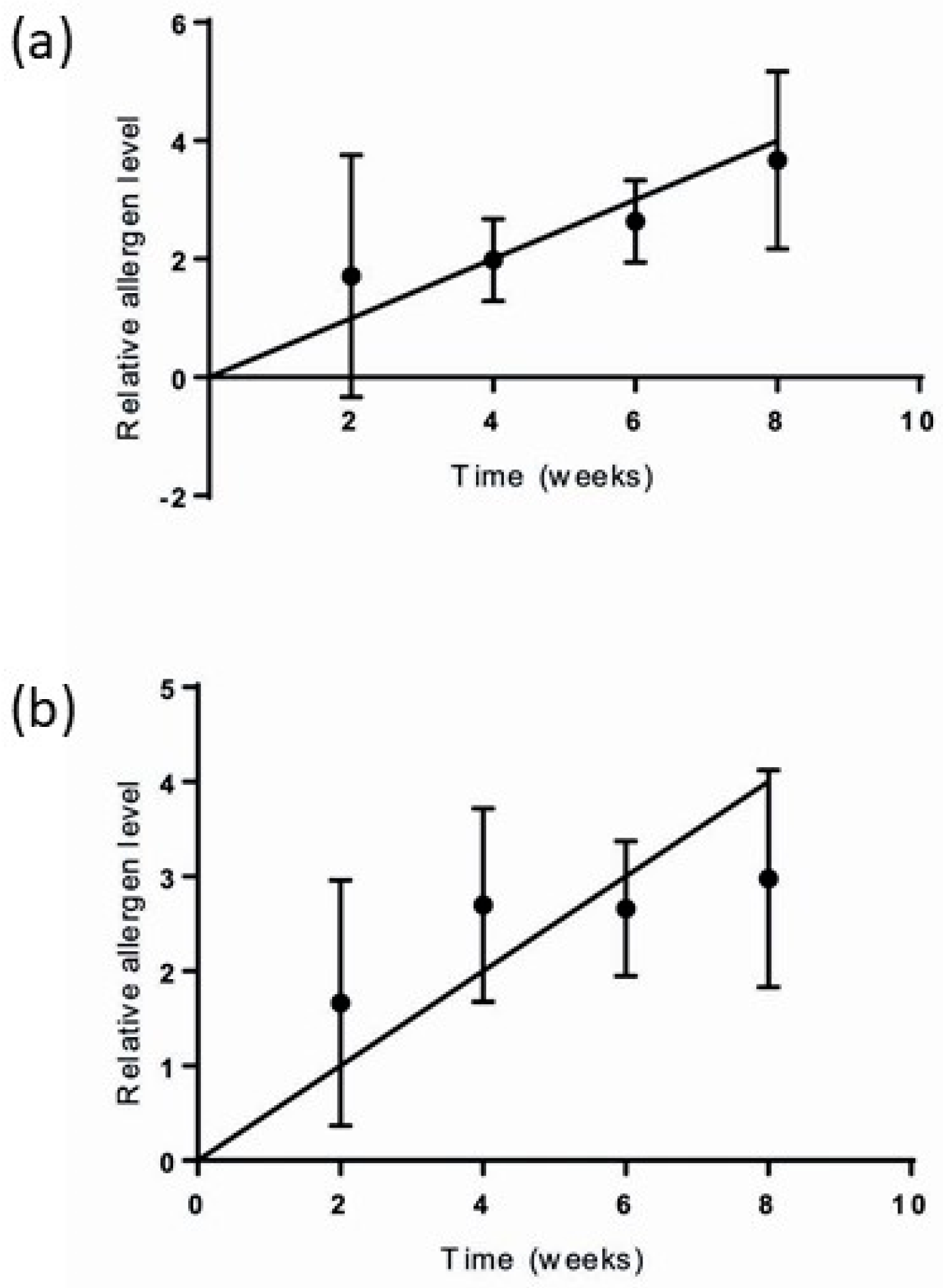
3.1. Prior Determination of Duration of Sampling Period
3.2. Participants and Household Characteristics
3.3. Risk Factors Associated with Detectable Allergens in the House
3.4. Risk Factors Associated with Higher or Lower Allergen Levels
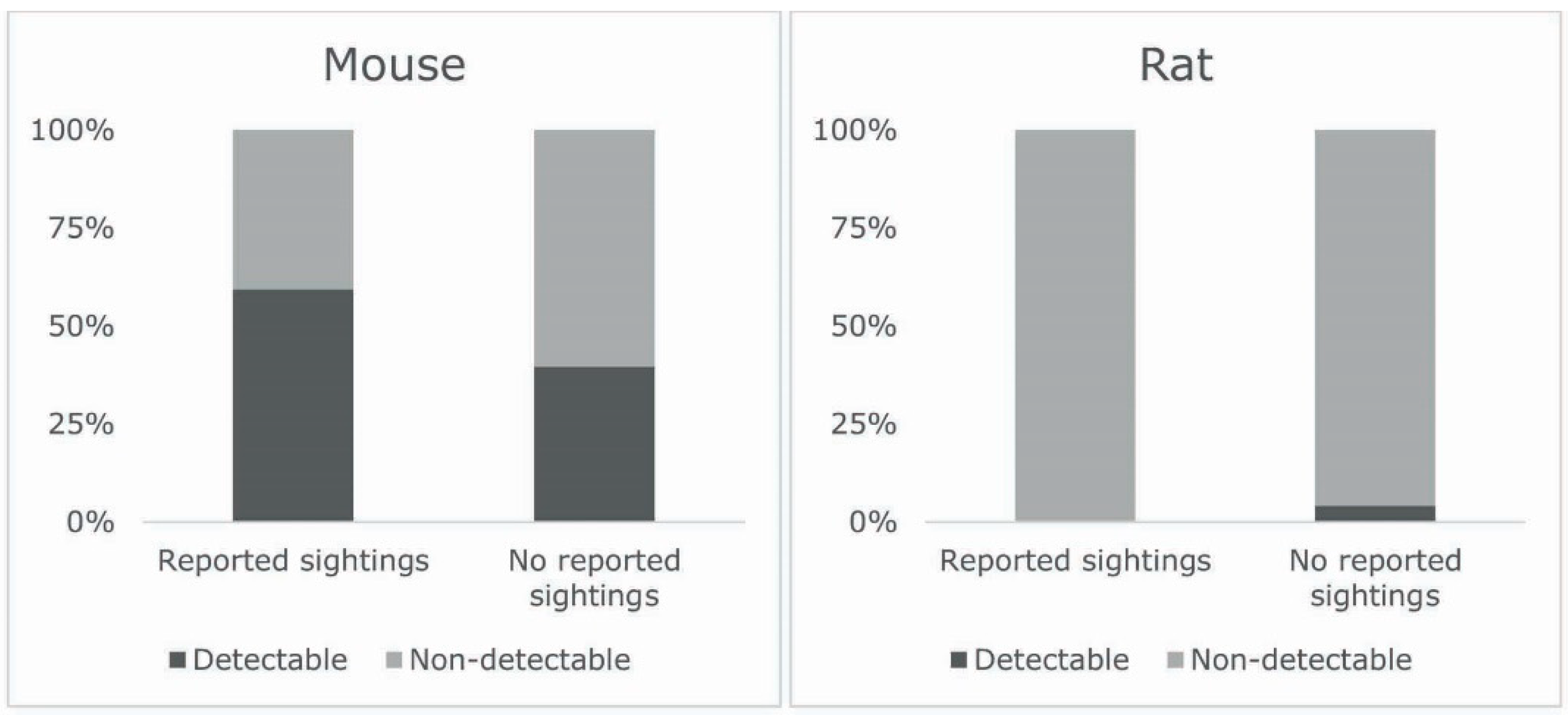
3.5. Relation between Sightings and Detectable Allergens
3.6. Relation between Allergens and Asthma
4. Discussion
4.1. Demographics
4.2. Building Location and Characteristics
4.3. Household Cleaning Habits
4.4. Relation between Mouse Sightings and Allergen Levels
4.5. Pets in the Home
4.6. Exposure to Mouse Allergen and Asthma
4.7. Strengths and Limitations of this Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
Research Ethics
References
- Ahluwalia, S.K.; Peng, R.D.; Breysse, P.N.; Diette, G.B.; Curtin-Brosnan, J.; Aloe, C.; Matsui, E.C. Mouse allergen is the major allergen of public health relevance in Baltimore City. J. Allergy Clin. Immunol. 2013, 132, 830–835. [Google Scholar] [CrossRef]
- Phipatanakul, W.; Eggleston, P.A.; Wright, E.C.; Wood, R.A. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J. Allergy Clin. Immunol. 2000, 106, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Matsui, E.C. Management of rodent exposure and allergy in the pediatric population. Curr. Allergy Asthma Rep. 2013, 13, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Cohn, R.D.; Arbes, S.J., Jr.; Yin, M.; Jaramillo, R.; Zeldin, D.C. National prevalence and exposure risk for mouse allergen in US households. J. Allergy Clin. Immunol. 2004, 113, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Salo, P.M.; Arbes, S.J., Jr.; Crockett, P.W.; Thorne, P.S.; Cohn, R.D.; Zeldin, D.C. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J. Allergy Clin. Immunol. 2008, 121, 678–684.e2. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Detjen, P.; Nimmagadda, S.; Rogers, L.; Patel, S.; Thompson, J.; Reboulet, R.; Gandhi, P. Bedroom exposure to airborne allergens in the Chicago area using a patient-operated sampling device. Ann. Allergy Asthma Immunol. 2018, 121, 211–217.e3. [Google Scholar] [CrossRef] [PubMed]
- Gruchalla, R.S.; Pongracic, J.; Plaut, M.; Evans, R.; Visness, C.M.; Walter, M.; Crain, E.F.; Kattan, M.; Morgan, W.J.; Steinbach, S.; et al. Inner City Asthma Study: Relationships among sensitivity, allergen exposure, and asthma morbidity. J. Allergy Clin. Immunol. 2005, 115, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Matsui, E.C.; Simons, E.; Rand, C.; Butz, A.; Buckley, T.J.; Breysse, P.; Eggleston, P.A. Airborne mouse allergen in the homes of inner-city children with asthma. J. Allergy Clin. Immunol. 2005, 115, 358–363. [Google Scholar] [CrossRef]
- Hernandez-Cadena, L.; Zeldin, D.C.; Barraza-Villarreal, A.; Sever, M.L.; Sly, P.D.; London, S.J.; Escamilla-Nunez, M.C.; Romieu, I. Indoor determinants of dustborne allergens in Mexican homes. Allergy Asthma Proc. 2015, 36, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zahradnik, E.; Raulf, M. Respiratory allergens from furred mammals: Environmental and occupational exposure. Vet. Sci. 2017, 4, 38. [Google Scholar] [CrossRef]
- Muti, D.; Purohit, A.; Dazy, A.; Verot, A.; de Blay, F. Mouse (Mus m1) and rat (Rat n1) allergen levels in dust from private and public houses in Strasbourg, France are lower than houses in the U.S.A. Eur. Ann. Allergy Clin. Immunol. 2012, 44, 93–95. [Google Scholar] [PubMed]
- Krop, E.J.M.; Jacobs, J.H.; Sander, I.; Raulf-Heimsoth, M.; Heederik, D.J.J. Allergens and β-Glucans in Dutch Homes and Schools: Characterizing Airborne Levels. PLoS ONE 2014, 9, e88871. [Google Scholar] [CrossRef]
- Lipman, S.A.; Burt, S.A. Self-reported prevalence of pests in Dutch households and the use of the health belief model to explore householders’ intentions to engage in pest control. PLoS ONE 2017, 12, e0190399. [Google Scholar] [CrossRef] [PubMed]
- Noss, I.; Wouters, I.M.; Visser, M.; Heederik, D.J.J.; Thorne, P.S.; Brunekreef, B.; Doekes, G. Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl. Environ. Microbiol. 2008, 74, 5621–5627. [Google Scholar] [CrossRef] [PubMed]
- Korpi, A.; Mäntyjärvi, R.; Rautiainen, J.; Kaliste, E.; Kalliokoski, P.; Renström, A.; Pasanen, A.-L. Detection of mouse and rat urinary aeroallergens with an improved ELISA. J. Allergy Clin. Immunol. 2004, 113, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Borlée, F.; Yzermans, C.J.; van Dijk, C.E.; Heederik, D.; Smit, L.A.M. Increased respiratory symptoms in COPD patients living in the vicinity of livestock farms. Eur. Respir. J. 2015, 46, 1605–1614. [Google Scholar] [CrossRef]
- Stelmach, I.; Jerzynska, J.; Stelmach, W.; Majak, P.; Chew, G.; Kuna, P. The prevalence of mouse allergen in inner-city homes. Pediatr. Allergy Immunol. 2002, 13, 299–302. [Google Scholar] [CrossRef]
- Berg, J.; McConnell, R.; Milam, J.; Galvan, J.; Kotlerman, J.; Thorne, P.; Jones, C.; Ferdman, R.; Eggleston, P.; Rand, C.; et al. Rodent allergen in Los Angeles inner city homes of children with asthma. J. Urban Health 2008, 85, 52–61. [Google Scholar] [CrossRef][Green Version]
- Global Invasive Species Database. Available online: http://www.iucngisd.org/gisd/search.php (accessed on 13 January 2017).
- The Mammal Society, Wood Mouse–Apodemus sylvaticus. Available online: http://www.mammal.org.uk/species-hub/full-species-hub/discover-mammals/species-wood-mouse/ (accessed on 2 February 2019).
- Van Adrichem, M.H.C.; Buijs, J.A.; Goedhart, P.W.; Verboom, J. Factors influencing the density of the brown rat (Rattus norvegicus) in and around houses in Amsterdam. Lutra 2013, 56, 77–91. [Google Scholar]
- Phipatanakul, W.; Cronin, B.; Wood, R.A.; Eggleston, P.A.; Shih, M.C.; Song, L.; Tachdjian, R.; Oettgen, H.C. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann. Allergy Asthma Immunol. 2004, 92, 420–425. [Google Scholar] [CrossRef]
- Torjusen, E.N.; Diette, G.B.; Breysse, P.N.; Curtin-Brosnan, J.; Aloe, C.; Matsui, E.C. Dose-response Relationships between Mouse Allergen Exposure and Asthma Morbidity Among Urban Children and Adolescents. Indoor Air 2013, 23, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Meijer, K.; Burggraaff, P.; Kamerich, W.S.; Harmanus, C. Wild mice in and around the city of Utrecht, the Netherlands, are carriers of Clostridium difficile but not ESBL-producing Enterobacteriaceae, Salmonella spp. or MRSA. Lett. Appl. Microbiol. 2018, 67, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Matsui, E.; Perzanowski, M.; Peng, R.D.; Wise, R.A.; Balcer-Whaley, S.; Newman, M.; Cunningham, A.; Divjan, A.; Bollinger, M.E.; Zhai, S.; et al. Effect of an Integrated Pest Management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma: A randomized clinical trial. J. Am. Med. Assoc. 2017, 317, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
| Demographics of the Population Included in Study | Frequency (n = 80) | % | Utrecht (n = 63) | % | Other (n = 17) | % |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 24 | 30 | 21 | 33.3 | 3 | 17.6 |
| Female | 56 | 70 | 42 | 66.7 | 14 | 82.4 |
| Age | ||||||
| Between 20 and 40 | 20 | 25 | 13 | 20.6 | 7 | 41.2 |
| Between 41 and 65 | 46 | 57.5 | 36 | 57.1 | 10 | 58.8 |
| Over 65 | 14 | 17.5 | 14 | 22.2 | 0 | 0 |
| Education | ||||||
| Lower education | 13 | 16.3 | 8 | 12.7 | 5 | 29.4 |
| Higher education | 67 | 83.3 | 55 | 87.3 | 12 | 70.6 |
| Smoke indoors (sometimes) (two missing values) | ||||||
| Sometimes | 7 | 9 | 4 | 6.6 | 3 | 17.6 |
| Participants that suffered from asthma | 9 | 11.25 | 7 | 11.1 | 2 | 11.8 |
| Pets at home (yes) (one missing value) | 39 | 49.5 | 28 | 45.2 | 11 | 64.7 |
| Cat | 21 | 26.25 | 15 | 24.2 | 6 | 35.3 |
| Dog | 11 | 13.75 | 8 | 12.9 | 3 | 17.6 |
| Mouse | 0 | 0 | 0 | 0 | 0 | 0 |
| Rat | 1 | 1.25 | 0 | 0 | 1 | 5.9 |
| Sightings | ||||||
| Cockroaches sightings (yes) | 0 | 0 | 0 | 0 | 0 | 0 |
| Mouse sightings at home (yes) | 32 | 40 | 26 | 41.3 | 6 | 35.3 |
| Rat sightings at home (yes) | 8 | 10 | 2 | 3.2 | 6 | 35.3 |
| Detectable mouse allergens in the house | 38 | 47.5 | 27 | 42.9 | 11 | 64.7 |
| Detectable rat allergens in the house | 3 | 3.8 | 1 | 1.6 | 2 | 11.8 |
| Household Characteristics and Reported Cleaning Habits | Frequency (n = 80) | % |
|---|---|---|
| Distance from home to green area (m) | ||
| Less than 250 | 47 | 58.7 |
| More than 250 | 33 | 41.3 |
| Distance from home to surface water (m) | ||
| Less than 250 | 45 | 56.3 |
| More than 250 | 35 | 43.7 |
| Private garden (yes) | 68 | 85 |
| Age of the building | ||
| Before 1920 | 28 | 35 |
| Between 1920–1960 | 32 | 40 |
| After 1960 | 20 | 25 |
| Type of dwelling | ||
| Terraced house | 51 | 63.7 |
| Other | 29 | 36.3 |
| Floor of the living room | ||
| Below ground or ground floor | 63 | 78.7 |
| First floor or higher | 17 | 21.3 |
| Type of floor in the living room | ||
| Smooth (tiles, parquet, laminate) | 61 | 76.3 |
| Other | 19 | 23.7 |
| Insulated living room floor (yes) | 41 | 51.2 |
| Double glazed windows (yes) | 65 | 81.3 |
| Ventilation | ||
| Through a ventilation grid (yes) | 28 | 35 |
| Through an open window (yes) | 41 | 51.2 |
| Through an open exterior door (yes) | 50 | 62.5 |
| Mechanical ventilation system (yes) | 26 | 32.5 |
| Household conditions | ||
| Water damage or leakage (yes) | 7 | 8.8 |
| Mildew or mould spots (yes) | 16 | 20 |
| Smell of mildew or mould (yes) | 9 | 11.3 |
| Usage of cleaning agents (sometimes) | ||
| Bleach/chlorine | 60 | 75 |
| Ammonia | 18 | 22.5 |
| Acids, liquid descaler | 45 | 56.3 |
| Solvents | 35 | 43.8 |
| Furniture polish | 9 | 11.3 |
| Cleaning spray | 65 | 81.3 |
| Degreasing sprays | 52 | 65 |
| Polishes or wax for the floor or furniture | 48 | 60 |
| Multipurpose cleaner | 50 | 62.5 |
| High frequency of cleaning habits (more than 1x per week) | ||
| Cleaning in general | 49 | 61.2 |
| Vacuuming | 56 | 70 |
| Sweeping | 35 | 43.8 |
| Dusting | 30 | 37.5 |
| Wet cleaning of the floor | 21 | 26.2 |
| Univariate Analysis | Multivariate Analysis (All Cases, n = 80) | Multivariate Analysis (Cases in Utrecht only, n = 63) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables * | Exp(B) OR | 95% C.I. for EXP(B) | Sig. | Exp(B) OR | 95% C.I. for EXP(B) | Sig. | Exp(B) OR | 95% C.I. for EXP(B) | Sig. | |||
| Lower | Upper | lower | Upper | lower | upper | |||||||
| Less than 250 m from green area | 2.17 | 0.87 | 5.26 | 0.097 | 5.56 | 1.59 | 20.00 | 0.008 | 7.25 | 1.41 | 37.04 | 0.018 |
| Less than 250 m from surface water | 0.50 | 0.20 | 1.22 | 0.130 | 0.24 | 0.07 | 0.84 | 0.025 | ||||
| House built before 1920 | 0.77 | 0.24 | 2.46 | 0.658 | 6.11 | 1.20 | 31.17 | 0.03 | ||||
| House built between 1920–1960 | 0.35 | 0.11 | 1.11 | 0.074 | ||||||||
| House built after 1960 | 1 | |||||||||||
| House has a private garden | 0.89 | 0.26 | 3.03 | 0.851 | 7.00 | 0.68 | 71.66 | 0.10 | ||||
| Living room floor is insulated | 2.04 | 0.84 | 4.99 | 0.116 | 3.28 | 1.04 | 10.36 | 0.042 | 13.25 | 2.52 | 69.57 | 0.002 |
| Living room is ventilated by an open window | 3.13 | 1.25 | 7.80 | 0.015 | 6.10 | 1.89 | 19.71 | 0.002 | 7.65 | 1.51 | 38.91 | 0.014 |
| Smell of mildew/mould in the last 12 months | 1.44 | 0.36 | 5.81 | 0.609 | ||||||||
| Furniture polish used | 0.51 | 0.12 | 2.22 | 0.373 | 0.11 | 0.01 | 0.81 | 0.03 | ||||
| Multipurpose cleaner used | 0.90 | 0.05 | 14.95 | 0.943 | ||||||||
| Degreasing spray used | 1.07 | 0.43 | 2.68 | 0.888 | ||||||||
| Solvents used | 0.88 | 0.36 | 2.13 | 0.778 | ||||||||
| Dusting at least once a week | 1.17 | 0.47 | 2.91 | 0.729 | ||||||||
| Vacuuming at least once a week | 1.40 | 0.53 | 3.68 | 0.495 | ||||||||
| Wet cleaning of floor at least once a week | 2.21 | 0.80 | 6.14 | 0.128 | ||||||||
| Mouse sightings in the last 12 months | 2.23 | 0.90 | 5.56 | 0.085 | 2.90 | 0.888 | 9.46 | 0.078 | 4.78 | 1.14 | 20.06 | 0.033 |
| Cat as pet | 0.25 | 0.08 | 0.79 | 0.017 | ||||||||
| Univariate Analysis | Multivariate Analysis (All Cases, n = 80) | Multivariate Analysis (Cases in Utrecht only, n = 63) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables * | GMR | 95.0% Confidence Interval for GMR | Sig. | GMR | 95.0% Confidence Interval for GMR | Sig. | GMR | 95.0% Confidence Interval for GMR | Sig. | |||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Less than 250 m from green area | 1.14 | 0.79 | 1.75 | 0.430 | 1.43 | 1.20 | 1.93 | 0.001 | 1.65 | 1.38 | 2.32 | 0 |
| Less than 250 m from surface water | 0.89 | 0.60 | 1.27 | 0.476 | 0.73 | 0.54 | 0.88 | 0.003 | 0.77 | 0.54 | 0.95 | 0.021 |
| House built before 1920 | 1.06 | 0.76 | 1.49 | 0.692 | ||||||||
| House built between 1920–1960 | 0.85 | 0.60 | 1.15 | 0.256 | ||||||||
| House built after 1960 (= reference) | 1.00 | |||||||||||
| House has a private garden | 0.84 | 0.46 | 1.26 | 0.278 | ||||||||
| Living room floor is insulated | 1.03 | 0.70 | 1.52 | 0.850 | 1.23 | 1.00 | 1.65 | 0.048 | ||||
| Living room is ventilated by an open window | 0.90 | 0.59 | 1.31 | 0.536 | 1.28 | 1.06 | 1.67 | 0.014 | ||||
| Smell of mildew/mould in the last 12 months | 0.89 | 0.47 | 1.43 | 0.481 | 0.81 | 0.46 | 1.01 | 0.053 | 0.78 | 0.34 | 0.91 | 0.021 |
| Furniture polish used | 0.99 | 0.49 | 1.97 | 0.961 | 0.77 | 0.41 | 0.90 | 0.013 | ||||
| Multipurpose cleaner used | 0.73 | 0.11 | 1.03 | 0.057 | 0.81 | 0.23 | 0.97 | 0.042 | ||||
| Degreasing spray used | 0.75 | 0.49 | 1.04 | 0.076 | 0.82 | 0.63 | 1.00 | 0.053 | ||||
| Solvents used | 0.82 | 0.55 | 1.16 | 0.222 | 0.76 | 0.56 | 0.93 | 0.013 | ||||
| Dusting at least once a week | 0.64 | 0.43 | 0.86 | 0.006 | 0.68 | 0.47 | 0.84 | 0.003 | ||||
| Vacuuming at least once a week | 0.93 | 0.59 | 1.4 | 0.669 | 1.22 | 0.95 | 1.75 | 0.1 | ||||
| Wet cleaning of floor at least once a week | 0.74 | 0.48 | 1.03 | 0.072 | ||||||||
| Mouse sightings in the last 12 months | 1.11 | 0.77 | 1.63 | 0.552 | 1.20 | 0.96 | 1.55 | 0.09 | 1.22 | 0.98 | 1.65 | 0.071 |
| Cat as pet | 1.05 | 0.61 | 1.9 | 0.787 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burt, S.A.; Parramon Dolcet, L.I.; Wouters, I.M. Airborne Rodent Allergen Levels in Dutch Households: A Pilot Study. Int. J. Environ. Res. Public Health 2019, 16, 3736. https://doi.org/10.3390/ijerph16193736
Burt SA, Parramon Dolcet LI, Wouters IM. Airborne Rodent Allergen Levels in Dutch Households: A Pilot Study. International Journal of Environmental Research and Public Health. 2019; 16(19):3736. https://doi.org/10.3390/ijerph16193736
Chicago/Turabian StyleBurt, Sara A., Lidia I. Parramon Dolcet, and Inge M. Wouters. 2019. "Airborne Rodent Allergen Levels in Dutch Households: A Pilot Study" International Journal of Environmental Research and Public Health 16, no. 19: 3736. https://doi.org/10.3390/ijerph16193736
APA StyleBurt, S. A., Parramon Dolcet, L. I., & Wouters, I. M. (2019). Airborne Rodent Allergen Levels in Dutch Households: A Pilot Study. International Journal of Environmental Research and Public Health, 16(19), 3736. https://doi.org/10.3390/ijerph16193736





