A Model of Phlebitis Associated with Peripheral Intravenous Catheters in Orthopedic Inpatients
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Participants
2.3. Data Collection
2.4. Ethical Considerations
2.5. Data Analysis
3. Results
3.1. Incidence and Severity of Phlebitis Based on the Modified INS Scale
3.2. Association between Intravenous Injection-Related Factors and the Incidence of Phlebitis
3.3. Predictive Factors for PIVC-Related Phlebitis in the 95% CI Model
3.4. Effect Sizes of Predictive Factors for PIVC-Related Phlebitis in the 95% CI Model
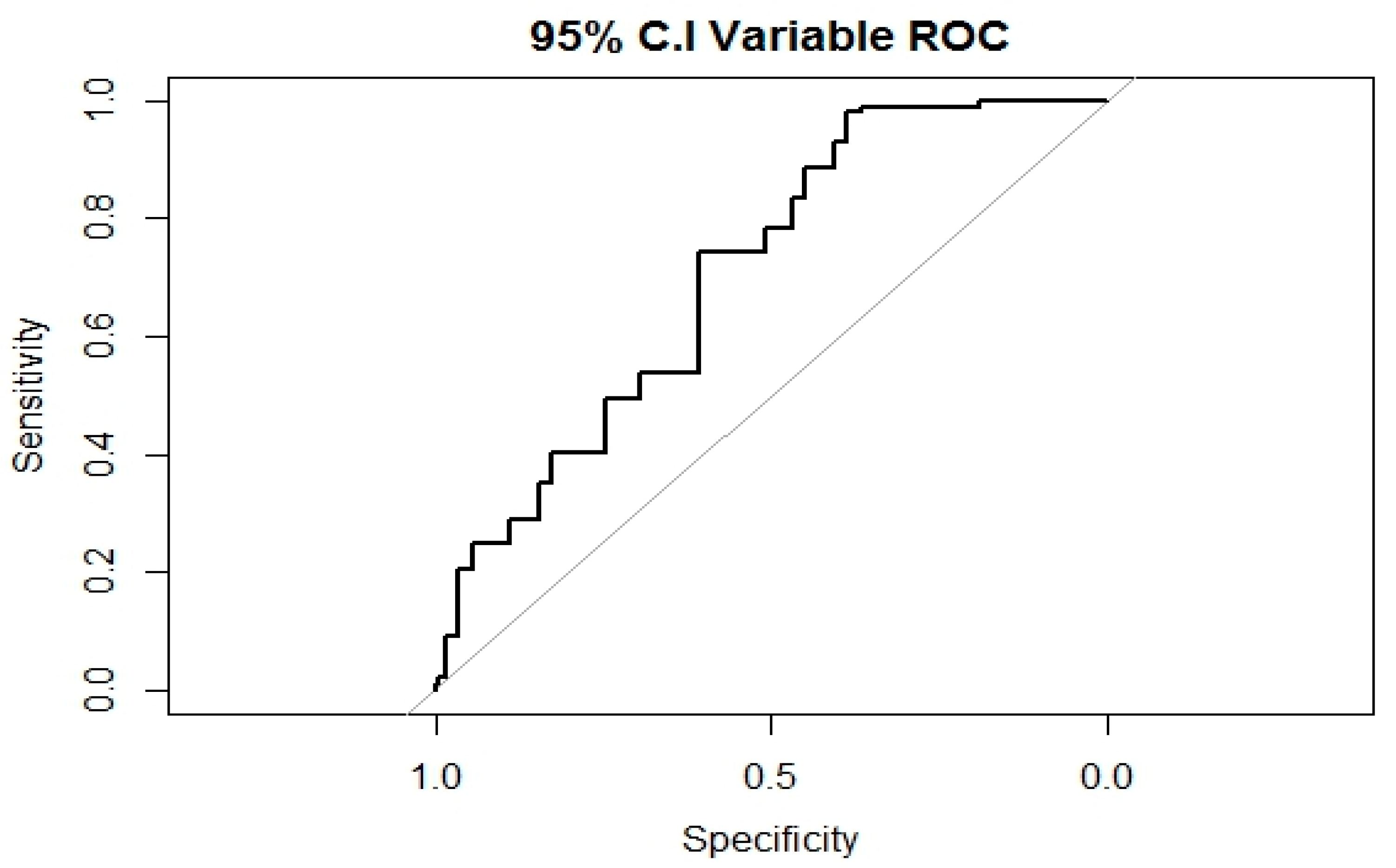
3.5. Fit of the Prediction Model for PIVC-related Phlebitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vukovic, A.A.; Frey, M.; Byczkowski, T.; Taylor, R.; Kerrey, B.T. Video-based Assessment of Peripheral Intravenous Catheter Insertion in the Resuscitation Area of a Pediatric Emergency Department. Acad. Emerg. Med. 2016, 23, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.E.; Klausner, J.D.; Klemperer, J.D.; Flint, L.M.; Huang, E. Accepted but unacceptable: Peripheral IV catheter failure. J. Infus. Nurs. 2015, 38, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; Carretero, A.; Díaz, D.; Fuentes, C.; González, J.I.; García-Reyne, A.; Aguado, J.M.; López-Medrano, F. Hospital-wide survey of the adequacy in the number of vascular catheters and catheter lumens. J. Hosp. Med. 2014, 9, 35–41. [Google Scholar] [CrossRef] [PubMed]
- New, K.A.; Webster, J.; Marsh, N.M.; Hewer, B. Intravascular device use, management, documentation and complications: A point prevalence survey. Aust. Health Rev. 2014, 38, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Piredda, M.; Biagioli, V.; Barrella, B.; Carpisassi, I.; Ghinelli, R.; Giannarelli, D.; De Marinis, M.G. Factors affecting difficult peripheral intravenous cannulation in adults: A prospective observational study. J. Clin. Nurs. 2017, 26, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Lillis, C.; Lynn, P. Fundamentals of Nursing: The Art and Science of Person-Centered Care; Wolters Kluwer: Philadelphia, PA, USA, 2015. [Google Scholar]
- Pires Nobre, A.S.; da Silva Martins, M.D. Prevalence of peripheral intravenous catheter-related phlebitis: Associated factors. Rev. Enferm. Ref. 2018, 4, 127–138. [Google Scholar] [CrossRef]
- Washington, G.T.; Barrett, R. Peripheral phlebitis: A point-prevalence study. J. Infus. Nurs. 2012, 35, 252–258. [Google Scholar] [CrossRef] [PubMed]
- do Rego Furtado, L.C. Maintenance of peripheral venous access and its impact on the development of phlebitis: A survey of 186 catheters in a general surgery department in Portugal. J. Infus. Nurs. 2011, 34, 382–390. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.R. Incidence of phlebitis according to intravenous therapy in inpatients. Keimyung J. Nurs. Sci. 2010, 14, 29–39. [Google Scholar]
- Salgueiro-Oliveira, A.; Parreira, P.; Veiga, P. Incidence of phlebitis in patients with peripheral intravenous catheters: The influence of some risk factors. Aust. J. Adv. Nurs. 2012, 30, 32–39. [Google Scholar]
- Rego Furtado, L.C. Incidence and predisposing factors of phlebitis in a surgery department. Br. J. Nurs. 2011, 20, S16–S25. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Osborne, S.; Rickard, C.M.; New, K. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst. Rev. 2015, 8, CD007798. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.D.; Crawford, B.S.; Phillips, G.; Berger, M.J.; Wesolowski, R. Incidence of infusion-site reactions associated with peripheral intravenous administration of fosaprepitant. Support. Care Cancer 2014, 22, 1461–1466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O’grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef] [PubMed]
- Roca, G.M.; Bertolo, C.B.; Lopez, P.T.; Samaranch, G.G.; Ramirez, M.C.A.; Buqueras, J.C.; Rodríguez-Baño, J.; Martinez, J.A. Assessing the influence of risk factors on rates and dynamics of peripheral vein phlebitis: An observational cohort study. Med. Clin. 2012, 139, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Pittet, D.; Allegranzi, B.; Boyce, J. World Health Organization World Alliance for Patient Safety First Global Patient Safety Challenge Core Group of Experts. The World Health Organization guidelines on hand hygiene in health care and their consensus recommendations. Infect. Control. Hosp. Epidemiol. 2009, 30, 611–622. [Google Scholar] [CrossRef]
- Rickard, C.M.; Marsh, N.M.; Webster, J.; Gavin, N.C.; McGrail, M.R.; Larsen, E.; Corley, A.; Long, D.; Gowardman, J.R.; Murgo, M.; et al. Intravascular device administration sets: Replacement after standard versus prolonged use in hospitalised patients—A study protocol for a randomised controlled trial (The RSVP Trial). BMJ Open 2015, 5, e007257. [Google Scholar] [CrossRef]
- Arias-Fernandez, L.; Suerez-Mier, B.; Martinez-Ortega, M.D.; Lana, A. Incidence and risk factors of phlebitis associated to peripheral intravenous catheters. Enferm. Clin. 2017, 27, 79–86. [Google Scholar] [CrossRef]
- Wallis, M.C.; McGrail, M.; Webster, J.; Marsh, N.; Gowardman, J.; Playford, E.G.; Rickard, C.M. Risk factors for peripheral intravenous catheter failure: A multivariate analysis of data from a randomized controlled trial. Infect. Control. Hosp. Epidemiol. 2014, 35, 63–68. [Google Scholar] [CrossRef]
- Korea Health Industry Development Institute. 2013 Statistics for Hospital Management. Available online: https://www.khidi.or.kr/kps (accessed on 13 March 2018).
- Roszell, S.; Jones, C. Intravenous administration issues: A comparison of intravenous insertions and complications in vancomycin versus other antibiotics. J. Infus. Nurs. 2010, 33, 112–118. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, J.S. Use of antimicrobial agents for the treatment of inpatients in Chonbuk National University Hospital. Pediatr. Infect. Vaccine 2000, 7, 225. [Google Scholar] [CrossRef]
- Regueiro Pose, M.A.; Souto Rodríguez, B.; Iglesias Maroño, M.; Outón Fernández, I.; Cambeiro Nuñez, J.; Pértega Díaz, S.; Pita Fernández, S. Peripheral venous catheters: Incidence of phlebitis and its determining factors. Rev. Enferm. 2005, 28, 21–28. [Google Scholar]
- Webster, J.; Morris, H.L.; Robinson, K.; Sanderson, U. Development and validation of a vein assessment tool (VAT). Aust. J. Adv. Nurs. 2007, 24, 5–7. [Google Scholar] [PubMed]
- Denkinger, M.D.; Coll-Planas, L.; Jamour, M.; Nikolaus, T. The assessment of physical activity in inpatient rehabilitation—An important aspect of the identification of frailty in hospitalized older people. J. Am. Geriatr. Soc. 2007, 55, 967–968. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.A.; Yee, B.H. Incidence and severity of phlebitis in patients receiving peripherally infused amiodarone. Crit. Care Nurse 2012, 32, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Neal, R.M. MCMC using Hamiltonian dynamics. Handb. Markov Chain Monte Carlo 2011, 2, 11. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model. Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer Science & Business Media: Berlin, Germany, 2003. [Google Scholar]
- McFadden, D. Conditional Logit Analysis of Qualitative Choice Behavior. In Frontiers in Econometrics; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Congdon, P. Bayesian Statistical Modelling; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398. [Google Scholar]
- Everitt, B.; Skrondal, A. The Cambridge Dictionary of Statistics, 4th ed.; Cambridge University Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Ray-Barruel, G.; Polit, D.F.; Murfield, J.E.; Rickard, C.M. Infusion phlebitis assessment measures: A systematic review. J. Eval. Clin. Pract. 2014, 20, 191–202. [Google Scholar] [CrossRef]
- Mowry, J.L.; Hartman, L.S. Intravascular thrombophlebitis related to the peripheral infusion of amiodarone and vancomycin. West. J. Nurs. Res. 2011, 33, 457–471. [Google Scholar] [CrossRef]
- Hadaway, L.C.; Millam, D.A. On the road to successful I.V. starts. Nursing 2005, 37, 1–14. [Google Scholar] [CrossRef]
- Plumer, A.L. Plumer’s Principles and Practice of Intravenous Therapy; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Chee, S.; Tan, W. Reducing infusion phlebitis in Singapore hospitals using extended life end-line filters. J. Infus. Nurs. 2002, 25, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hadaway, L. Extravasation injuries from contrast media in radiology. J. Leg. Nurs. Consult. 2017, 28, 13–16. [Google Scholar]
- Bernard, L.; Biron, A.; Lavigne, G.; Frechette, J.; Bernard, A.; Mitchell, J.; Lavoie-Tremblay, M. An exploratory study of safety culture, biological risk management and hand hygiene of healthcare professionals. J. Adv. Nurs. 2018, 74, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, K. Hand Hygiene Promotion from the US Perspective: Putting WHO and CDC Guidelines into Practice. In Hand Hygiene: A Handbook for Medical Professionals; Pittet, D., Boyce, J.M., Allegranzi, B., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; Volume 9, p. 221. [Google Scholar]
- Pires, D.; Soule, H.; Bellissimo-Rodrigues, F.; Gayet-Ageron, A.; Pittet, D. Hand hygiene with alcohol-based hand rub: How long is long enough? Infect. Control. Hosp. Epidemiol. 2017, 38, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.M.; Pittet, D. Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm. Rep. 2002, 51, 1–45. [Google Scholar] [PubMed]
- World Health Organization. Guidelines on Hand Hygiene in Health Care; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Chen, S.; Yao, J.; Chen, J.; Liu, L.; Miu, A.; Jiang, Y.; Zhu, J.; Tang, S.; Chen, Y. Knowledge of “Guidelines for the prevention of intravascular catheter-related infections (2011)”: A survey of intensive care unit nursing staffs in China. Int. J. Nurs. Sci. 2015, 2, 383–388. [Google Scholar] [CrossRef]
- Webster, J.; McGrail, M.; Marsh, N.; Wallis, M.C.; Ray-Barruel, G.; Rickard, C.M. Postinfusion Phlebitis: Incidence and Risk Factors. Nurs. Res. Pract. 2015, 2015, 691934. [Google Scholar] [CrossRef]
- da Silva, G.A.; Priebe, S.; Dias, F.N. Benefits of establishing an intravenous team and the standardization of peripheral intravenous catheters. J. Infus. Nurs. 2010, 33, 156–160. [Google Scholar] [CrossRef]
- Cho, M.; Cho, Y.; Kim, K.; Kwon, I.; Kim, M.; Lee, J. Development of clinical ladder system model for nurses: For tertiary care hospitals. J. Korean Clin. Nurs. Res. 2015, 21, 277–292. [Google Scholar]
| Grade | Criteria | n (%) |
|---|---|---|
| 0 | No clinical symptoms | 173 (64.1) |
| 0+ | Pain at site, but no clinical symptoms | 49 (18.2) |
| 1+ | Erythema at access site with or without pain | 29 (10.7) |
| 2+ | Pain at access site with erythema and/or edema | 16 (5.9) |
| 3+ | Pain at access site with erythema and/or edema, streak formation, palpable venous cord | 3 (1.1) |
| 4+ | Pain at access site with erythema and/or edema, or palpable venous cord > 1 inch, purulent drainage | 0 (0) |
| Characteristics | Categories | Occurrence of Phlebitis, n (%) | χ2 | p | |||
|---|---|---|---|---|---|---|---|
| Total (n = 270) | Yes (n = 97) | No (n = 173) | |||||
| Individual | Sex | Male | 136 (50.4) | 47 (48.5) | 89 (51.4) | 0.12 | 0.730 |
| Female | 134 (49.6) | 50 (51.5) | 84 (48.6) | ||||
| Age (years) | <60 | 144 (53.3) | 48 (49.5) | 96 (55.5) | 0.68 | 0.411 | |
| ≥60 | 126 (46.7) | 49 (50.5) | 77 (44.5) | ||||
| Underlying conditions | Yes | 122 (45.2) | 46 (47.4) | 76 (43.9) | 0.18 | 0.670 | |
| No | 148 (54.8) | 51 (52.6) | 97 (56.1) | ||||
| Diagnostic areas | Upper limb | 98 (36.3) | 42 (43.3) | 56 (32.4) | 5.37 | 0.068 | |
| Lower limb | 145 (53.7) | 43 (44.3) | 102 (59.0) | ||||
| Spine | 27 (10.0) | 12 (12.4) | 15 (8.7) | ||||
| Underwent surgery | Yes | 106 (39.3) | 38 (39.2) | 68 (39.3) | 0.00 | 1.000 | |
| No | 164 (60.7) | 59 (60.8) | 105 (60.7) | ||||
| Vein quality | Good | 141 (52.2) | 36 (37.1) | 105 (60.7) | 17.40 | <0.001 | |
| Fair | 100 (37.0) | 43 (44.3) | 57 (32.9) | ||||
| Poor | 29 (10.7) | 18 (18.6) | 11 (6.4) | ||||
| Caregiver residence | Yes | 157 (58.1) | 54 (55.7) | 103 (59.5) | 0.24 | 0.624 | |
| No | 113 (41.9) | 43 (44.3) | 70 (40.5) | ||||
| Use a walking device | Yes | 72 (26.7) | 23 (23.8) | 49 (28.3) | 0.46 | 0.497 | |
| No | 198 (73.3) | 74 (76.3) | 124 (71.7) | ||||
| Activity level | Inactive | 130 (48.1) | 38 (39.2) | 92 (53.2) | 4.33 | 0.037 | |
| Active | 140 (51.9) | 59 (60.8) | 81 (46.8) | ||||
| Chemical | Fluid therapy | Yes | 229 (84.8) | 89 (91.8) | 140 (80.9) | 4.85 | 0.028 |
| No | 41 (15.2) | 8 (8.2) | 33 (19.1) | ||||
| Drugs mixed with fluids | Yes | 198 (73.3) | 78 (80.4) | 120 (69.4) | 3.34 | 0.068 | |
| No | 72 (26.7) | 19 (19.6) | 53 (30.6) | ||||
| Number of IV drugs | <3 | 180 (66.7) | 59 (60.8) | 121 (69.9) | 1.93 | 0.164 | |
| ≥3 | 90 (33.3) | 38 (39.2) | 52 (30.1) | ||||
| Types of antibiotics | Cephalosporin | 195 (72.2) | 66 (68.0) | 129 (74.6) | 5.91 | 0.116 | |
| Penicillin | 21 (7.8) | 11 (11.3) | 10 (5.8) | ||||
| Others | 12 (4.4) | 7 (7.2) | 5 (2.9) | ||||
| Did not use | 42 (15.6) | 13 (13.4) | 29 (16.8) | ||||
| Drugs with high osmolality | Yes | 30 (11.1) | 19 (19.6) | 11 (6.4) | 9.72 | 0.002 | |
| No | 240 (88.9) | 78 (80.4) | 162 (93.6) | ||||
| Use of contrast medium | Yes | 37 (13.7) | 28 (28.9) | 9 (5.2) | 27.46 | <0.001 | |
| No | 233 (86.3) | 69 (71.1) | 164 (94.8) | ||||
| Method of infusion | Continuous | 22 (8.1) | 4 (4.1) | 18 (10.4) | 10.18 | 0.006 | |
| Intermittent | 41 (15.2) | 8 (8.2) | 33 (19.1) | ||||
| Continuous and intermittent | 207 (76.7) | 85 (87.6) | 122 (70.5) | ||||
| Total daily dose (mL) | <1000 | 172 (63.7) | 57 (58.8) | 115 (66.5) | 1.28 | 0.257 | |
| ≥1000 | 98 (36.3) | 40 (41.2) | 58 (33.5) | ||||
| Mechanical | Catheter dwell time (h) | ≤24 | 48 (17.8) | 22 (22.7) | 26 (15.0) | 16.84 | 0.001 |
| 25–48 | 122 (45.2) | 54 (55.7) | 68 (39.3) | ||||
| 49–72 | 63 (23.3) | 16 (16.5) | 47 (27.2) | ||||
| 73–96 | 37 (13.7) | 5 (5.2) | 32 (18.5) | ||||
| Side of catheter insertion | Right | 129 (47.8) | 47 (48.5) | 82 (47.4) | 0.00 | 0.968 | |
| Left | 141 (52.2) | 50 (51.5) | 91 (52.6) | ||||
| Site of catheter insertion | Hand | 52 (19.3) | 24 (24.7) | 28 (16.2) | 3.20 | 0.202 | |
| Arm | 211 (78.1) | 70 (72.2) | 141 (81.5) | ||||
| Lower limb | 7 (2.6) | 3 (3.1) | 4 (2.3) | ||||
| Catheter gauge | 18 | 123 (45.6) | 48 (49.5) | 75 (43.4) | 0.71 | 0.399 | |
| ≤20 | 147 (54.4) | 49 (50.5) | 98 (56.6) | ||||
| Infectious | Hand hygiene duration (s) | <10 | 98 (36.3) | 64 (66.0) | 34 (19.7) | 60.02 | <0.001 |
| ≥10 to <20 | 92 (34.1) | 32 (33.0) | 60 (34.7) | ||||
| ≥20 to <30 | 71 (26.3) | 1 (1.0) | 70 (40.5) | ||||
| ≥30 | 9 (3.3) | 0 (0.0) | 9 (5.2) | ||||
| Period of nursing clinical experience (years) | <1 year | 44 (16.3) | 18 (18.6) | 26 (15.0) | 24.66 | <0.001 | |
| ≥1 to <3 | 74 (27.4) | 42 (43.3) | 32 (18.5) | ||||
| ≥3 to <5 | 31 (11.5) | 5 (5.2) | 26 (15.0) | ||||
| ≥5 to <7 | 96 (35.6) | 24 (24.7) | 72 (41.6) | ||||
| ≥7 | 25 (9.3) | 8 (8.2) | 17 (9.8) | ||||
| Factors | Variables | Category | Reference Category | Coefficient | 95% CI | Post. Prob | MCSE | ESS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | 2.5% | 97.5% | |||||||
| Individual | Vein quality | Fair | Good | 0.922 | 0.926 | 0.416 | 0.095 | 1.728 | 0.95 | 2.94−3 | 100.0 |
| Poor | 2.114 | 2.098 | 0.646 | 0.880 | 3.420 | 0.95 | 4.57−3 | 99.8 | |||
| Chemical | Use of contrast medium | No | Yes | −2.591 | −2.566 | 0.659 | −3.958 | −1.366 | 0.95 | 4.81−3 | 93.9 |
| Infectious | Hand hygiene (s) | ≥10 to <20 | <10 | −1.595 | −1.590 | 0.404 | −2.413 | −0.828 | 0.95 | 2.86−3 | 100.0 |
| ≥20 to <30 | −5.549 | −5.407 | 1.205 | −8.363 | −3.617 | 0.95 | 1.02−2 | 69.8 | |||
| ≥30 | −26.158 | v10.335 | 46.000 | −168.176 | −2.738 | 0.95 | 2.93 | 1.2 | |||
| Period of nursing clinical experience (years) _cons | ≥1 to <3 | <1 | 0.210 | 0.138 | 0.421 | −0.557 | 1.156 | NA | 3.05−3 | 95.6 | |
| ≥3 to <5 | −1.989 | −1.965 | 0.739 | −3.480 | −0.561 | 0.95 | 5.43−3 | 92.8 | |||
| ≥5 to <7 | −0.775 | −0.772 | 0.495 | −1.753 | 0.079 | NA | 3.57−3 | 96.5 | |||
| ≥7 | 0.715 | 0.627 | 0.704 | −0.399 | 2.246 | NA | 5.04−3 | 97.7 | |||
| 2.837 | 2.821 | 0.766 | 1.395 | 4.377 | NA | NA | NA | ||||
| Factors | Variables | Category | Reference Category | OR | 95% CI | Post. Prob | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | SD | |||||||
| Individual | Vein quality | Fair | Good | 2.514 | 2.525 | 1.155 | 1.100 | 5.630 | 0.95 |
| Poor | 8.286 | 8.150 | 7.186 | 2.413 | 30.585 | 0.95 | |||
| Chemical | Use of contrast medium | No | Yes | 0.074 | 0.076 | 0.060 | 0.019 | 0.255 | 0.95 |
| Infectious | Hand hygiene (s) | ≥10 to <20 | <10 | 0.202 | 0.203 | 0.088 | 0.089 | 0.436 | 0.95 |
| ≥20 to <30 | 0.003 | 0.004 | 0.006 | 0.000 | 0.026 | 0.95 | |||
| period of nursing clinical experience (years) _cons | ≥3 to <5 | <1 | 0.136 | 0.140 | 0.137 | 0.030 | 0.570 | 0.95 | |
| 17.074 | 16.809 | 19.291 | 4.035 | 79.657 | NA | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kim, K.; Kim, J.-S. A Model of Phlebitis Associated with Peripheral Intravenous Catheters in Orthopedic Inpatients. Int. J. Environ. Res. Public Health 2019, 16, 3412. https://doi.org/10.3390/ijerph16183412
Lee S, Kim K, Kim J-S. A Model of Phlebitis Associated with Peripheral Intravenous Catheters in Orthopedic Inpatients. International Journal of Environmental Research and Public Health. 2019; 16(18):3412. https://doi.org/10.3390/ijerph16183412
Chicago/Turabian StyleLee, Sookhee, Kyunghee Kim, and Ji-Su Kim. 2019. "A Model of Phlebitis Associated with Peripheral Intravenous Catheters in Orthopedic Inpatients" International Journal of Environmental Research and Public Health 16, no. 18: 3412. https://doi.org/10.3390/ijerph16183412
APA StyleLee, S., Kim, K., & Kim, J.-S. (2019). A Model of Phlebitis Associated with Peripheral Intravenous Catheters in Orthopedic Inpatients. International Journal of Environmental Research and Public Health, 16(18), 3412. https://doi.org/10.3390/ijerph16183412





