Effect of Endogenous Selenium on Arsenic Uptake and Antioxidative Enzymes in As-Exposed Rice Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice
2.2. As and Se Treatments
2.3. Sample Preparation and Analysis of ASCONTENT
2.4. Sample Preparation and Analysis of Antioxidant Enzyme Activities and MDA Content
2.5. Data Analysis
2.6. Statistical Analysis
3. Results
3.1. Effect of Se on As Uptake Kinetics by Rice Seedlings
3.2. Effect of Se Pretreatment on As(III) Uptake and Translocation in Seedlings of Rice
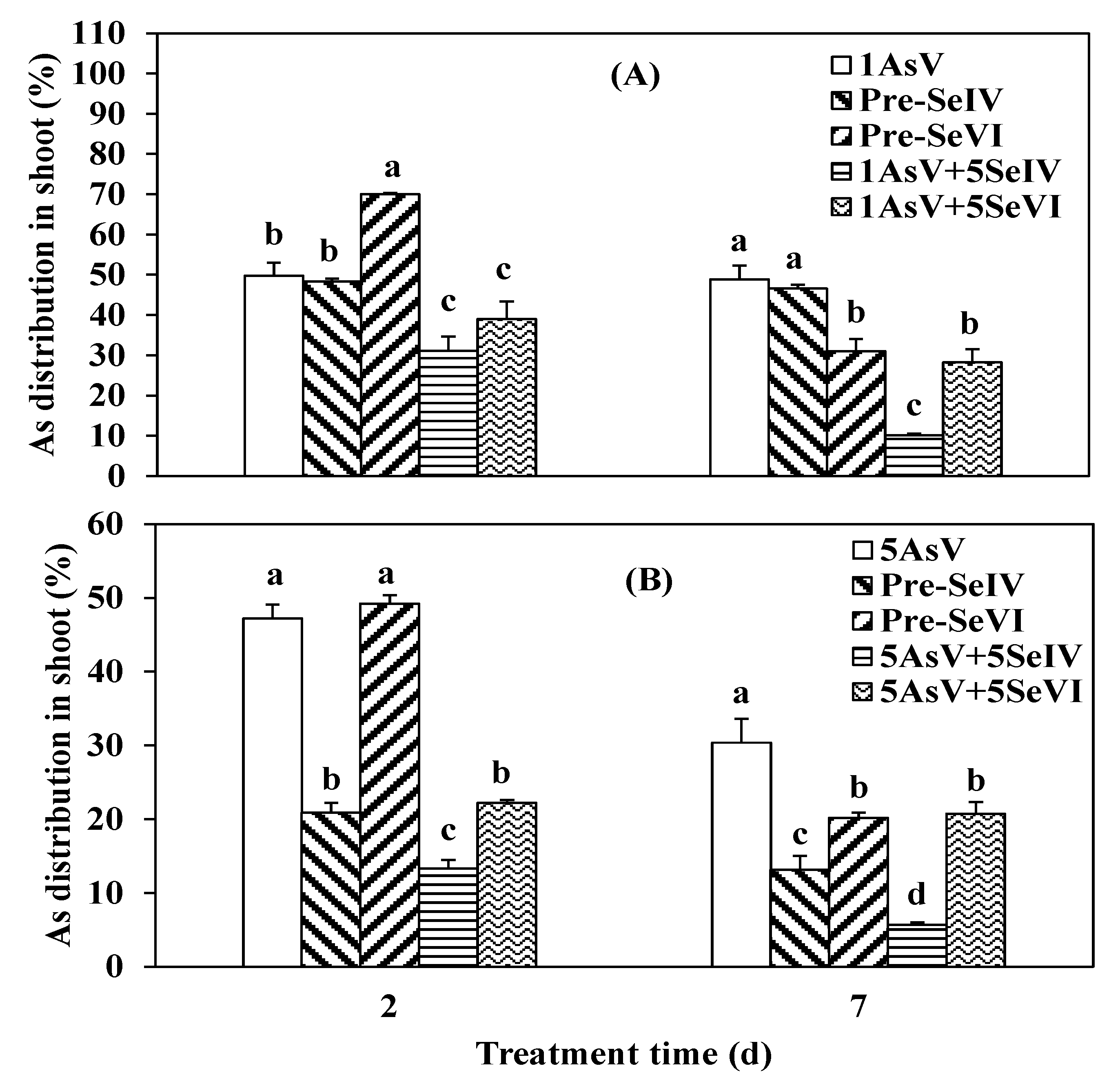
3.3. Impact of Se Pretreatment on As(V) Accumulation and Translocation in the Rice
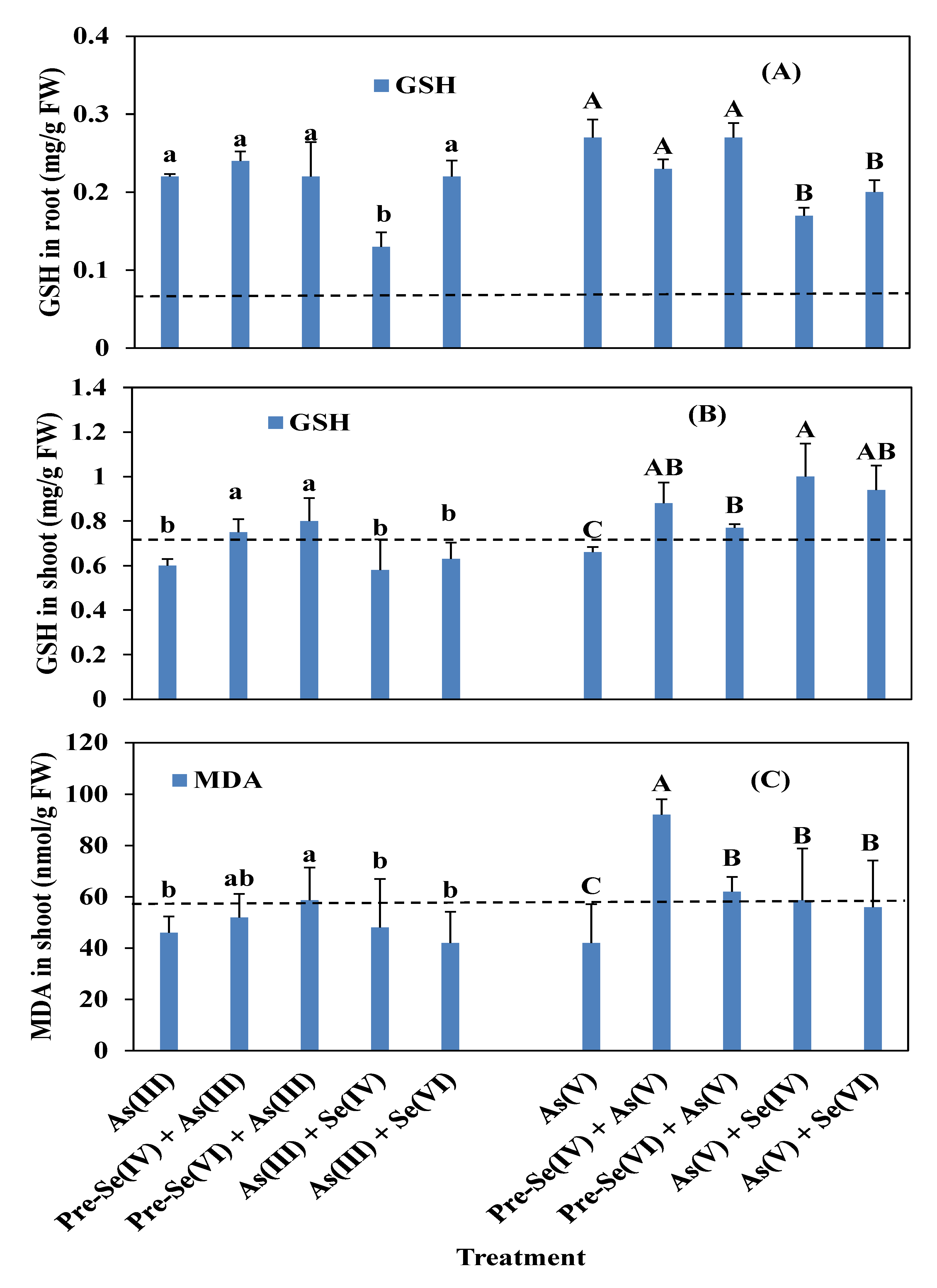
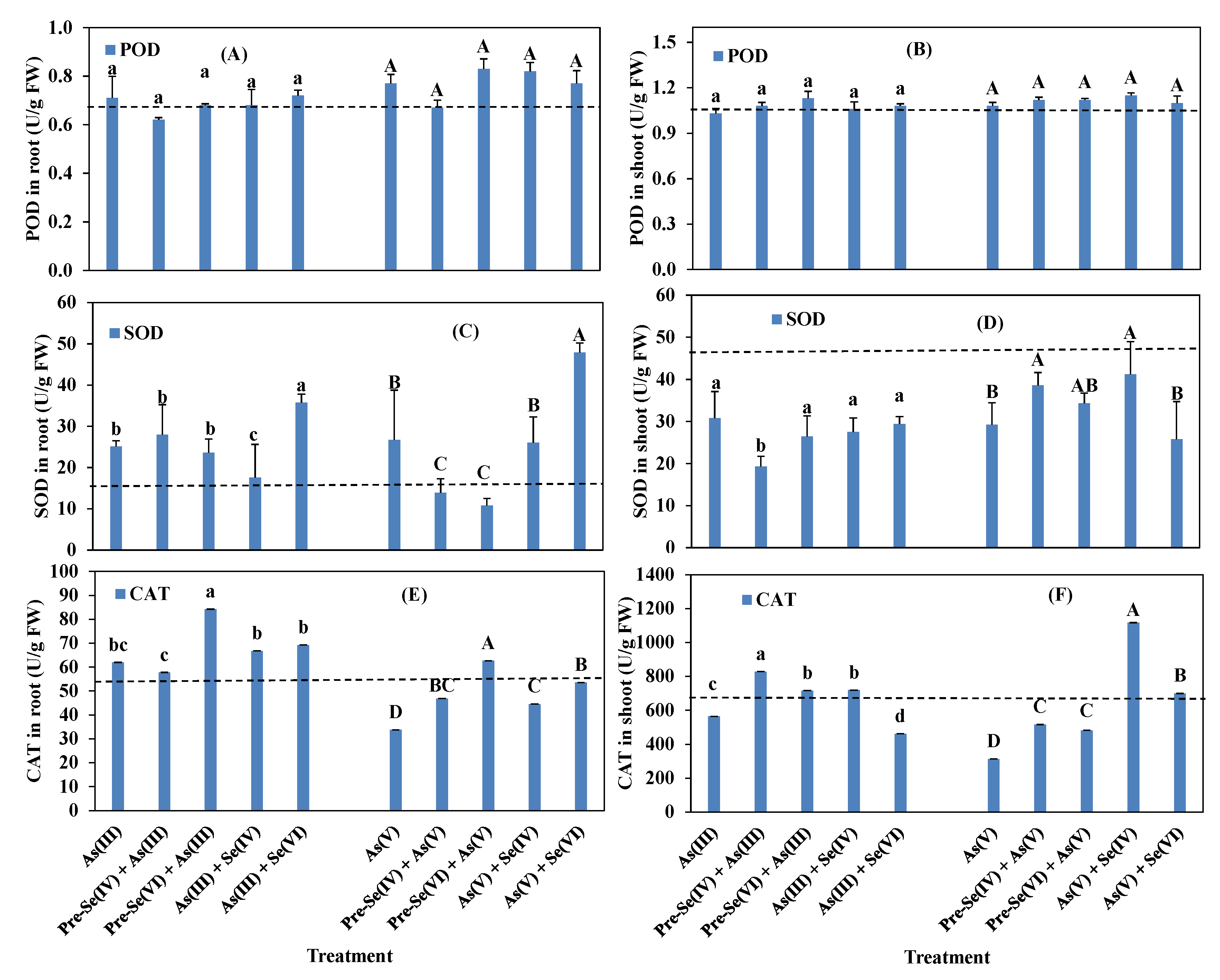
3.4. Rice Root and Shoot POD, SOD, and CAT Activities and GSH and MDA Contents
4. Discussion
4.1. Se Enhances/Reduces As Uptake by Rice Seedlings
4.2. As Translocation from Root to Shoot in Rice Seedlings
4.3. Enzymatic Activities in Rice Seedlings
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Goh, K.-H.; Lim, T.-T. Geochemistry of inorganic arsenic and selenium in a tropical soil: Effect of reaction time, pH, and competitive anions on arsenic and selenium adsorption. Chemosphere 2004, 55, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Bastías, J.M.; Beldarrain, T. Arsenic translocation in rice cultivation and its implication for human health. Chil. J. Agric. Res. 2016, 76, 114–122. [Google Scholar] [CrossRef]
- Kumar, N.; Mallick, S.; Yadava, R.N.; Singh, A.P.; Sinha, S. Co-application of selenite and phosphate reduces arsenite uptake in hydroponically grown rice seedlings: Toxicity and defence mechanism. Ecotoxicol. Environ. Saf. 2013, 91, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Navin, K.; Dubey, A.K.; Jaiswal, P.K.; Nayan, S.; Behera, S.K.; Tripathi, R.D.; Shekhar, M. Selenite supplementation reduces arsenate uptake greater than phosphate but compromises the phosphate level and physiological performance in hydroponically grown Oryza sativa L. Environ. Toxicol. Chem. 2016, 35, 163–172. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Srivastava, S.; Mishra, S.; Singh, N.; Tuli, R.; Gupta, D.K.; Maathuis, F.J.M. Arsenic hazards: Strategies for tolerance and remediation by plants. Trends Biotechnol. 2007, 25, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.; Mukherjee, A. Arsenic fate and transport in the groundwater-soil-plant system: An understanding of suitable rice paddy cultivation in arsenic enriched areas. In Recent Trends in Modelling of Environmental Contaminants; Springer: New Delhi, India, 2014; pp. 21–44. [Google Scholar]
- Mkandawire, M.; Taubert, B.; Dudel, E.G. Resource manipulation in uranium and arsenic attenuation by Lemna gibba L. (duckweed) in tailing water of a former uranium mine. Water Air Soil Pollut. 2005, 166, 83–101. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, A.-J.; Zhao, F.-J.; Xu, G.-Z.; Duan, G.-L.; Zhu, Y.-G. Arsenic accumulation by the aquatic fern Azolla: Comparison of arsenate uptake, speciation and efflux by A. caroliniana and A. filiculoides. Environ. Pollut. 2008, 156, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mishra, S.; Tripathi, R.D.; Dwivedi, S.; Trivedi, P.K.; Tandon, P.K. Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (L.f) Royle. Environ. Sci. Technol. 2007, 41, 2930–2936. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FA, USA, 2010. [Google Scholar]
- Chen, T.; Wong, Y.-S. Selenocystine induces reactive oxygen species–mediated apoptosis in human cancer cells. Biomed. Pharmacother. 2009, 63, 105–113. [Google Scholar] [CrossRef]
- Carroll, L.; Davies, M.J.; Pattison, D. Reaction of low-molecular-mass organoselenium compounds (and their sulphur analogues) with inflammation-associated oxidants. Free Radic. Res. 2015, 49, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Nadgórska-Socha, A.; Kafel, A.; Kandziora-Ciupa, M.; Gospodarek, J.; Zawisza-Raszka, A. Accumulation of heavy metals and antioxidant responses in Vicia faba plants grown on monometallic contaminated soil. Environ. Sci. Pollut. Res. 2013, 20, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- El-Sharaky, A.S.; Newairy, A.A.; Badreldeen, M.M.; Eweda, S.M.; Sheweita, S.A. Protective role of selenium against renal toxicity induced by cadmium in rats. Toxicology 2007, 235, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in higher plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Turakainen, M.; Hartikainen, H.; Seppanen, M.M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 2004, 52, 5378–5382. [Google Scholar] [CrossRef]
- Xue, T.L.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Lazarus, M.; Orct, T.; Aladrovic, J.; Ljubic, B.B.; Jurasovic, J.; Blanusa, M. Effect of Selenium Pre-treatment on Antioxidative Enzymes and Lipid Peroxidation in Cd-exposed Suckling Rats. Biol. Trace Elem. Res. 2011, 142, 611–622. [Google Scholar] [CrossRef]
- Hu, Y.; Duan, G.-L.; Huang, Y.-Z.; Liu, Y.-X.; Sun, G.-X. Interactive effects of different inorganic As and Se species on their uptake and translocation by rice (Oryza sativa L.) seedlings. Environ. Sci. Pollut. Res. 2014, 21, 3955–3962. [Google Scholar] [CrossRef]
- CAMARA, A.Y.; Wan, Y.; Yu, Y.; Wang, Q.; Li, H. Effect of selenium on uptake and translocation of arsenic in rice seedlings (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2018, 148, 869–875. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, Y.; Wang, Q.; Qiao, Y.; Li, H. Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotoxicol. Environ. Saf. 2016, 133, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Li, L.; Ali, B.; Gill, R.A.; Wang, J.; Ali, S.; Gill, M.B.; Zhou, W. Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ. Sci. Pollut. Res. 2015, 22, 10699–10712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, C.; Zhao, X.; Tan, Q.; Sun, X.; Cao, A.; Cui, M.; Zhang, Y. Molybdenum improves antioxidant and osmotic-adjustment ability against salt stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis). Plant Soil 2012, 355, 375–383. [Google Scholar] [CrossRef]
- Allan, C.B.; Lacourciere, G.M.; Stadtman, T.C. Responsiveness of selenoproteins to dietary selenium. Ann. Rev. Nutr. 1999, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, F.M.; Zhang, G.D.; Green, K.; Liu, X.P. Soil, grain and water chemistry in relation to human selenium-responsive diseases in Enshi District, China. Appl. Geochem. 2000, 15, 117–132. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.L. The promotive effect of selenium on plant growth as triggered by ultraviolet irradiation. J. Environ. Qual. 1999, 28, 1372–1375. [Google Scholar] [CrossRef]
- Ebbs, S.; Weinstein, L. Alteration of selenium transport and volatilization in barley (Hordeum vulgare) by arsenic. J. Plant Physiol. 2001, 158, 1231–1233. [Google Scholar] [CrossRef]
- Yathavakilla, S.K.V.; Caruso, J.A. A study of Se-Hg antagonism in Glycine max (soybean) roots by size exclusion and reversed phase HPLC-ICPMS. Anal. Bioanal. Chem. 2007, 389, 715–723. [Google Scholar] [CrossRef]
- Malik, J.A.; Goel, S.; Kaur, N.; Sharma, S.; Singh, I.; Nayyar, H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012, 77, 242–248. [Google Scholar] [CrossRef]
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Filek, M.; Keskinen, R.; Hartikainen, H.; Szarejko, I.; Janiak, A.; Miszalski, Z.; Golda, A. The protective role of selenium in rape seedlings subjected to cadmium stress. J. Plant Physiol. 2008, 165, 833–844. [Google Scholar] [CrossRef]
- Li, H.-F.; McGrath, S.P.; Zhao, F.-J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. N. Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Meharg, A.A.; Macnair, M.R. The mechanisms of arsenate tolerance in deschampsia-cespitosa (L.) beauv and agrostis-capillaris L. N. Phytol. 1991, 119, 291–297. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. N. Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef]
- Bluemlein, K.; Klimm, E.; Raab, A.; Feldmann, J. Selenite enhances arsenate toxicity in Thunbergia alata. Environ. Chem. 2009, 6, 486–494. [Google Scholar] [CrossRef]
- Fargasova, A.; Pastierova, J.; Svetkova, K. Effect of Se-metal pair combinations (Cd, Zn, Cu, Pb) on photosynthetic pigments production and metal accumulation in Sinapis alba L. seedlings. Plant Soil Environ. 2006, 52, 8–15. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, M. Influence of selenium on uptake and toxicity of copper and cadmium in pea (Pisum sativum) and wheat (Triticum aestivum). Physiol. Plant. 1994, 90, 637–644. [Google Scholar] [CrossRef]
- Afton, S.E.; Catron, B.; Caruso, J.A. Elucidating the selenium and arsenic metabolic pathways following exposure to the non-hyperaccumulating Chlorophytum comosum, spider plant. J. Exp. Bot. 2009, 60, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Stolz, J.F.; Oremland, R.S. Bacterial respiration of arsenic and selenium. FEMS Microbiol. Rev. 1999, 23, 615–627. [Google Scholar] [CrossRef]
- Tu, C.; Ma, L.Q. Effects of arsenate and phosphate on their accumulation by an arsenic-hyperaccumulator Pteris vittata L. Plant Soil 2003, 249, 373–382. [Google Scholar] [CrossRef]
- Srivastava, M.; Ma, L.Q.; Singh, N.; Singh, S. Antioxidant responses of hyper-accumulator and sensitive fern species to arsenic. J. Exp. Bot. 2005, 56, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Zhu, Y.G.; Smith, F.A. Effects of iron and manganese plaques on arsenic uptake by rice seedlings (Oryza sativa L.) grown in solution culture supplied with arsenate and arsenite. Plant Soil 2005, 277, 127–138. [Google Scholar] [CrossRef]
- Salt, D.E.; Prince, R.C.; Pickering, I.J.; Raskin, I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995, 109, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Lou-Hing, D.; Zhang, B.; Price, A.H.; Meharg, A.A. Effects of phosphate on arsenate and arsenite sensitivity in two rice (Oryza sativa L.) cultivars of different sensitivity. Environ. Exp. Bot. 2011, 72, 47–52. [Google Scholar] [CrossRef]
- Hu, Y.; Norton, G.J.; Duan, G.; Huang, Y.; Liu, Y. Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant Soil 2014, 384, 131–140. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M. Selenium Pretreatment Upregulates the Antioxidant Defense and Methylglyoxal Detoxification System and Confers Enhanced Tolerance to Drought Stress in Rapeseed Seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Mittova, V.; Theodoulou, F.L.; Kiddle, G.; Gomez, L.; Volokita, M.; Tal, M.; Foyer, C.H.; Guy, M. Coordinate induction of glutathione biosynthesis and glutathione-metabolizing enzymes is correlated with salt tolerance in tomato. FEBS Lett. 2003, 554, 417–421. [Google Scholar] [CrossRef]
- Kadioglu, A.; Saruhan, N.; Saglam, A.; Terzi, R.; Acet, T. Exogenous salicylic acid alleviates effects of long term drought stress and delays leaf rolling by inducing antioxidant system. Plant Growth Regul. 2011, 64, 27–37. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Han, D.; Li, X.; Xiong, S.; Tu, S.; Chen, Z.; Li, J.; Xie, Z. Selenium uptake, speciation and stressed response of Nicotiana tabacum L. Environ. Exp. Bot. 2013, 95, 6–14. [Google Scholar] [CrossRef]
- Srivastava, M.; Ma, L.Q.; Rathinasabapathi, B.; Srivastava, P. Effects of selenium on arsenic uptake in arsenic hyperaccumulator Pteris vittata L. Bioresour. Technol. 2009, 100, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Shri, M.; Kumar, S.; Chakrabarty, D.; Trivedi, P.K.; Mallick, S.; Misra, P.; Shukla, D.; Mishra, S.; Srivastava, S.; Tripathi, R.D.; et al. Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol. Environ. Saf. 2009, 72, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Jha, A.B.; Dubey, R.S. Arsenite treatment induces oxidative stress, upregulates antioxidant system, and causes phytochelatin synthesis in rice seedlings. Protoplasma 2011, 248, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yan, X.; Liao, X.; Wen, Y.; Chong, Z.; Liang, T. Interactions of arsenic and phenanthrene on their uptake and antioxidative response in Pteris vittata L. Environ. Pollut. 2011, 159, 3398–3405. [Google Scholar] [CrossRef]
- Tripathi, P.; Mishra, A.; Dwivedi, S.; Chakrabarty, D.; Trivedi, P.K.; Singh, R.P.; Tripathi, R.D. Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotoxicol. Environ. Saf. 2012, 79, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, R.P.; Singh, P.K.; Awasthi, S.; Chakrabarty, D.; Trivedi, P.K.; Tripathi, R.D. Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.). Ecotoxicology 2014, 23, 1153–1163. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Gupta, M. Exposure of Brassica juncea (L) to arsenic species in hydroponic medium: Comparative analysis in accumulation and biochemical and transcriptional alterations. Environ. Sci. Pollut. Res. 2013, 20, 8141–8150. [Google Scholar] [CrossRef]
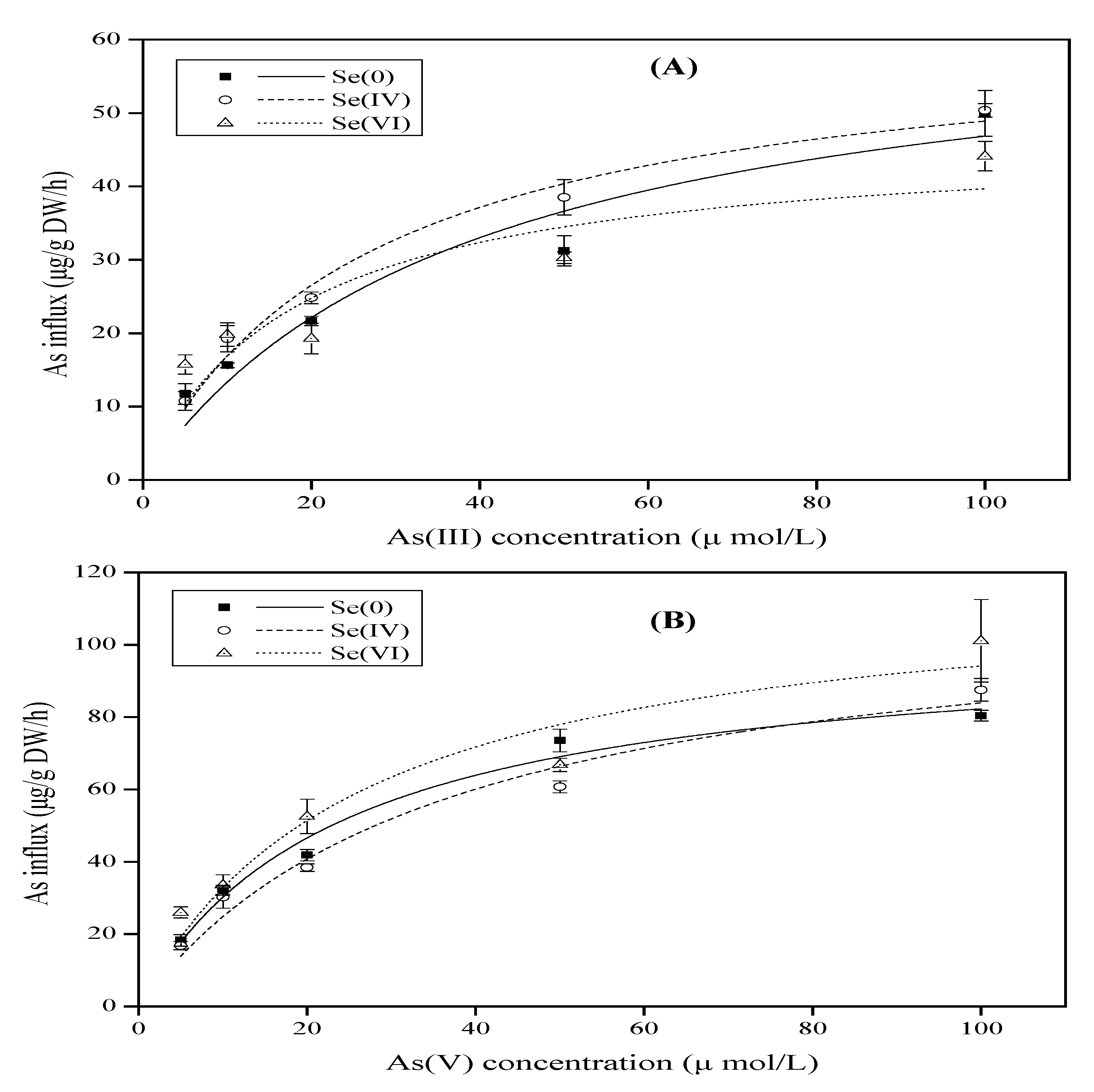
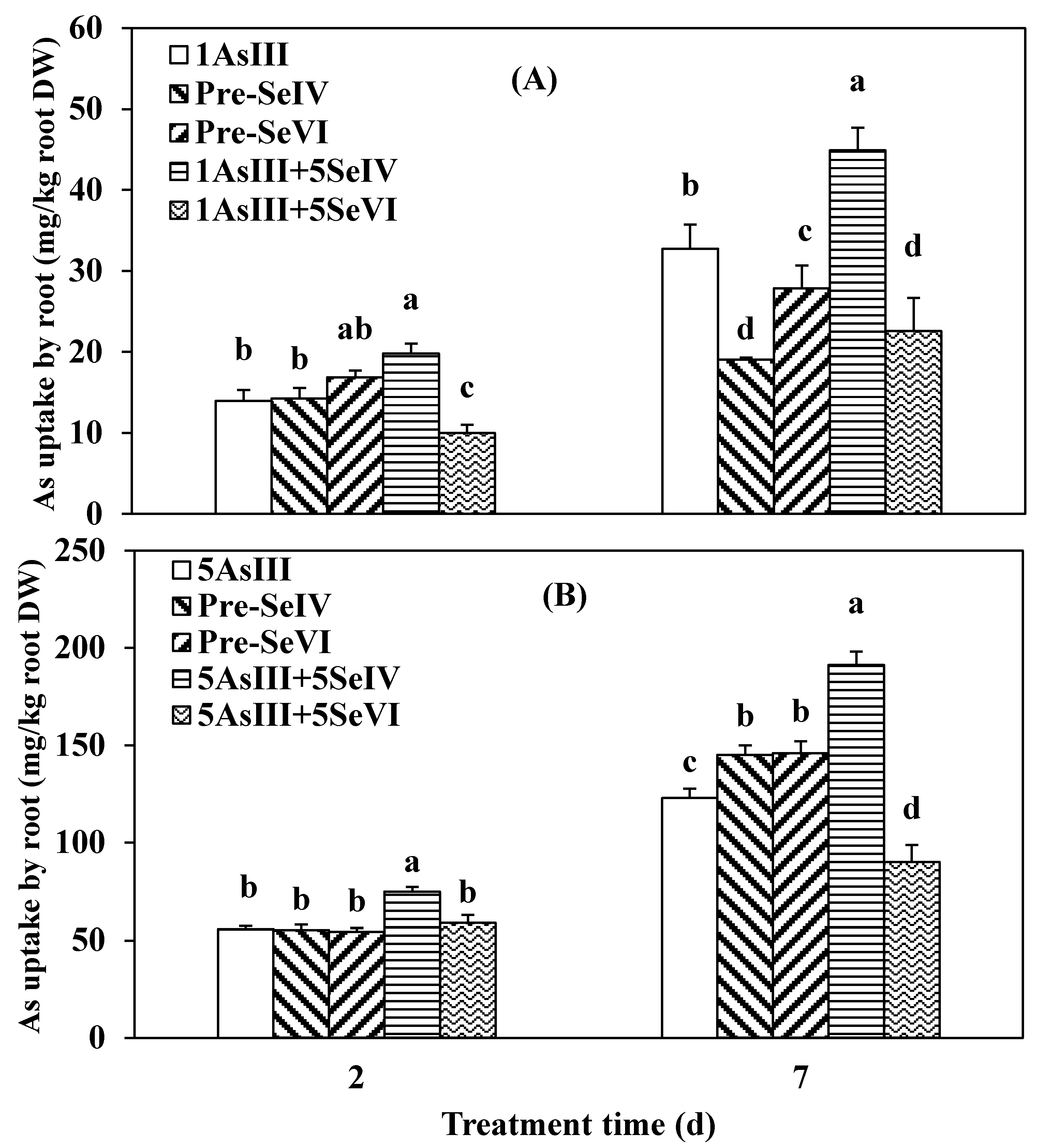
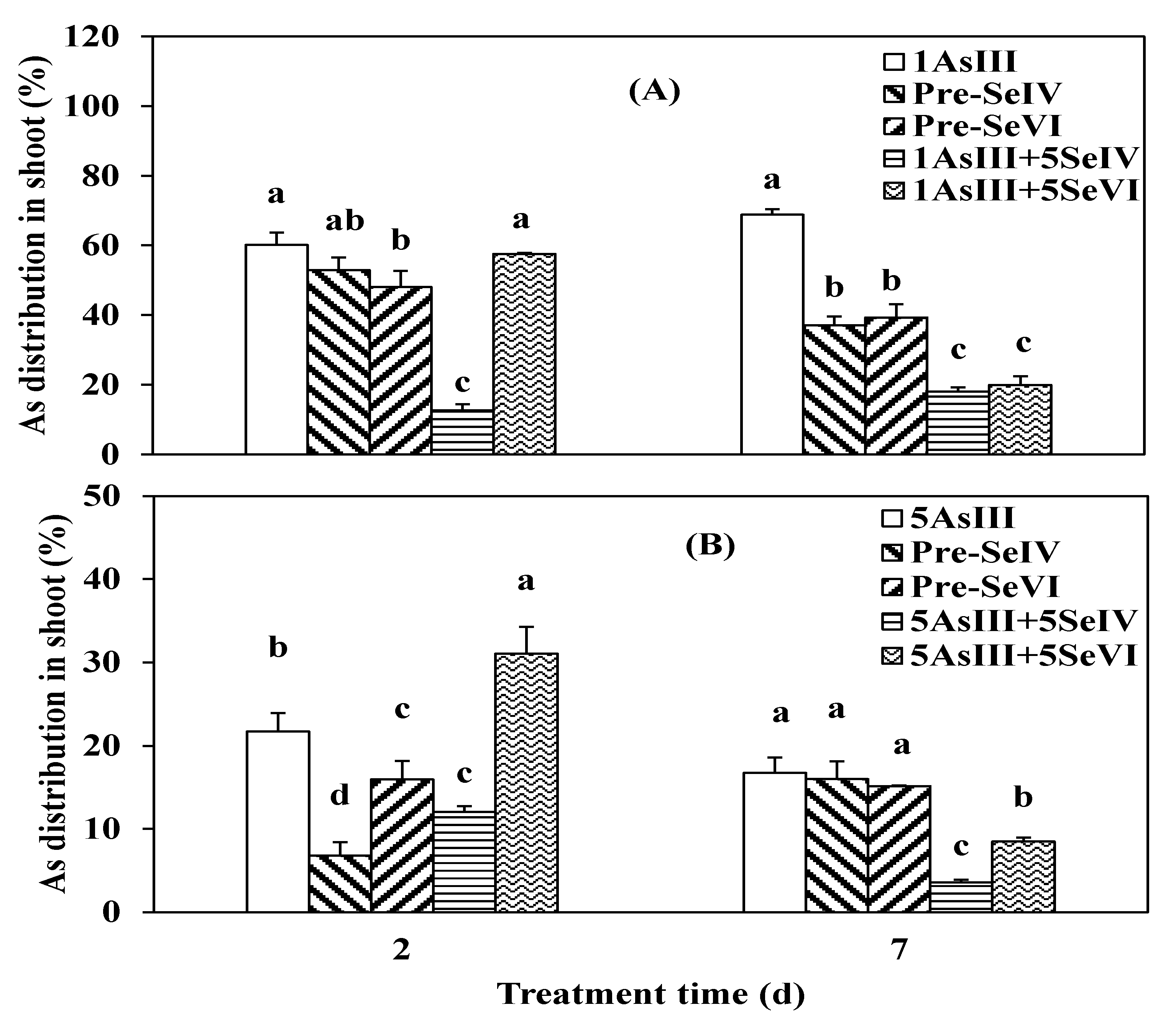
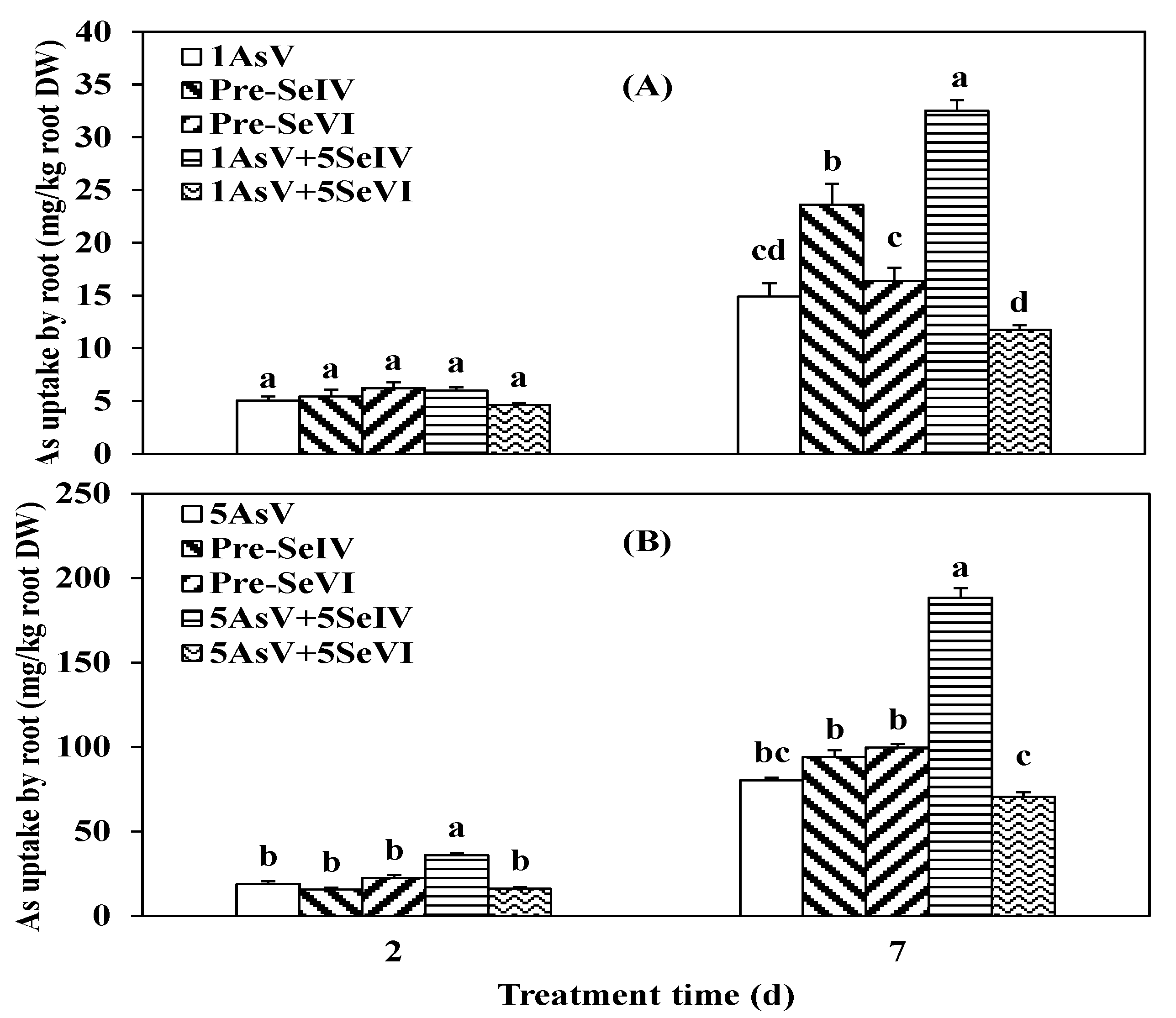
| As Species | Se Concentration (µM) | Vmax (µg/g rood/h DW) | Km (µM) | R |
|---|---|---|---|---|
| As(III) | Se0 | 64.97 ± 12.39 | 38.71 ± 17.10 | 0.9657 |
| Se(IV) 5 | 61.96 ± 4.45 | 26.70 ± 4.99 | 0.9923 | |
| Se(VI) 5 | 46.68 ± 8.91 | 17.68 ± 9.97 | 0.8952 | |
| As(V) | Se0 | 101.75 ± 7.45 | 23.68 ± 4.68 | 0.9911 |
| Se(IV) 5 | 114.26 ± 13.75 | 36.07 ± 10.28 | 0.9857 | |
| Se(VI) 5 | 118.91 ± 16.83 | 26.28 ± 9.74 | 0.9683 |
| Treatment (1 µM As) | Exposure Time (days) | Treatment (5 µM As) | Exposure Time (days) | ||
|---|---|---|---|---|---|
| 2 | 7 | 2 | 7 | ||
| 1AsIII | 0.54 ± 0.91 a | 0.93 ± 0.05 a | 5AsIII | 0.10 ± 0.01 a | 0.09 ± 0.01 a |
| Pre-SeIV + AsIII | 0.42 ± 0.08 a | 0.28 ± 0.03 b | Pre-SeIV + AsIII | 0.03 ± 0.01 a | 0.09 ± 0.01 a |
| Pre-SeVI + AsIII | 0.33 ± 0.05 a | 0.40 ± 0.08 b | Pre-SeVI + AsIII | 0.07 ± 0.01 a | 0.07 ± 0.00 a |
| 1AsIII + 5SeIV | 0.05 ± 0.01 b | 0.10 ± 0.01 c | 5AsIII + 5SeIV | 0.05 ± 0.01 a | 0.02 ± 0.00 a |
| 1AsIII + 5SeVI | 0.47 ± 0.01 a | 0.10 ± 0.01 c | 5AsIII + 5SeVI | 0.15 ± 0.02 a | 0.03 ± 0.00 a |
| Treatment (1 µM As) | Exposure Time (days) | Treatment (5 µM As) | Exposure time (days) | ||
|---|---|---|---|---|---|
| 2 | 7 | 2 | 7 | ||
| 1AsV | 0.26 ± 0.03b | 0.27 ± 0.02a | 5AsV | 0.23 ± 0.02a | 0.14 ± 0.02a |
| Pre-SeIV + AsV | 0.24 ± 0.01b | 0.25 ± 0.01a | Pre-SeIV + AsV | 0.06 ± 0.01b | 0.04 ± 0.01b |
| Pre-SeVI + AsV | 0.53 ± 0.02a | 0.13 ± 0.02b | Pre-SeVI + AsV | 0.21 ± 0.01a | 0.07 ± 0.00b |
| 1AsV + 5SeIV | 0.11 ± 0.02c | 0.02 ± 0.00c | 5AsV + 5SeIV | 0.04 ± 0.00b | 0.02 ± 0.00b |
| 1AsV + 5SeVI | 0.17 ± 0.03c | 0.11 ± 0.01b | 5AsV + 5SeVI | 0.07 ± 0.00b | 0.06 ± 0.01b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camara, A.Y.; Wan, Y.; Yu, Y.; Wang, Q.; Wang, K.; Li, H. Effect of Endogenous Selenium on Arsenic Uptake and Antioxidative Enzymes in As-Exposed Rice Seedlings. Int. J. Environ. Res. Public Health 2019, 16, 3350. https://doi.org/10.3390/ijerph16183350
Camara AY, Wan Y, Yu Y, Wang Q, Wang K, Li H. Effect of Endogenous Selenium on Arsenic Uptake and Antioxidative Enzymes in As-Exposed Rice Seedlings. International Journal of Environmental Research and Public Health. 2019; 16(18):3350. https://doi.org/10.3390/ijerph16183350
Chicago/Turabian StyleCamara, Aboubacar Younoussa, Yanan Wan, Yao Yu, Qi Wang, Kang Wang, and Huafen Li. 2019. "Effect of Endogenous Selenium on Arsenic Uptake and Antioxidative Enzymes in As-Exposed Rice Seedlings" International Journal of Environmental Research and Public Health 16, no. 18: 3350. https://doi.org/10.3390/ijerph16183350
APA StyleCamara, A. Y., Wan, Y., Yu, Y., Wang, Q., Wang, K., & Li, H. (2019). Effect of Endogenous Selenium on Arsenic Uptake and Antioxidative Enzymes in As-Exposed Rice Seedlings. International Journal of Environmental Research and Public Health, 16(18), 3350. https://doi.org/10.3390/ijerph16183350




