Seasonal Release Potential of Sediments in Reservoirs and its Impact on Water Quality Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Experimental Methods
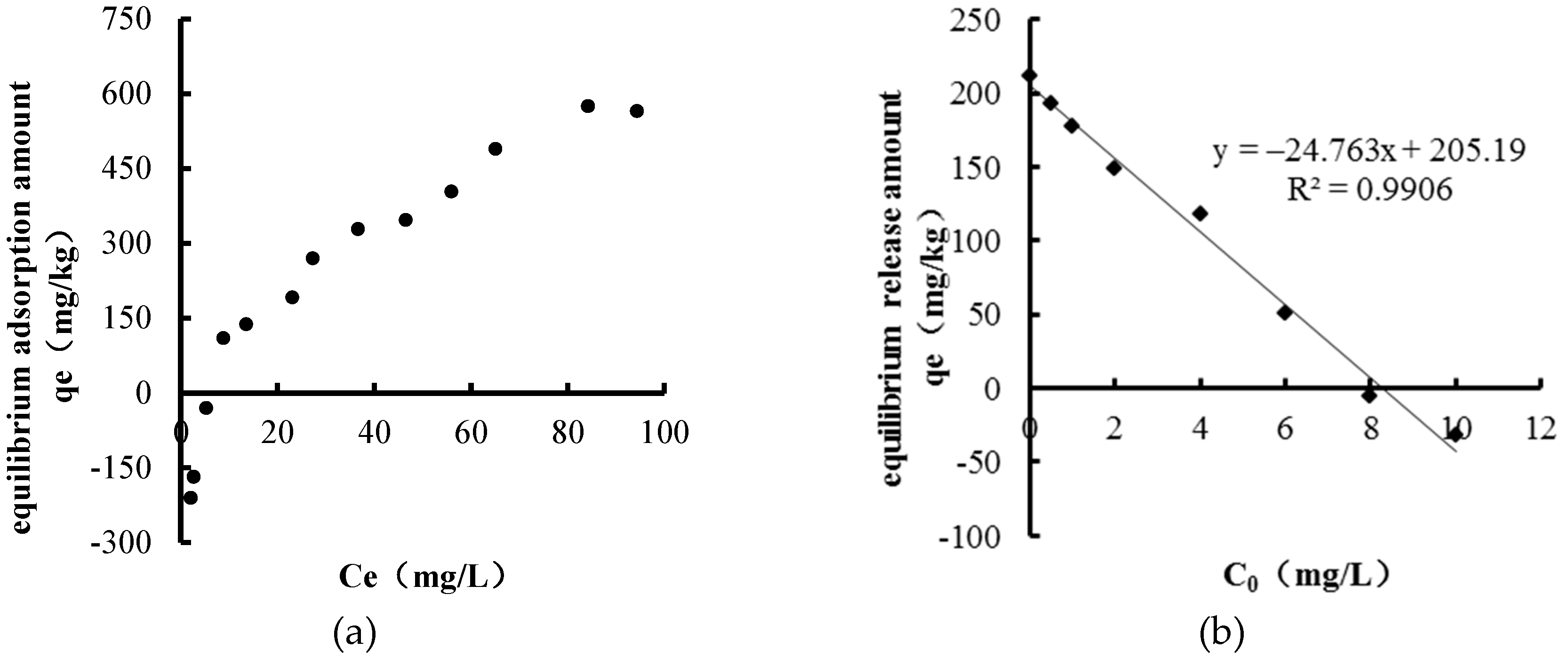
2.2.1. Sediments Adsorption Experiments
2.2.2. Sediments Release Experiments
2.2.3. Sediments Release Experiments under Different TW, DO, and pH
2.3. Water Quality Assessment Methods Considering Sediments Release Potential
2.3.1. Assessment Index System
2.3.2. Indicator Normalization
2.3.3. Comprehensive Relative Membership Degree
2.3.4. Comprehensive Assessment Level of Water Quality
3. Results and Discussion
3.1. Identification of Source–Sink Characteristics of Sediments
3.1.1. Sediments NH4+–N Source–Sink Identification
3.1.2. Sediments TP Source–Sink Identification
3.2. Sediments Release Characteristics under Different TW, DO, and pH
3.2.1. Water Temperature
3.2.2. Dissolved Oxygen
3.2.3. pH
3.3. Seasonal Variations of Sediments Release Potential
3.3.1. Seasonal Variations of Environmental Factors in Biliuhe Reservoir
3.3.2. Sediments Release Potential in Different Seasons
3.4. Seasonal Assessment of Water Quality Considering Sediments Release Potential
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ni, Z.; Wang, S. Historical accumulation and environmental risk of nitrogen and phosphorus in sediments of Erhai Lake, Southwest China. Ecol. Eng. 2015, 79, 42–53. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, F.; Yan, W.; Zhang, F.; Lv, S.; Li, Y. Carbon and Nitrogen Burial and Response to Climate Change and Anthropogenic Disturbance in Chaohu Lake, China. Int. J. Environ. Res. Public Health 2018, 15, 2734. [Google Scholar] [CrossRef] [PubMed]
- Portielje, R.; Lijklema, L. Estimation of sediment–water exchange of solutes in Lake Veluwe, The Netherlands. Water Res. 1999, 33, 279–285. [Google Scholar] [CrossRef]
- He, J.; Deng, W.; Chen, C.; Xu, X.; Wang, S.; Liu, W.; Wu, X. Ammonia nitrogen adsorption and release characteristics of surface sediments in Dianchi Lake, China. Environ. Earth Sci. 2015, 74, 3917–3927. [Google Scholar] [CrossRef]
- Hoch, M. Organotin compounds in the environment: An overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Ahlgren, J.; Reitzel, K.; De Brabandere, H.; Gogoll, A.; Rydin, E. Release of organic P forms from lake sediments. Water Res. 2011, 45, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Han, W.M. Phosphorus release from the sediments of West Lake in Hangzhou and its effects on lake eutrophication. J. Lake Sci. 1993, 5, 71–77, (In Chinese with English abstract). [Google Scholar]
- Rydin, E.; Brunberg, A.K. Seasonal dynamics of phosphorous in Lake Erken surface sediments. Arch. Hydrobiol. L: Spec. Issues Adv. Limno. 1998, 51, 157–167. [Google Scholar]
- James, R.T.; Martin, J.; Wool, T.; Wang, P.F. A Sediment Resuspension and Water Quality Model of Lake Okeechobee 1. J. Am. Water Resour. Assoc. 1997, 33, 661–678. [Google Scholar] [CrossRef]
- Cheng, X.; Zeng, Y.; Guo, Z.; Zhu, L. Diffusion of nitrogen and phosphorus across the sediment-water interface and in seawater at aquaculture areas of Daya Bay, China. Int. J. Environ. Res. Public Health 2014, 11, 1557–1572. [Google Scholar] [CrossRef]
- Abrams, M.M.; Jarrell, W.M. Soil phosphorus as a potential nonpoint source for elevated stream phosphorus levels. J. Environ. Qual. 1995, 24, 132–138. [Google Scholar] [CrossRef]
- Pan, X.; Lin, L.; Huang, Z.; Liu, M.; Dong, L.; Chen, J.; Crittenden, J. Distribution characteristics and pollution risk evaluation of the nitrogen and phosphorus species in the sediments of Lake Erhai, Southwest China. Environ. Sci. Pollut. Res. 2019, 26, 22295–22304. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, C.T.A. Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res. 2012, 46, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Pang, Y.; Wang, H.; Wang, X.; Wu, X.; Bao, K.; Liu, Q. Simulation study on water quality based on sediment release flume experiment in Lake Taihu, China. Ecol. Eng. 2010, 37, 607–615. [Google Scholar] [CrossRef]
- Wang, F.E.; Yang, J.; Li, Y.N.; Yang, X.Q.; Zhong, X.H.; Bian, J.Y. Modification of WASP model based on release of sediment phosphorus. Acta Scientiae Circumstantiae 2013, 33, 3301–3308, (In Chinese with English abstract). [Google Scholar]
- Wang, T.; Xu, S.; Liu, J. Dynamic Assessment of Comprehensive Water Quality Considering the Release of Sediment Pollution. Water 2017, 9, 275. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Bu, Q.; Zhou, X.; Wu, F. Effects of particle size, organic matter and ionic strength on the phosphate sorption in different trophic lake sediments. J. Hazard. Mater. 2006, 128, 95–105. [Google Scholar] [CrossRef]
- Kim, L.H.; Choi, E.; Stenstrom, M.K. Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. Chemosphere 2003, 50, 53–61. [Google Scholar] [CrossRef]
- Dursun, A.Y.; Kalayci, C.S. Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto chitin. J. Hazard. Mater. 2005, 123, 151–157. [Google Scholar] [CrossRef]
- Kang, M.; Peng, S.; Tian, Y.; Zhang, H. Effects of dissolved oxygen and nutrient loading on phosphorus fluxes at the sediment–water interface in the Hai River Estuary, China. Mar. Pollut. Bull. 2018, 130, 132–139. [Google Scholar] [CrossRef]
- Tao, Y.; Geng, J.; Wang, R.; Chen, Z.; Pan, C.; Yu, R.; Ren, H. Effect of environmental variations on release of three nitrogen in sediments from river. Environ. Sci. 2013, 36, 41–44, (In Chinese with English abstract). [Google Scholar]
- Jin, X.C.; Jia, X. Effects of light and oxygen on the uptake and distribution of phosphorus at the sediment-water interface. Environ. Sci. Technol. 2006, 357, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chao, J.; Zhuang, W.; Wang, P.; Wang, C.; Hou, J.; Wu, Z.; Wang, L.; Gao, G.; Wang, Y. Spatial and temporal distribution of particulate phosphorus and their correlation with environmental factors in a shallow eutrophic Chinese lake (Lake Taihu). Int. J. Environ. Res. Public Health 2018, 15, 2355. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B. Water quality protection strategy of Tanghe Reservoir in Liaoning Province. Heilongjiang Water Res. 2016, 2, 65–67, (In Chinese with English abstract). [Google Scholar]
- Yin, H.; Chang, J.; Zhang, G.; Sun, X. The reason and control measures of Xinlicheng reservoir eutrophication. J. Northeast Norm. Univ. (Nat. Sci. Ed.) 2010, 42, 152–156, (In Chinese with English abstract). [Google Scholar]
- Xu, S.; Wang, T.; Hu, S. Dynamic Assessment of Water Quality Based on a Variable Fuzzy Pattern Recognition Model. Int. J. Environ. Res. Public Health 2015, 12, 2230–2248. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, S.; Liu, J. Analysis of accumulation formation of sediment contamination in reservoirs after decades of running: A case study of nitrogen accumulation in Biliuhe Reservoir. Environ. Sci. Pollut. Res. 2018, 25, 9165–9175. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Xu, S.; Qin, G.; Sun, Y.; Wang, F. Spatial distribution, adsorption/release characteristics, and environment influence of phosphorus on sediment in reservoir. Water 2017, 9, 724. [Google Scholar] [CrossRef]
- Shidan, B. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2002; pp. 21–22, (In Chinese with English abstract). [Google Scholar]
- Zhu, Y.; Jin, X.; Tang, W.; Meng, X.; Shan, B. Comprehensive analysis of nitrogen distributions and ammonia nitrogen release fluxes in the sediments of Baiyangdian Lake, China. J. Environ. Sci. 2019, 76, 319–328. [Google Scholar] [CrossRef]
- Hu, W.F.; Lo, W.; Chua, H.; Sin, S.N.; Yu, P.H.F. Nutrient release and sediment oxygen demand in a eutrophic land-locked embayment in Hong Kong. Environ. Int. 2001, 26, 369–375. [Google Scholar] [CrossRef]
- Jalali, M.; Peikam, E.N. Phosphorus sorption–desorption behaviour of river bed sediments in the Abshineh river, Hamedan, Iran, related to their composition. Environ. Monit. Assess. 2013, 185, 537–552. [Google Scholar] [CrossRef] [PubMed]
- State Environmental Protection Administration (SEPA). Standard Methods for the Examination of Water and Wastewater, 4th ed.; China Environmental Science Publish Press: Beijing, China, 2002; pp. 243–284, (In Chinese with English abstract).
- Zhang, B.; Fang, F.; Guo, J.; Chen, Y.; Li, Z.; Guo, S. Phosphorus fractions and phosphate sorption-release characteristics relevant to the soil composition of water-level-fluctuating zone of Three Gorges Reservoir. Ecol. Eng. 2012, 40, 153–159. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, Y. Variation laws and release characteristics of phosphorus on surface sediment of Dongting Lake. Environ. Sci. Pollut. Res. 2018, 25, 12342–12351. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Xu, X.; Chen, C.; He, J.; Xu, D.; Wang, L. Sorption and desorption characteristics of ammonium in the surface sediments of Lake Dianchi. J. Lake Sci. 2016, 28, 75–85, (In Chinese with English abstract). [Google Scholar]
- Chen, S.; Guo, Y. Variable fuzzy sets and its application in comprehensive risk evaluation for flood control engineering system. Fuzzy Optim. Decis. Mak. 2006, 5, 153–162. [Google Scholar]
- Sun, C.; Sun, L.; Sun, X. Graphical Evaluation of the Favorability of Adsorption Processes by Using Conditional Langmuir Constant. Ind. Eng. Chem. Res. 2013, 52, 14251–14260. [Google Scholar] [CrossRef]
- Pan, G.; Krom, M.D.; Herut, B. Adsorption−Desorption of Phosphate on Airborne Dust and Riverborne Particulates in East. Mediterr. Seawater Environ. Sci. Technol. 2002, 36, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 470. [Google Scholar]
- Wang, S.; Jin, X.; Zhao, H.; Zhou, X.; Wu, F. Effect of organic matter on the sorption of dissolved organic and inorganic phosphorus in lake sediments. Colloids Surf. A: Physicochem. Eng. Asp. 2007, 297, 154–162. [Google Scholar] [CrossRef]
- Pant, H.K.; Reddy, K.R. Phosphorus sorption characteristics of estuarine sediments under different redox conditions. J. Environ. Qual. 2001, 30, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Xu, W. Effects of environmental factors on NH4+ release from Chaohu Lake sediments. Anhui Agric. Sci. 2008, 36, 12870–12872, (In Chinese with English abstract). [Google Scholar]
- Cao, X.; Liu, X.; Zhu, J.; Wang, L.; Liu, S.; Yang, G. Characterization of phosphorus sorption on the sediments of Yangtze River estuary and its adjacent areas. Mar. Pollut. Bull. 2017, 114, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, K.; Liang, R.; Han, S.; Li, Y. Distribution and Release Characteristics of Phosphorus in a Reservoir in Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 303. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, D.; Bauer, H.D. A remark on microorganisms in lake sediments with emphasis on polyphosphate-accumulating bacteria. Internationale Revue der gesamten Hydrobiologie und Hydrographie 1988, 73, 703–708. [Google Scholar] [CrossRef]
- Papaspyrou, S.; Kristensen, E.; Christensen, B. Arenicola marina (Polychaeta) and organic matter mineralisation in sandy marine sediments: In situ and microcosm comparison. Estuar. Coast. Shelf Sci. 2007, 72, 213–222. [Google Scholar] [CrossRef]
- Zoumis, T.; Schmidt, A.; Grigorova, L.; Calmano, W. Contaminants in sediments: Remobilisation and demobilisation. Sci. Total Environ. 2001, 266, 195–202. [Google Scholar] [CrossRef]
- Sun, Y. Study on Adsorption and Release of Nutrient Salts in Lake Sediment. Master’s Thesis, Hefei University of Technology, Hefei, China, 2000. (In Chinese with English abstract). [Google Scholar]
- Ye, L.; Pan, C.; Zhang, Z.; Zheng, Z.; Liu, J.; Wang, J. Characteristics of N forms in Wabu Lake sediments and effects of environmental factors on NH4+-N Release. J. Agric. Environ. Sci. 2006, 25, 1333–1336, (In Chinese with English abstract). [Google Scholar]
- Christophoridis, C.; Fytianos, K. Conditions affecting the release of phosphorus from surface lake sediments. J. Environ. Qual. 2006, 35, 1181–1192. [Google Scholar] [CrossRef]
- Andersen, J.M. Influence of pH on release of phosphorus from lake sediments. Arch. Hydrobiol. 1975, 76, 411–419. [Google Scholar]
- Boers, P.C.M. The influence of pH on phosphate release from lake sediments. Water Res. 1991, 25, 309–311. [Google Scholar] [CrossRef]
- Du, G.L.; Rinklebe, J.; Vandecasteele, B.; Meers, E.; Tack, F.M. Trace metal behaviour in estuarine and riverine floodplain soils and sediments:A review. Sci. Total Environ. 2009, 407, 3972–3985. [Google Scholar]
- Jiang, X.; Zhu, L.; Xu, S.; Xie, Z. Effects of seasonal stratification and suspended sediment behaviors on the mobilization of manganese and iron in a drinking water reservoir—A case of Biliuhe Reservoir, Liaoning Province. J. Lake Sci. 2019, 31, 375–385, (In Chinese with English abstract). [Google Scholar]
- Bi, W.; Weng, B.; Yuan, Z.; Ye, M.; Zhang, C.; Zhao, Y.; Yan, D.; Xu, T. Evolution Characteristics of Surface Water Quality Due to Climate Change and LUCC under Scenario Simulations: A Case Study in the Luanhe River Basin. Int. J. Environ. Res. Public Health 2018, 15, 1724. [Google Scholar] [CrossRef] [PubMed]








| Indicator | EC0 (mg/L) | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Equation | R2 | Qmax (mg/kg) | KL (L/mg) | Equation | R2 | 1/n | K (L/mg) | ||
| NH4+–N | 8.29 | 1/q = 0.0766/C+0.0011 | 0.9735 | 909.1 | 0.014 | Ln(q) = 0.7408ln(C)+3.0403 | 0.9846 | 0.7408 | 20.9115 |
| Indicator | EC0 (mg/L) | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Equation | R2 | Qmax (mg/kg) | KL (L/mg) | Equation | R2 | 1/n | K (L/mg) | ||
| TP | 0.025 | 1/q = 0.0077/C+0.0032 | 0.9899 | 312.5 | 0.415584 | Ln(q) = 0.4489ln(C)+4.3439 | 0.9117 | 0.4489 | 77.0073 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Wang, T.; Xu, S.; Ma, L.; Sun, a.X. Seasonal Release Potential of Sediments in Reservoirs and its Impact on Water Quality Assessment. Int. J. Environ. Res. Public Health 2019, 16, 3303. https://doi.org/10.3390/ijerph16183303
Hu S, Wang T, Xu S, Ma L, Sun aX. Seasonal Release Potential of Sediments in Reservoirs and its Impact on Water Quality Assessment. International Journal of Environmental Research and Public Health. 2019; 16(18):3303. https://doi.org/10.3390/ijerph16183303
Chicago/Turabian StyleHu, Suduan, Tianxiang Wang, Shiguo Xu, Lingxiao Ma, and and Xinguo Sun. 2019. "Seasonal Release Potential of Sediments in Reservoirs and its Impact on Water Quality Assessment" International Journal of Environmental Research and Public Health 16, no. 18: 3303. https://doi.org/10.3390/ijerph16183303
APA StyleHu, S., Wang, T., Xu, S., Ma, L., & Sun, a. X. (2019). Seasonal Release Potential of Sediments in Reservoirs and its Impact on Water Quality Assessment. International Journal of Environmental Research and Public Health, 16(18), 3303. https://doi.org/10.3390/ijerph16183303





