Adsorption of Malachite Green with Sodium Dodecylbenzene Sulfonate Modified Sepiolite: Characterization, Adsorption Performance and Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Organically Modified Sepiolite
2.3. Characterization
2.4. Adsorption Test
2.5. Acid Regeneration Test
3. Results and Discussion
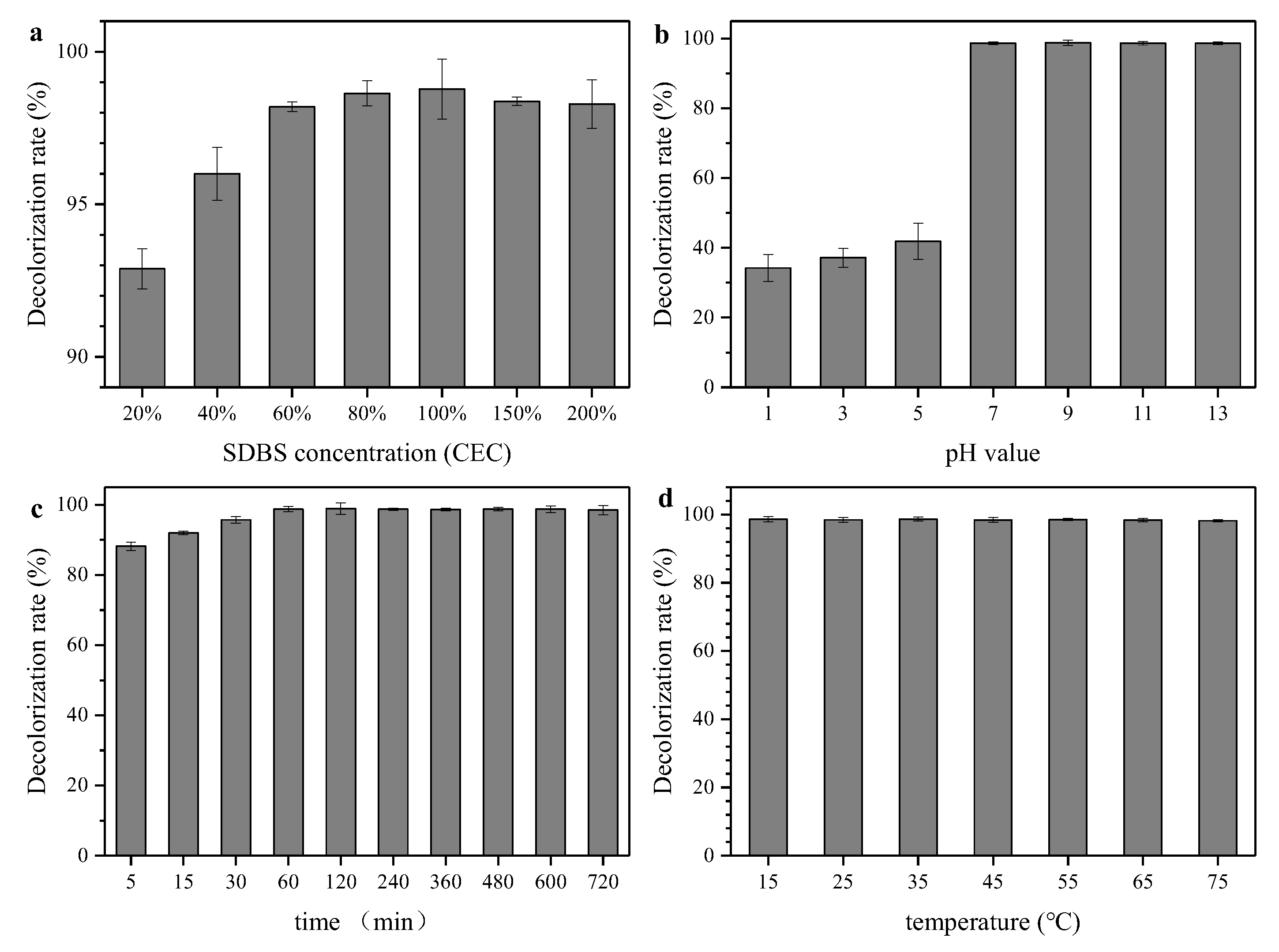
3.1. Optimization of the Modification Process
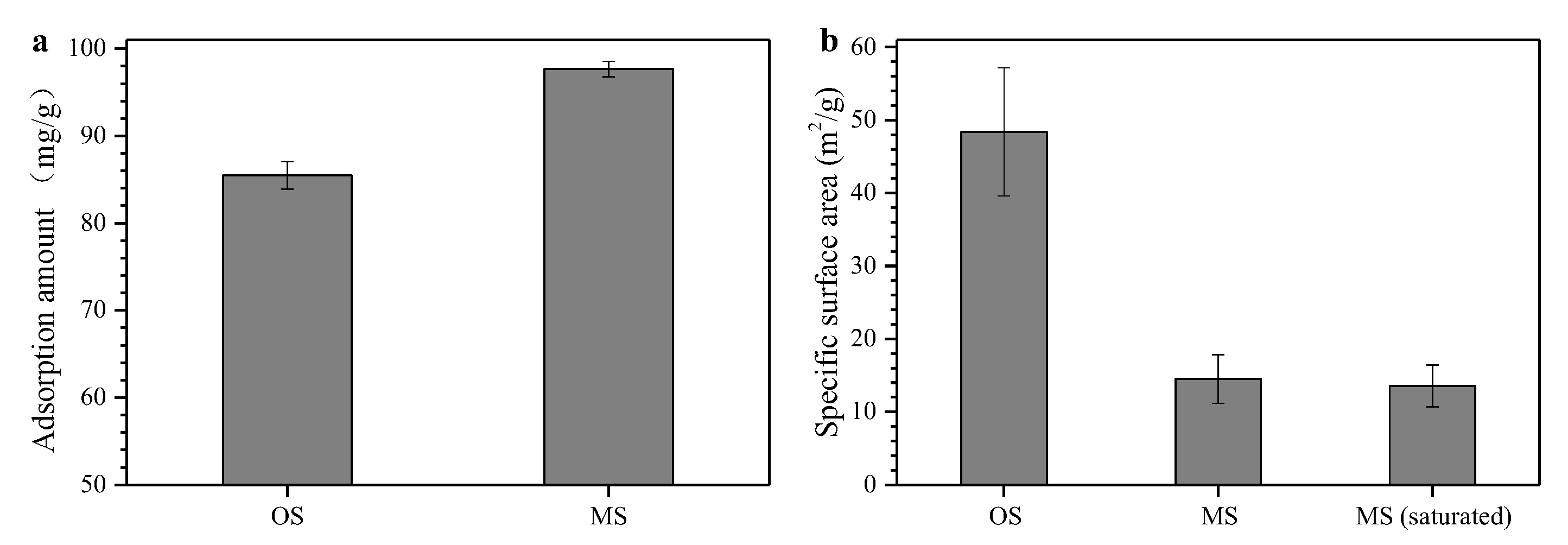
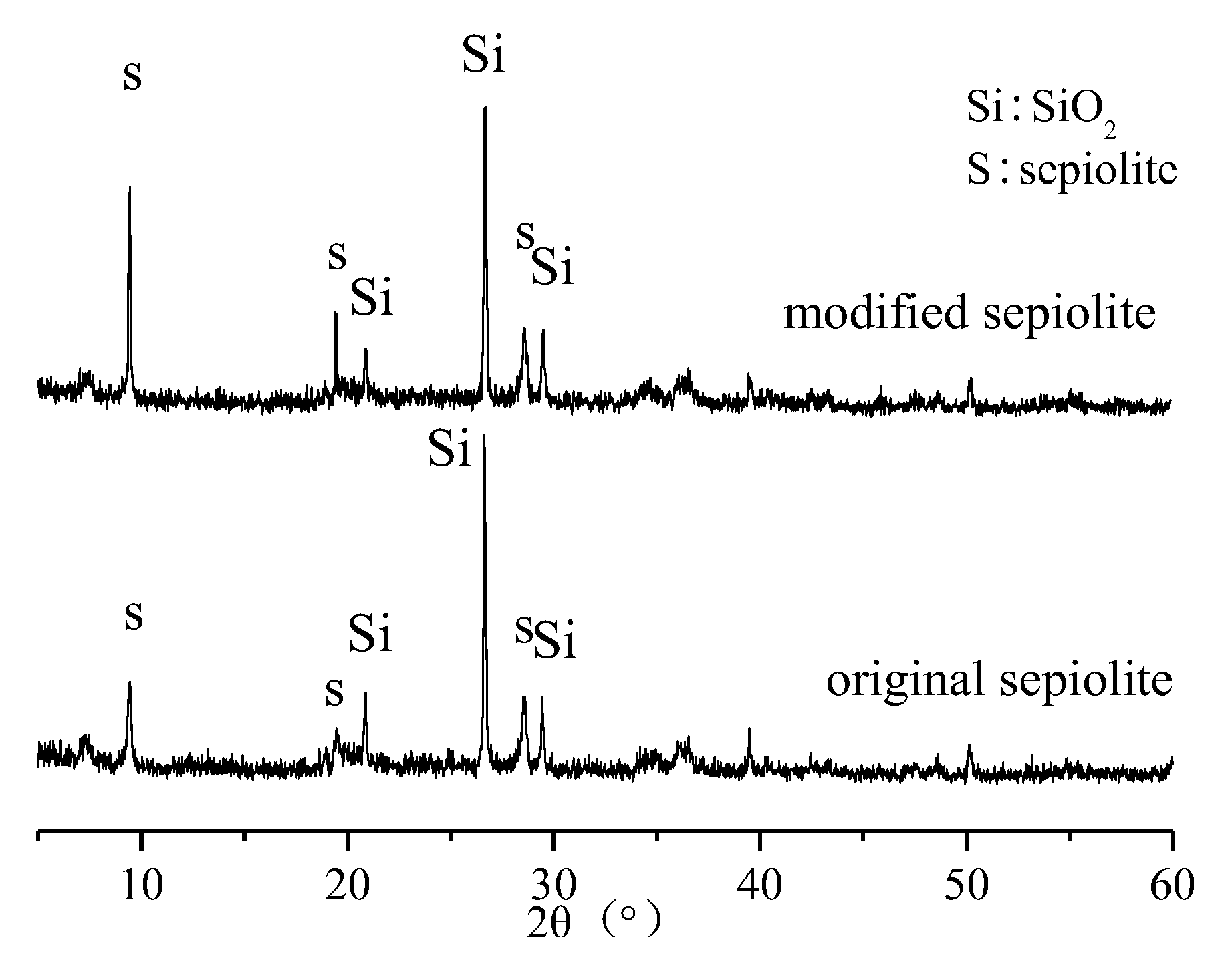
3.2. Characterization of Modified Sepiolite
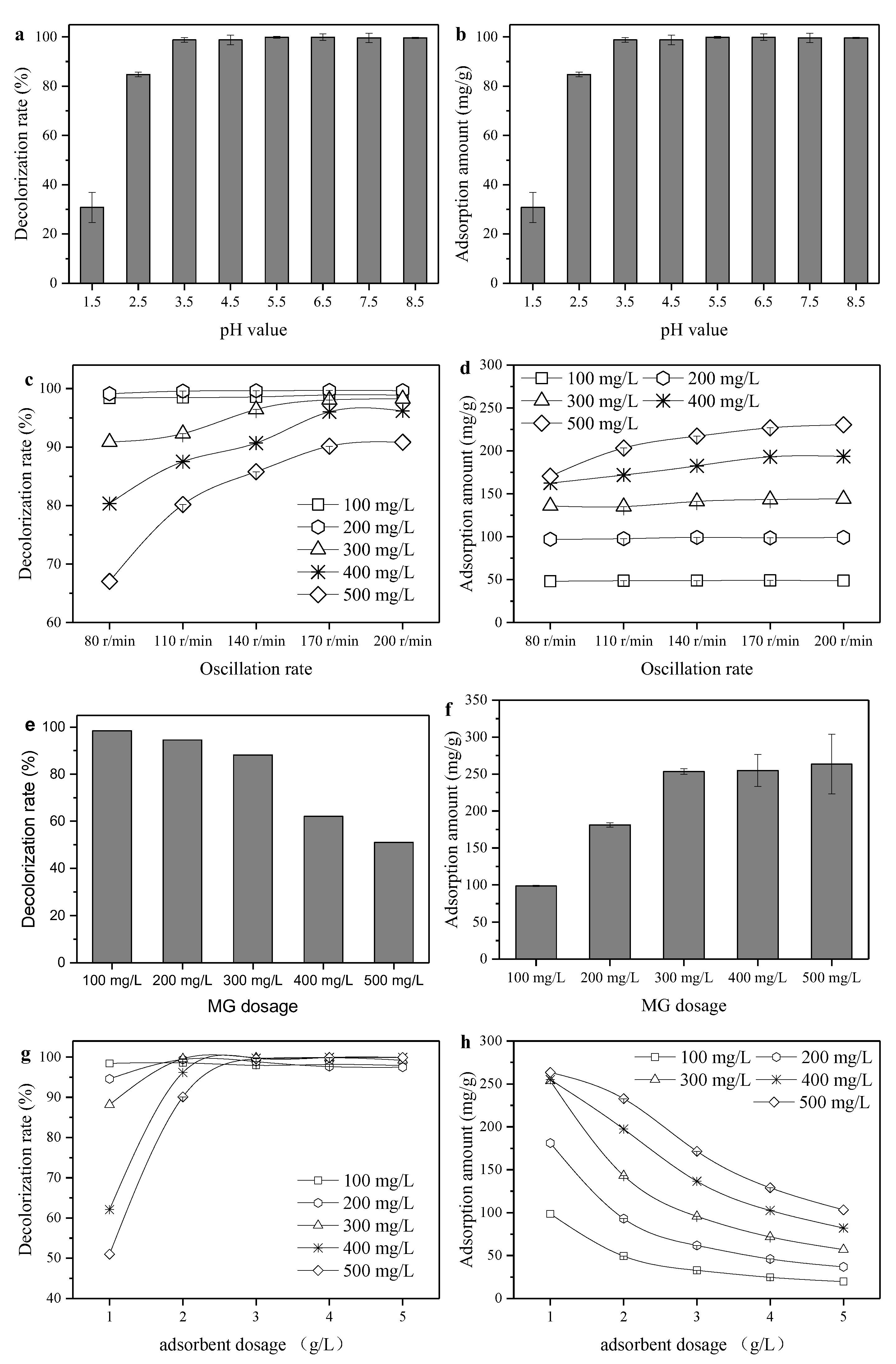
3.3. Impact of the Operation Condition of Adsorption Performance
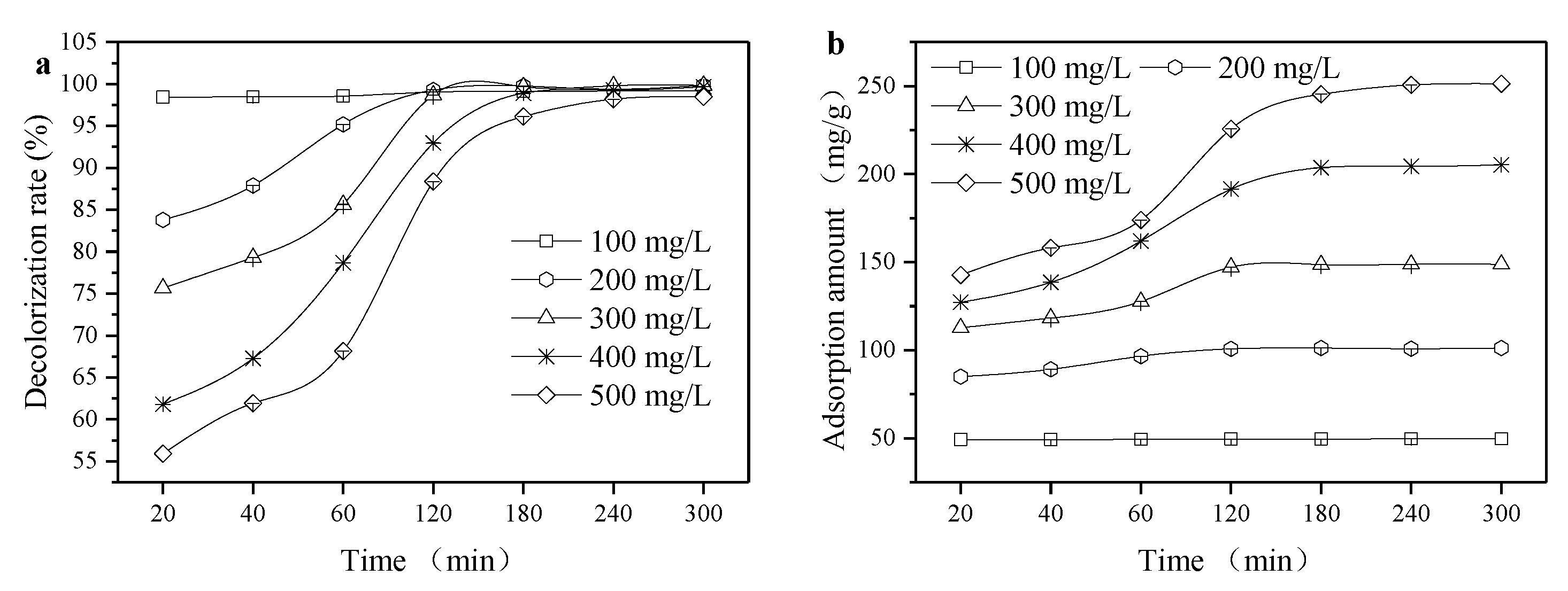
3.4. Adsorption Thermodynamics and Adsorption Kinetics
3.5. Regeneration of Organic Sepiolite Adsorbent
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307. [Google Scholar] [CrossRef]
- Li, G.; Zhou, S.; Shi, Z.; Meng, X.; Li, L.; Liu, B. Electrochemical degradation of ciprofloxacin on BDD anode using a differential column batch reactor: Mechanisms, kinetics and pathways. Environ. Sci. Pollut. R. 2019, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, D.; Ren, W.; Liu, B. Transport of Enterococcus faecalis in granular activated carbon column: Potential energy, migration, and release. Colloids Surf. B Biointerfaces 2019, 183, 110415. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Parra-Saldivar, R.; Hu, H.; Wang, W.; Zhang, X.; Iqbal, H.M. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants–A review. Sci. Total Environ. 2017, 576, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Sayğılı, H.; Güzel, F.; Önal, Y. Conversion of grape industrial processing waste to activated carbon sorbent and its performance in cationic and anionic dyes adsorption. J. Clean. Prod. 2015, 93, 84–93. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Kumar, A.; Rana, S.; Sharma, S.; Bhatnagar, A.; Stadler, F.J.; Ghfar, A.A.; Khan, M.R. Efficient removal of coomassie brilliant blue R-250 dye using starch/poly (alginic acid-cl-acrylamide) nanohydrogel. Process Saf. Environ. Prot. 2017, 109, 301–310. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Pathania, D.; Kumar, A. A multifunctional nanocomposite pectin thorium (IV) tungstomolybdate for heavy metal separation and photoremediation of malachite green. Desalin Water Treat 2016, 57, 19443–19455. [Google Scholar] [CrossRef]
- Pathania, D.; Katwal, R.; Sharma, G.; Naushad, M.; Khan, M.R.; Ala’a, H. Novel guar gum/Al2O3 nanocomposite as an effective photocatalyst for the degradation of malachite green dye. Int. J. Biol. Macromol. 2016, 87, 366–374. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, X.; Zhang, G. Core-shell Ag@ Pt nanoparticles supported on sepiolite nanofibers for the catalytic reduction of nitrophenols in water: Enhanced catalytic performance and DFT study. Appl. Catal. B: Environ. 2017, 205, 262–270. [Google Scholar] [CrossRef]
- Wang, Z.; Liao, L.; Hursthouse, A.; Song, N.; Ren, B. Sepiolite-Based Adsorbents for the Removal of Potentially Toxic Elements from Water: A Strategic Review for the Case of Environmental Contamination in Hunan, China. Int. J. Environ. Res. Public Health 2018, 15, 1653. [Google Scholar] [CrossRef] [PubMed]
- Ning, Q.; Yin, Z.; Liu, Y.; Tan, X.; Zeng, G.; Jiang, L.; Liu, S.; Tian, S.; Liu, N.; Wang, X. Fabrication of Stabilized Fe–Mn Binary Oxide Nanoparticles: Effective Adsorption of 17β-Estradiol and Influencing Factors. Int. J. Environ. Res. Public Health 2018, 15, 2218. [Google Scholar] [CrossRef] [PubMed]
- Alhujaily, A.; Yu, H.; Zhang, X.; Ma, F. Highly Efficient and Sustainable Spent Mushroom Waste Adsorbent Based on Surfactant Modification for the Removal of Toxic Dyes. Int. J. Environ. Res. Public Health 2018, 15, 1421. [Google Scholar] [CrossRef]
- Liu, B.; Qu, F.; Yu, H.; Tian, J.; Chen, W.; Liang, H.; Li, G.; Van der Bruggen, B. Membrane Fouling and Rejection of Organics during Algae-Laden Water Treatment Using Ultrafiltration: A Comparison between in Situ Pretreatment with Fe (II)/Persulfate and Ozone. Environ. Sci. Technol. 2018, 52, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Yaumi, A.; Bakar, M.A.; Hameed, B. Reusable nitrogen-doped mesoporous carbon adsorbent for carbon dioxide adsorption in fixed-bed. Energy 2017, 138, 776–784. [Google Scholar] [CrossRef]
- Schuster, K.D.; Mohammadi, M.; Cahill, K.B.; Matte, S.L.; Maillet, A.D.; Vashisth, H.; Cote, R.H. Pharmacological and molecular dynamics analyses of differences in inhibitor binding to human and nematode PDE4: Implications for management of parasitic nematodes. PLoS ONE 2019, 14, e0214554. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mohammadiarani, H.; Shaw, V.S.; Neubig, R.R.; Vashisth, H. Interplay of cysteine exposure and global protein dynamics in small-molecule recognition by a regulator of G-protein signaling protein. Proteins Struct. Funct. Bioinform. 2019, 87, 146–156. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Z.; Zhang, J.; Wu, C. The strengthening role of the amino group in metal–organic framework MIL-53 (Al) for methylene blue and malachite green dye adsorption. J. Chem. Eng. Data 2015, 60, 3414–3422. [Google Scholar] [CrossRef]
- Baldermann, A.; Mavromatis, V.; Frick, P.M.; Dietzel, M. Effect of aqueous Si/Mg ratio and pH on the nucleation and growth of sepiolite at 25 °C. Geochim. Cosmochim. Acta 2018, 227, 211–226. [Google Scholar] [CrossRef]
- Ge, X.; Li, H.; Wu, L.; Li, P.; Mu, X.; Jiang, Y. Improved mechanical and barrier properties of starch film with reduced graphene oxide modified by SDBS. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Du, X.; Han, Q.; Li, J.; Li, H. The behavior of phosphate adsorption and its reactions on the surfaces of Fe–Mn oxide adsorbent. J. Taiwan Inst. Chem. Eng. 2017, 76, 167–175. [Google Scholar] [CrossRef]
- Shao, S.; Fu, W.; Li, X.; Shi, D.; Jiang, Y.; Li, J.; Gong, T.; Li, X. Membrane fouling by the aggregations formed from oppositely charged organic foulants. Water Res. 2019, 159, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.; Ghaedi, M.; Taghdiri, M.; Asfaram, A. Application of ZnO nanorods loaded on activated carbon for ultrasonic assisted dyes removal: Experimental design and derivative spectrophotometry method. Ultrason. Sonochemistry 2016, 33, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Gora, S.L.; Andrews, S.A. Adsorption of natural organic matter and disinfection byproduct precursors from surface water onto TiO2 nanoparticles: pH effects, isotherm modelling and implications for using TiO2 for drinking water treatment. Chemosphere 2017, 174, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Alqadami, A.A.; Naushad, M.; Alothman, Z.; Ahamad, T. Adsorptive performance of MOF nanocomposite for methylene blue and malachite green dyes: Kinetics, isotherm and mechanism. J. Environ. Manag. 2018, 223, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Lin, X.; Xie, J.; Huang, X. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskite nano-composites. RSC Adv. 2017, 7, 4492–4500. [Google Scholar] [CrossRef]
- Zhou, C.H.; Li, G.L.; Zhuang, X.Y.; Wang, P.P.; Tong, D.S.; Yang, H.M.; Lin, C.X.; Li, L.; Zhang, H.; Ji, S.F. Roles of texture and acidity of acid-activated sepiolite catalysts in gas-phase catalytic dehydration of glycerol to acrolein. Mol. Catal. 2017, 434, 219–231. [Google Scholar] [CrossRef]
- Sturini, M.; Speltini, A.; Maraschi, F.; Profumo, A.; Tarantino, S.; Gualtieri, A.F.; Zema, M. Removal of fluoroquinolone contaminants from environmental waters on sepiolite and its photo-induced regeneration. Chemosphere 2016, 150, 686–693. [Google Scholar] [CrossRef] [PubMed]

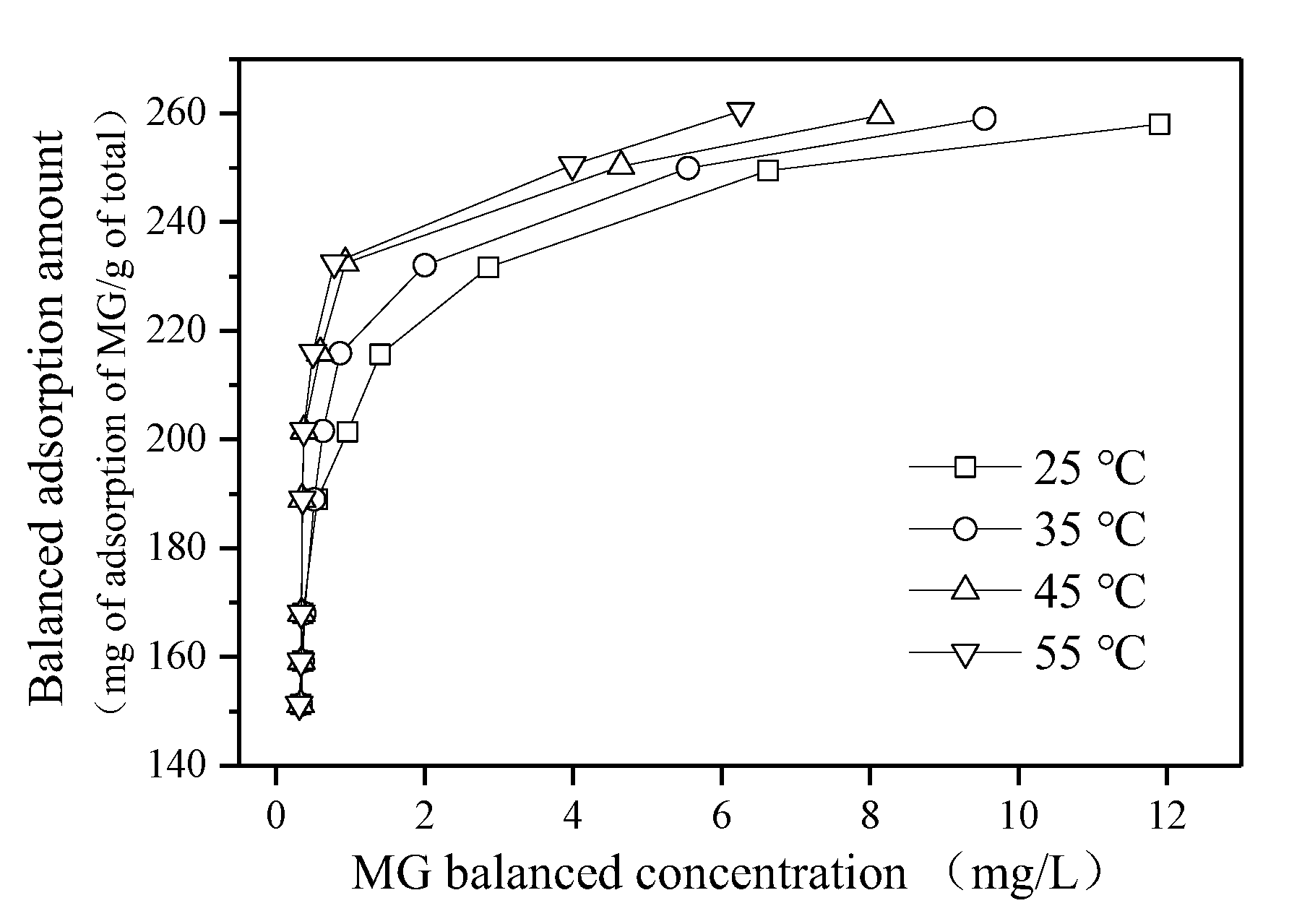

| Temperature (°C) | Regression Equation | b (L/mg) | ||
|---|---|---|---|---|
| 25 | Ce/qe = 0.0039 Ce + 0.001 | 0.9976 | 256.4 | 3.9 |
| 35 | Ce/qe = 0.00375 Ce + 0.0008 | 0.9998 | 266.7 | 4.7 |
| 45 | Ce/qe = 0.0037 Ce + 0.0007 | 0.9997 | 270.3 | 5.3 |
| 55 | Ce/qe = 0.0036 Ce + 0.0006 | 0.9993 | 277.8 | 6.1 |
| Temperature (°C) | Regression Equation | |||
|---|---|---|---|---|
| 25 | ln qe = 0.1409 ln Ce + 5.2632 | 0.9172 | 193.098 | 7.097 |
| 35 | ln qe = 0.146 Ce + 5.2901 | 0.8506 | 198.383 | 6.849 |
| 45 | ln qe = 0.1351 Ce + 5.3297 | 0.7075 | 206.376 | 7.402 |
| 55 | ln qe = 0.1439 Ce + 5.3458 | 0.6952 | 209.726 | 6.949 |
| Adsorbent | Experimental Conditions | qm, (mg/g) | Ref. |
|---|---|---|---|
| Fe3O4@AMCA-MIL53(Al) | C0: 25–400 mg/L; pH 6.8; T: 45 °C; m: 20 mg | 329.61 | (Alqadami et al., 2018) [25] |
| MIL-53(Al)-NH2 | C0: 5 mg/L; T: 35 °C; m: 10 mg | 164.9 | (Li et al., 2015) [18] |
| This work | C0: 500 mg/L; T: 45 °C; m: 10 mg | 270.3 |
| Temperature (K) | |||||
|---|---|---|---|---|---|
| 318 | 3.90 | 1423.19 | 11.50 | −37.46 | 153.96 |
| 328 | 4.69 | 1710.56 | 11.50 | −39.14 | 154.39 |
| 338 | 5.29 | 1928.86 | 11.50 | −40.67 | 154.35 |
| 348 | 6 | 2189.52 | 11.50 | −42.24 | 154.42 |
| C0 (mg/L) | Quasi-First-Order Reaction Kinetics Model | Quasi-Secondary Reaction Kinetics Model | Intraparticle Diffusion Model | |||||
|---|---|---|---|---|---|---|---|---|
(mg/g) | (1/min) | (mg/g) | (1/min) | (1/min) | ||||
| 100 | 2.72 | 0.6 × 10−3 | 0.8319 | 49.75 | 0.947 × 10−4 | 1 | 0.0339 | 0.9027 |
| 200 | 13.05 | 15 × 10−3 | 0.8183 | 103.09 | 0.895 × 10−4 | 0.9999 | 1.2067 | 0.7663 |
| 300 | 51.04 | 22.2 × 10−3 | 0.9367 | 149.25 | 0.731 × 10−4 | 0.9994 | 0.9196 | 0.6648 |
| 400 | 145.98 | 21.8 × 10−3 | 0.9872 | 222.22 | 2.109 × 10−3 | 0.9989 | 6.521 | 0.9005 |
| 500 | 270.10 | 23.2 × 10−3 | 0.9809 | 277.78 | 2.197 × 10−3 | 0.9974 | 9.4062 | 0.9356 |
| Temperature (°C) | Quasi-First-Order Reaction Kinetics Model | Quasi-Secondary Reaction Kinetics Model | Intraparticle Diffusion Model | |||||
|---|---|---|---|---|---|---|---|---|
(mg/g) | (1/min) | (mg/g) | (1/min) | (1/min) | ||||
| 25 | 51.04 | 22.2 × 10−3 | 0.9367 | 149.25 | 7.3 × 10−4 | 0.9994 | 0.9196 | 0.6648 |
| 35 | 149.01 | 24.3 × 10−3 | 0.8840 | 151.51 | 2 × 10−3 | 0.9999 | 1.1494 | 0.7108 |
| 45 | 149.11 | 24.8 × 10−3 | 0.8950 | 152.67 | 2.7 × 10−3 | 1 | 1.4461 | 0.7542 |
| 55 | 149.23 | 26.7 × 10−3 | 0.9092 | 153.85 | 3.5 × 10−3 | 1 | 3.0565 | 0.8563 |
| HCl Concentration (mol/L) | Decolorization Ratio (%) |
|---|---|
| 0 | 27.14 |
| 0.05 | 33.64 |
| 0.1 | 50.34 |
| 0.2 | 73.56 |
| 0.3 | 60.28 |
| 0.5 | 40.38 |
| 1.0 | 30.88 |
| Numbers of Regeneration | Decolorization Ratio (%) |
|---|---|
| 0 | 99.34 |
| 1 | 73.56 |
| 2 | 62.08 |
| 4 | 48.56 |
| 6 | 18.15 |
| 8 | 10.58 |
| 10 | 10.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhang, L.; Liu, B. Adsorption of Malachite Green with Sodium Dodecylbenzene Sulfonate Modified Sepiolite: Characterization, Adsorption Performance and Regeneration. Int. J. Environ. Res. Public Health 2019, 16, 3297. https://doi.org/10.3390/ijerph16183297
Yu J, Zhang L, Liu B. Adsorption of Malachite Green with Sodium Dodecylbenzene Sulfonate Modified Sepiolite: Characterization, Adsorption Performance and Regeneration. International Journal of Environmental Research and Public Health. 2019; 16(18):3297. https://doi.org/10.3390/ijerph16183297
Chicago/Turabian StyleYu, Jian, Lirong Zhang, and Bin Liu. 2019. "Adsorption of Malachite Green with Sodium Dodecylbenzene Sulfonate Modified Sepiolite: Characterization, Adsorption Performance and Regeneration" International Journal of Environmental Research and Public Health 16, no. 18: 3297. https://doi.org/10.3390/ijerph16183297
APA StyleYu, J., Zhang, L., & Liu, B. (2019). Adsorption of Malachite Green with Sodium Dodecylbenzene Sulfonate Modified Sepiolite: Characterization, Adsorption Performance and Regeneration. International Journal of Environmental Research and Public Health, 16(18), 3297. https://doi.org/10.3390/ijerph16183297






