Effects of Exogenous N-Acyl-Homoserine Lactone as Signal Molecule on Nitrosomonas Europaea under ZnO Nanoparticle Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Chemicals and Nano-ZnO Characterization
2.3. Anti-Toxicity Experiment Design
2.4. Analytical Methods
2.5. RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Test
3. Results
3.1. Nano-ZnO Characterization
3.2. Cell Growth and Membrane Integrity
3.3. Ammonia Oxidation Rate (AOR), Ammonia Monooxygenase (AMO) Activity, and amoA Expression
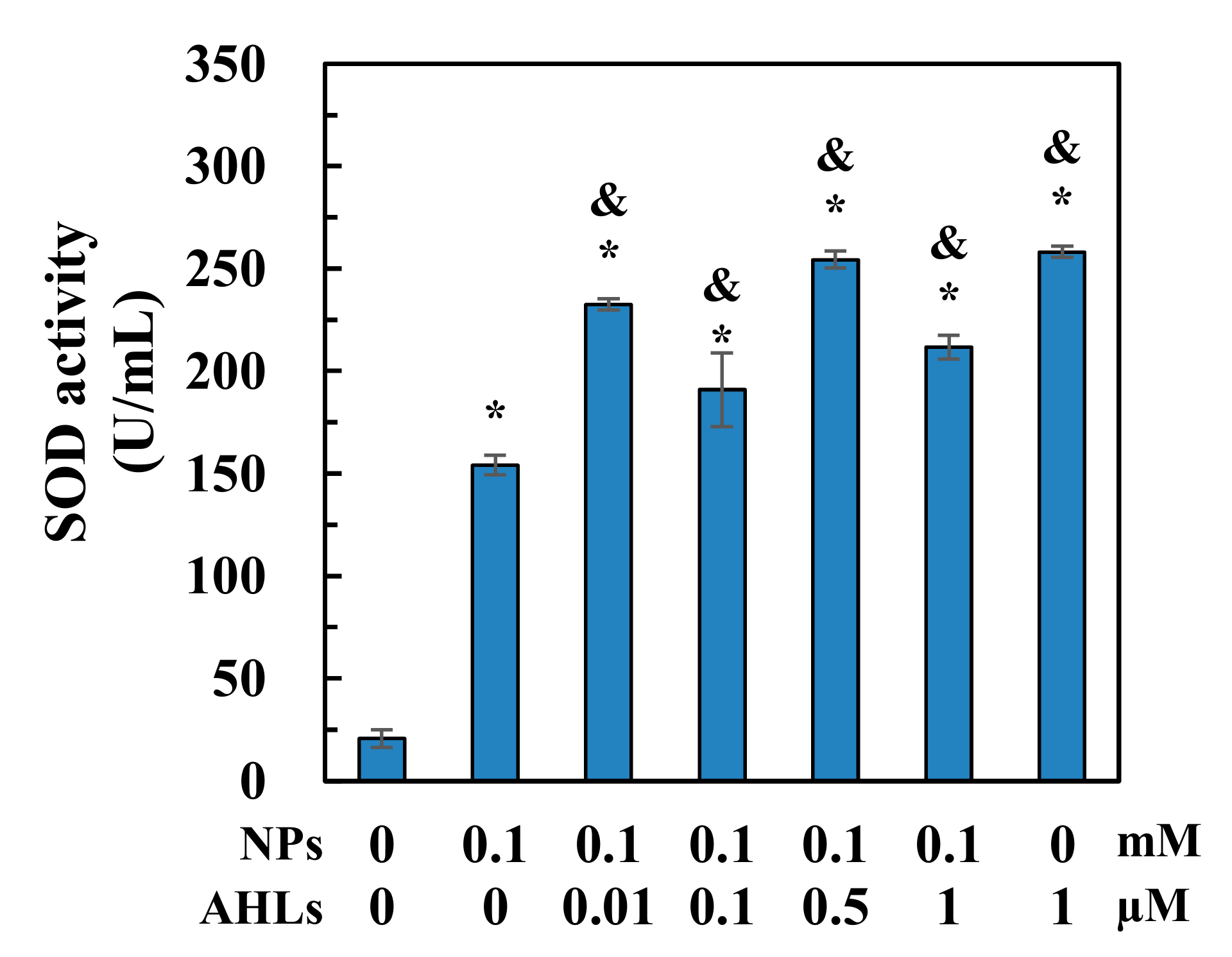
3.4. Superoxide Dismutase (SOD) Activity
3.5. Nano-ZnO Dissolution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MNPs | metallic nanoparticles |
| nano-ZnO | ZnO nanoparticle |
| BNR | biological nitrogen removal |
| AOB | ammonia-oxidizing bacteria |
| QS | quorum sensing |
| AI | autoinducer |
| 3-oxo-C6-HSL | N-3-oxo-hexanoyl-homoserine lactone |
| AHL | N-acyl-homoserine lactone |
| AOR | ammonia oxidation rate |
| DMSO | dimethylsulfoxide |
| DO | dissolved oxygen |
| SOD | superoxide dismutase |
| AMO | ammonia monooxygenase |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
References
- Roco, M.C. The long view of nanotechnology development: The National Nanotechnology Initiative at 10 years. J. Nanopart. Res. 2011, 13, 427–445. [Google Scholar] [CrossRef]
- Joo, S.H.; Zhao, D. Environmental dynamics of metal oxide nanoparticles in heterogeneous systems: A review. J. Hazard. Mater. 2017, 322, 29–47. [Google Scholar] [CrossRef] [PubMed]
- The JRC Nanomaterials Repository webpage. List of Nanomaterials in the JRC Nanomaterials (NM) Repository. 2016. Available online: https://ec.europa.eu/jrc/en/scientific-tool/jrc-nanomaterials-repository.
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticle—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; He, Y.; Chen, C.; Liu, X.; Wang, L.; Yang, B.; Leng, M.; Song, H.; Zeng, K.; Li, D.; et al. Magnetron sputtered ZnO buffer layer for Sb2Se3 thin film solar cells. Sol. Energy Mater. Sol. Cells 2017, 172, 74–81. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Peer, A.; Joshi, P.H.; Biswas, R.; Dalal, V.L. Blue photon management by inhouse grown ZnO:Al cathode for enhanced photostability in polymer solar cells. Sol. Energy Mater. Sol. Cells 2018, 179, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Leitch, M.E.; Casman, E.; Lowry, G.V. Nanotechnology patenting trends through an environmental lens: Analysis of materials and applications. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, G.; Yu, R. Fates and impacts of nanomaterial contaminants in biological wastewater treatment system: A review. Water Air Soil Pollut. 2018, 229. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y. Critical review of the influences of nanoparticles on biological wastewater treatment and sludge digestion. Crit. Rev. Biotechnol. 2016, 36, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Li, S.P.; Wang, W.Q.; You, H. The impact of zinc oxide nanoparticles on nitrification and the bacterial community in activated sludge in an SBR. RSC Adv. 2015, 5, 67335–67342. [Google Scholar] [CrossRef]
- Wilke, C.M.; Tong, T.; Gaillard, J.F.; Gray, K.A. Attenuation of microbial stress due to nano-Ag and nano-TiO2 interactions under dark conditions. Environ. Sci. Technol. 2016, 50, 11302–11310. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wu, J.; Liu, M.; Chen, L.; Zhu, G.; Lu, H. Physiological and transcriptional responses of Nitrosomonas europaea to TiO2 and ZnO nanoparticles and their mixtures. Environ. Sci. Pollut. Res. 2016, 23, 13023–13034. [Google Scholar] [CrossRef] [PubMed]
- Wilke, C.M.; Gaillard, J.-F.; Gray, K.A. The critical role of light in moderating microbial stress due to mixtures of engineered nanomaterials. Environ. Sci. Nano 2018, 5, 96–102. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C. Exploiting quorum sensing interfering strategies in gram-negative bacteria for the enhancement of environmental applications. Front. Microbiol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal–response systems in gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, A.; Cui, D.; Wang, Q.; Wu, D.; Cui, C.; Ma, F. N-Acyl-homoserine lactones and autoinducer-2-mediated quorum sensing during wastewater treatment. Appl. Microbiol. Biot. 2018, 102, 1119–1130. [Google Scholar] [CrossRef]
- Batchelor, S.E.; Cooper, M.; Chhabra, S.R.; Glover, L.A.; Stewart, G.S.; Williams, P.; Prosser, J.I. Cell density-regulated recovery of starved biofilm populations of ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 1997, 63, 2281–2286. [Google Scholar] [Green Version]
- Li, A.J.; Hou, B.L.; Li, M.X. Cell adhesion, ammonia removal and granulation of autotrophic nitrifying sludge facilitated by N-acyl-homoserine lactones. Bioresour. Technol. 2015, 196, 550–558. [Google Scholar] [CrossRef]
- Tan, C.H.; Koh, K.S.; Xie, C.; Tay, M.; Zhou, Y.; Williams, R.; Ng, W.J.; Rice, S.A.; Kjelleberg, S. The role of quorum sensing signalling in EPS production and the assembly of a sludge community into aerobic granules. ISME J. 2014, 8, 1186–1197. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, N.; Wang, M.; Feng, H.; Xu, C.; Xu, F. Interference of non-lethal levels of graphene oxide in biofilm formation and adaptive response of quorum sensing in bacteria. Environ. Sci. Nano 2018, 5, 2809–2818. [Google Scholar] [CrossRef]
- Mohanty, A.; Tan, C.H.; Cao, B. Impacts of nanomaterials on bacterial quorum sensing: Differential effects on different signals. Environ. Sci. Nano 2016, 3, 351–356. [Google Scholar] [CrossRef]
- Gu, A.Z.; Pedros, P.B.; Kristiansen, A.; Onnis-Hayden, A.; Schramm, A. Nitrifying community analysis in a single submerged attached-growth bioreactor for treatment of high-ammonia waste stream. Water Environ. Res. 2007, 79, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.O.; Read, H.W.; Pellitteri, M.C.; Hickey, W.J. Identification of acyl-homoserine lactone signal molecules produced by Nitrosomonas europaea strain schmidt. Appl. Environ. Microbiol. 2005, 71, 4906–4909. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhan, M.; Chang, Y.; Su, Q.; Yu, R. Adaption and recovery of Nitrosomonas europaea to chronic TiO2 nanoparticle exposure. Water Res. 2018, 147, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.; Kimyon, O.; Rice, S.A.; Kjelleberg, S.; Manefield, M. The presence and role of bacterial quorum sensing in activated sludge. Microb. Biotechnol. 2012, 5, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chang, Y.; Gao, H.; Liang, G.; Yu, R.; Ding, Z. Responses and recovery assessment of continuously cultured Nitrosomonas europaea under chronic ZnO nanoparticle stress: Effects of dissolved oxygen. Chemosphere 2018, 195, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wu, J.; Liu, M.; Zhu, G.; Chen, L.; Chang, Y.; Lu, H. Toxicity of binary mixtures of metal oxide nanoparticles to Nitrosomonas europaea. Chemosphere 2016, 153, 187–197. [Google Scholar] [CrossRef] [PubMed]
- SEPAC. Methods for Monitor and Analysis of Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002. (In Chinses) [Google Scholar]
- Liu, M.T.; Yu, R.; Chen, L.H.; Wu, J.K. Biological effects of typical metal oxide nanoparticles on Nitrosomonas europaea. Chin. Environ. Sci. 2015, 35, 190–195. [Google Scholar]
- Bhuvaneshwari, M.; Iswarya, V.; Archanaa, S.; Madhu, G.M.; Kumar, G.K.S.; Nagarajan, R.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aquat. Toxicol. 2015, 162, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, L.V.; Vijayaraghavan, R. Insight into the mechanism of antibacterial activity of ZnO: Surface defects mediated reactive oxygen species even in the dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.H. A review of microbial injury and recovery methods in food. Food Microbiol. 2008, 25, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide dismutase and stress tolerance. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Wu, J.; Lu, H.; Zhu, G.; Chen, L.; Chang, Y.; Yu, R. Regulation of membrane fixation and energy production/conversion for adaptation and recovery of ZnO nanoparticle impacted Nitrosomonas europaea. Appl. Microbiol. Biotechnol. 2017, 101, 2953–2965. [Google Scholar] [CrossRef] [PubMed]
- Radniecki, T.S.; Semprini, L.; Dolan, M.E. Expression of merA, amoA and hao in continuously cultured Nitrosomonas europaea cells exposed to zinc chloride additions. Biotechnol. Bioeng. 2009, 102, 546–553. [Google Scholar] [CrossRef]
- Chain, P.; Lamerdin, J.; Larimer, F.; Regala, W.; Lao, V.; Land, M.; Hauser, L.; Hooper, A.; Klotz, M.; Norton, J.; et al. Complete genome sequence of the ammonia-oxidizing bacterium and obligate chemolithoautotroph Nitrosomonas europaea. J. Bacteriol. 2003, 185, 2759–2773. [Google Scholar] [CrossRef]
- Leadbetter, J.R.; Greenberg, E.P. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 2000, 182, 6921–6926. [Google Scholar] [CrossRef]
- Huang, J.J.; Han, J.I.; Zhang, L.H.; Leadbetter, J.R. Utilization of acyl-homoserine lactone quorum signals for growth by a soil Pseudomonad and Pseudomonas aeruginosa PAO1. Appl. Environ. Microbiol. 2003, 69, 5941–5949. [Google Scholar] [CrossRef]
- Hommes, N.G.; Sayavedra-Soto, L.A.; Arp, D.J. Chemolithoorganotrophic growth of Nitrosomonas europaea on fructose. J. Bacteriol. 2003, 185, 6809. [Google Scholar] [CrossRef]




| DMSO Content (v/v) | Corresponding AHLs Content (µM) | Cell Density (lg(mL−1)) | Membrane Integrity (%) | AOR (10−2·mg N/L/min) |
|---|---|---|---|---|
| 0% (Control) | 0 | 8.49 ± 0.30 | 98.98 ± 0.07 | 9.07 ± 0.08 |
| 0.33% | 1 | 8.45 ± 0.48 * | 98.08 ± 0.23 * | 8.58 ± 0.58 * |
| 0.17% | 0.5 | 8.46 ± 0.20 * | 98.74 ± 0.43 * | 9.04 ± 0.30 * |
| 0.03% | 0.1 | 8.48 ± 0.50 * | 98.22 ± 1.35 * | 8.63 ± 0.52 * |
| Target Gene | Primer Sequence | Length (bp) | Amplification Procedure |
|---|---|---|---|
| amoA | F: GGACTTCACGCTGTATCTG R: GTGCCTTCTACAACGATTGG | 662 | Pre-denaturation: 95 °C, 3 min Denaturation: 95 °C, 20 s Annealing: 59 °C, 30 s Elongation: 72 °C, 20 s Cycle: 40 Melting curve: from 55 to 95 °C, 0.1 °C/s Final hold: 4 °C |
| 16S rRNA | F: TCCTACGGGAGGCAGCAGT R: GGACTACCAGGGTATCTAATCCTGTT | 1462 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Gao, H.; Ye, J.; Chang, Y.; Yu, R.; Ding, Z.; Zhu, G. Effects of Exogenous N-Acyl-Homoserine Lactone as Signal Molecule on Nitrosomonas Europaea under ZnO Nanoparticle Stress. Int. J. Environ. Res. Public Health 2019, 16, 3003. https://doi.org/10.3390/ijerph16163003
Wu J, Gao H, Ye J, Chang Y, Yu R, Ding Z, Zhu G. Effects of Exogenous N-Acyl-Homoserine Lactone as Signal Molecule on Nitrosomonas Europaea under ZnO Nanoparticle Stress. International Journal of Environmental Research and Public Health. 2019; 16(16):3003. https://doi.org/10.3390/ijerph16163003
Chicago/Turabian StyleWu, Junkang, Huan Gao, Jinyu Ye, Yan Chang, Ran Yu, Zhen Ding, and Guangcan Zhu. 2019. "Effects of Exogenous N-Acyl-Homoserine Lactone as Signal Molecule on Nitrosomonas Europaea under ZnO Nanoparticle Stress" International Journal of Environmental Research and Public Health 16, no. 16: 3003. https://doi.org/10.3390/ijerph16163003







