Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review
Abstract
1. Introduction
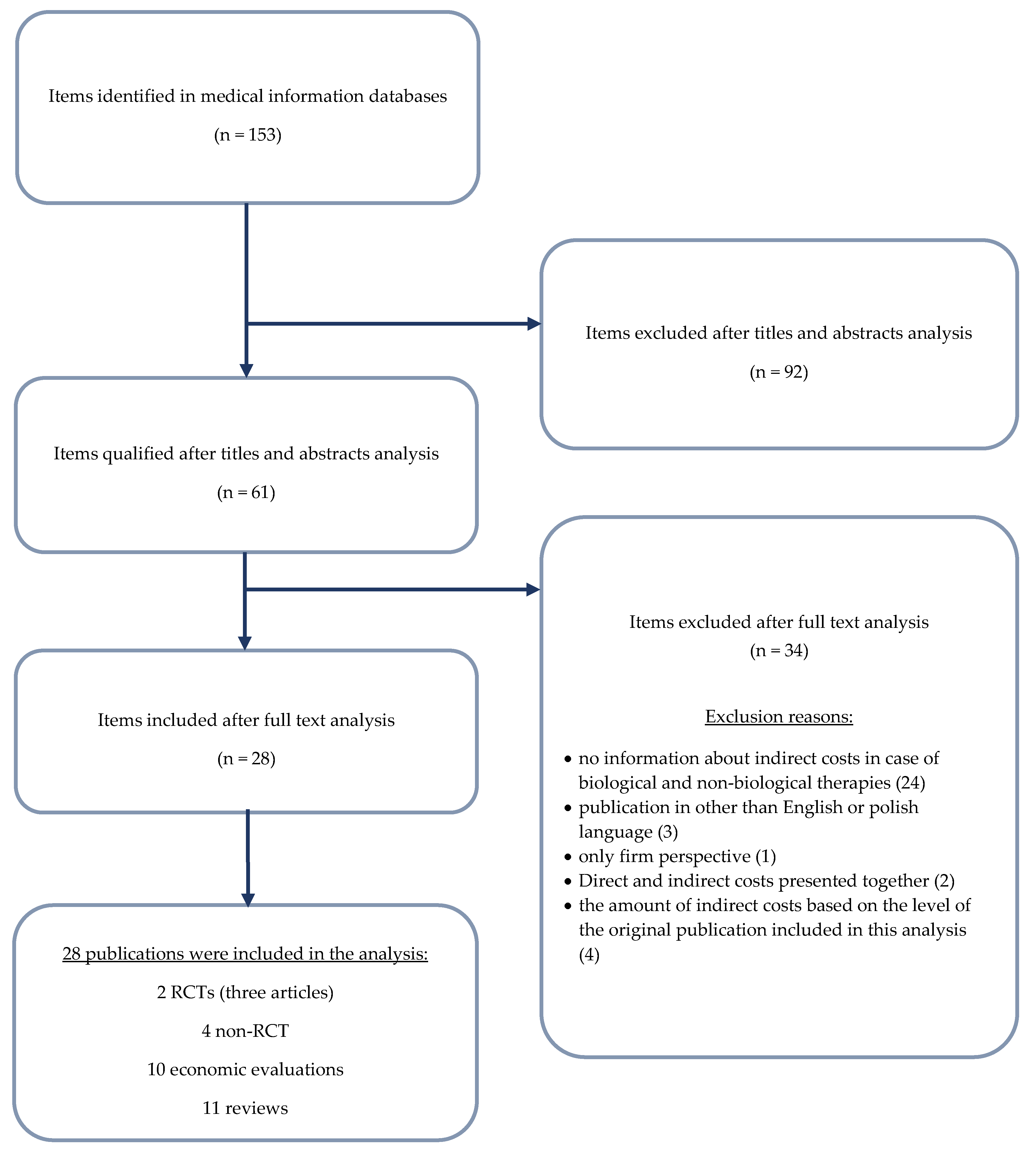
2. Materials and Methods
3. Results
3.1. Absenteeism and Presenteeism
3.2. Overview of Indirect Costs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| N. | Query | Items Found |
|---|---|---|
| #1 | adalimumab* OR Humira OR (D2E7 AND antibody) OR adalimumab [MeSH] | 6987 |
| #2 | etanercept* OR Enbrel OR Erelzi OR Benepali OR (TNR–001 AND "fusion protein") OR etanercept [MeSH] | 7804 |
| #3 | tocilizumab* OR RoActemra OR atlizumab OR tocilizumab [MeSH] | 2380 |
| #4 | certolizumab* OR Cimzia OR (CDP870 OR CDP–870) OR certolizumab [MeSH] | 1072 |
| #5 | rituximab* OR MabThera OR rituximab [MeSH] | 19,933 |
| #6 | anakinra OR Kineret OR anakinra [Mesh] | 5464 |
| #7 | abatacept OR Orencia OR abatacept [MeSH] | 3353 |
| #8 | infliximab OR Remicade OR infliximab [MeSH] | 13,165 |
| #9 | golimumab OR Simponi OR golimumab [MeSH] | 962 |
| #10 | biologic* OR bio-logic* OR "bio logic*" OR biosimilar* OR bio-similar* or "bio similar*" | 1,735,869 |
| #11 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 | 1,776,784 |
| #12 | "rheumatoid arthritis" OR RA | 159,750 |
| #13 | #11 AND #12 | 19,542 |
| #14 | (Indirect OR Productivity) AND (Cost OR Costs OR Cost* OR (Human AND Capital)) | 64,023 |
| #15 | absenteeism OR presenteeism | 11,562 |
| #16 | "human capital method"" OR HCM OR ""willingness to pay method"" OR WTP OR ""friction cost method"" OR FCM | 15,253 |
| #17 | #13 AND (#14 OR #15 OR #16) | 153 |
| Domain | BeST Allaart 2007 [22] | COMET Anis 2009 [23] |
|---|---|---|
| Random sequence generation | Unclear | Low |
| Allocation concealment | Unclear | Low |
| Blinding (participants and personnel) | High | Low |
| Blinding (outcome assessment) | Low | Low |
| Incomplete outcome data | Unclear | Unclear |
| Selective reporting | High | Low |
| Other sources of bias | Unclear | Low |
| Quality Assessment | Zhang 2008 [24] | Augustsson 2010 [25] | Hone 2013 [26] | Klimes 2014 [27] | Tanaka 2018 [28] |
|---|---|---|---|---|---|
| Case series collected in more than one centre, i.e., multi-center study | Yes | Yes | Yes | No | Yes |
| Is the hypothesis/aim/objective of the study clearly described? | Yes | Yes | Yes | No | Yes |
| Are the inclusion and exclusion criteria (case definition) clearly reported? | Yes/No | Yes/No | Yes | No | Yes |
| Is there a clear definition of the outcomes reported? | Yes | Yes | Yes | Yes | Yes |
| Were data collected prospectively? | Yes | Yes | Yes | No | Yes |
| Is there an explicit statement that patients were recruited consecutively? | Yes | Yes | Yes | Yes | Yes |
| Are the main findings of the study clearly described? | Yes | Yes | Yes | Yes | Yes |
| Are outcomes stratified? (e.g., by disease stage, abnormal test results, patient characteristics) | Yes | Yes | Yes | Yes | Yes |
References
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N.; et al. The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Batko, B.; Stajszczyk, M.; Świerkot, J.; Urbański, K.; Raciborski, F.; Jędrzejewski, M.; Wiland, P. Prevalence and clinical characteristics of rheumatoid arthritis in Poland: A nationwide study. Arch. Med. Sci. 2019, 15, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Rheumatology, 6th ed.; Hochberg, M.C., Ed.; Mosby/Elsevier: Philadelphia, PA, USA, 2015; ISBN 978-0-323-09138-1. [Google Scholar]
- Yang, D.-H.; Huang, J.-Y.; Chiou, J.-Y.; Wei, J.C.-C. Analysis of Socioeconomic Status in the Patients with Rheumatoid Arthritis. Int. J. Environ. Res. Public Health 2018, 15, 1194. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.; Fitzpatrick, C.; O’Toole, L.; Doran, M.; O’Shea, F.; Connolly, D.; Fitzpatrick, C.; O’Toole, L.; Doran, M.; O’Shea, F. Impact of Fatigue in Rheumatic Diseases in the Work Environment: A Qualitative Study. Int. J. Environ. Res. Public Health 2015, 12, 13807–13822. [Google Scholar] [CrossRef] [PubMed]
- Sokka, T. Work disability in early rheumatoid arthritis. Clin. Exp. Rheumatol. 2003, 21, S71–S74. [Google Scholar] [PubMed]
- McIntosh, E. The cost of rheumatoid arthritis. Br. J. Rheumatol. 1996, 35, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, H.; Pike, C.; Kaufman, R.; Maynchenko, M.; Kidolezi, Y.; Cifaldi, M. Societal cost of rheumatoid arthritis patients in the US. Curr. Med. Res. Opin. 2010, 26, 77–90. [Google Scholar] [CrossRef]
- Krishnan, E.; Lingala, B.; Bruce, B.; Fries, J.F. Disability in rheumatoid arthritis in the era of biological treatments. Ann. Rheum. Dis. 2012, 71, 213–218. [Google Scholar] [CrossRef]
- Pugner, K.M.; Scott, D.I.; Holmes, J.W.; Hieke, K. The costs of rheumatoid arthritis: an international long-term view. Semin. Arthritis Rheum. 2000, 29, 305–320. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lin, G.-J.; Chen, J.-W.; Wang, K.-C.; Tien, C.-H.; Hu, C.-F.; Chang, C.-N.; Hsu, W.-F.; Fan, H.-C.; Sytwu, H.-K.; et al. Immunopathogenic Mechanisms and Novel Immune-Modulated Therapies in Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 1332. [Google Scholar] [CrossRef]
- Hammond, A. The use of self-management strategies by people with rheumatoid arthritis. Clin. Rehabil. 1998, 12, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jo, C. Cost-of-illness studies: concepts, scopes, and methods. Clin. Mol. Hepatol. 2014, 20, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Her, M.; Kavanaugh, A. Critical analysis of economic tools and economic measurement applied to rheumatoid arthritis. Clin. Exp. Rheumatol. 2012, 30, S107–S111. [Google Scholar] [PubMed]
- Raciborski, F.; Kłak, A.; Kwiatkowska, B. Indirect costs of rheumatoid arthritis. Reumatologia 2015, 53, 268–275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smolen, J.; Aletaha, D. The burden of rheumatoid arthritis and access to treatment: a medical overview. Eur. J. Heal. Econ. 2008, 8, 39–47. [Google Scholar] [CrossRef]
- Kobelt, G.; Jönsson, B. The burden of rheumatoid arthritis and access to treatment: outcome and cost-utility of treatments. Eur. J. Heal. Econ. 2008, 8, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Burton, W.; Morrison, A.; Maclean, R.; Ruderman, E. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occup. Med. (Chic. Ill). 2006, 56, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gignac, M.A.M.; Anis, A.H. The Indirect Costs of Arthritis Resulting From Unemployment, Reduced Performance, and Occupational Changes While at Work. Med. Care 2006, 44, 304–310. [Google Scholar] [CrossRef]
- Goetzel, R.Z.; Long, S.R.; Ozminkowski, R.J.; Hawkins, K.; Wang, S.; Lynch, W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J. Occup. Environ. Med. 2004, 46, 398–412. [Google Scholar] [CrossRef]
- Collins, J.J.; Baase, C.M.; Sharda, C.E.; Ozminkowski, R.J.; Nicholson, S.; Billotti, G.M.; Turpin, R.S.; Olson, M.; Berger, M.L. The assessment of chronic health conditions on work performance, absence, and total economic impact for employers. J. Occup. Environ. Med. 2005, 47, 547–557. [Google Scholar] [CrossRef]
- Allaart, C.F.; Breedveld, F.C.; Dijkmans, B.A.C. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J. Rheumatol. Suppl. 2007, 80, 25–33. [Google Scholar]
- Anis, A.; Zhang, W.; Emery, P.; Sun, H.; Singh, A.; Freundlich, B.; Sato, R. The effect of etanercept on work productivity in patients with early active rheumatoid arthritis: results from the COMET study. Rheumatology 2009, 48, 1283–1289. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Bansback, N.; Guh, D.; Li, X.; Nosyk, B.; Marra, C.A.; Anis, A.H. Short-term influence of adalimumab on work productivity outcomes in patients with rheumatoid arthritis. J. Rheumatol. 2008, 35, 1729–1736. [Google Scholar]
- Augustsson, J.; Neovius, M.; Cullinane-Carli, C.; Eksborg, S.; van Vollenhoven, R.F. Patients with rheumatoid arthritis treated with tumour necrosis factor antagonists increase their participation in the workforce: potential for significant long-term indirect cost gains (data from a population-based registry). Ann. Rheum. Dis. 2010, 69, 126–131. [Google Scholar] [CrossRef]
- Hone, D.; Cheng, A.; Watson, C.; Huang, B.; Bitman, B.; Huang, X.-Y.; Gandra, S.R. Impact of etanercept on work and activity impairment in employed moderate to severe rheumatoid arthritis patients in the United States. Arthritis Care Res. (Hoboken). 2013, 65, 1564–1572. [Google Scholar] [CrossRef]
- Klimeš, J.; Vocelka, M.; Šedová, L.; Doležal, T.; Mlčoch, T.; Petříková, A.; Vlček, J. Medical and Productivity Costs of Rheumatoid Arthritis in The Czech Republic: Cost-of-Illness Study Based on Disease Severity. Value Heal. Reg. Issues 2014, 4, 75–81. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yamazaki, K.; Nakajima, R.; Komatsu, S.; Igarashi, A.; Tango, T.; Takeuchi, T. Economic impact of adalimumab treatment in Japanese patients with rheumatoid arthritis from the adalimumab non-interventional trial for up-verified effects and utility (ANOUVEAU) study. Mod. Rheumatol. 2018, 28, 39–47. [Google Scholar] [CrossRef]
- Eriksson, J.K.; Karlsson, J.A.; Bratt, J.; Petersson, I.F.; van Vollenhoven, R.F.; Ernestam, S.; Geborek, P.; Neovius, M. Cost-effectiveness of infliximab versus conventional combination treatment in methotrexate-refractory early rheumatoid arthritis: 2-year results of the register-enriched randomised controlled SWEFOT trial. Ann. Rheum. Dis. 2015, 74, 1094–1101. [Google Scholar] [CrossRef]
- Birnbaum, H.; Pike, C.; Kaufman, R.; Cifaldi, M. Employer Model of Workplace Impacts of Anti-TNF Therapy for Rheumatoid Arthritis. J. Occup. Environ. Med. 2009, 51, 1167–1176. [Google Scholar] [CrossRef]
- DAVIES, A.; CIFALDI, M.A.; SEGURADO, O.G.; WEISMAN, M.H. Cost-Effectiveness of Sequential Therapy with Tumor Necrosis Factor Antagonists in Early Rheumatoid Arthritis. J. Rheumatol. 2009, 36, 16–26. [Google Scholar] [CrossRef]
- Gissel, C.; Götz, G.; Repp, H. Cost-effectiveness of adalimumab for rheumatoid arthritis in Germany. Z. Rheumatol. 2016, 75, 1006–1015. [Google Scholar] [CrossRef][Green Version]
- Kobelt, G.; Jönsson, L.; Young, A.; Eberhardt, K. The cost-effectiveness of infliximab (Remicade®) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology 2003, 42, 326–335. [Google Scholar] [CrossRef]
- Kobelt, G. TNF inhibitors in the treatment of rheumatoid arthritis in clinical practice: costs and outcomes in a follow up study of patients with RA treated with etanercept or infliximab in southern Sweden. Ann. Rheum. Dis. 2004, 63, 4–10. [Google Scholar] [CrossRef]
- Merkesdal, S.; Kirchhoff, T.; Wolka, D.; Ladinek, G.; Kielhorn, A.; Rubbert-Roth, A. Cost-effectiveness analysis of rituximab treatment in patients in Germany with rheumatoid arthritis after etanercept-failure. Eur. J. Heal. Econ. 2010, 11, 95–104. [Google Scholar] [CrossRef]
- Soini, E.; Asseburg, C.; Taiha, M.; Puolakka, K.; Purcaru, O.; Luosujärvi, R. Modeled Health Economic Impact of a Hypothetical Certolizumab Pegol Risk-Sharing Scheme for Patients with Moderate-to-Severe Rheumatoid Arthritis in Finland. Adv. Ther. 2017, 34, 2316–2332. [Google Scholar] [CrossRef][Green Version]
- Valle-Mercado, C.; Cubides, M.-F.; Parra-Torrado, M.; Rosselli, D. Cost-effectiveness of biological therapy compared with methotrexate in the treatment for rheumatoid arthritis in Colombia. Rheumatol. Int. 2013, 33, 2993–2997. [Google Scholar] [CrossRef]
- Van den Hout, W.B.; Goekoop-Ruiterman, Y.P.M.; Allaart, C.F.; De Vries-Bouwstra, J.K.; Hazes, J.M.; Kerstens, P.J.S.M.; Van Zeben, D.; Hulsmans, H.M.J.; De Jonge-Bok, J.M.; De Sonnaville, P.B.J.; et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2009, 61, 291–299. [Google Scholar] [CrossRef]
- Benucci, M.; Rogai, V.; Atzeni, F.; Hammen, V.; Sarzti-Puttini, P.; Migliore, A. Costs associated with rheumatoid arthritis in Italy: past, present, and future. Clinicoecon. Outcomes Res. 2016, 8, 33–41. [Google Scholar] [CrossRef]
- Fautrel, B.; Verstappen, S.M.M.; Boonen, A. Economic consequences and potential benefits. Best Pract. Res. Clin. Rheumatol. 2011, 25, 607–624. [Google Scholar] [CrossRef]
- Lapadula, G.; Marchesoni, A.; Armuzzi, A.; Blandizzi, C.; Caporali, R.; Chimenti, S.; Cimaz, R.; Cimino, L.; Gionchetti, P.; Girolomoni, G.; et al. Adalimumab in the Treatment of Immune-Mediated Diseases. Int. J. Immunopathol. Pharmacol. 2014, 27, 33–48. [Google Scholar] [CrossRef]
- Furneri, G.; Mantovani, L.G.; Belisari, A.; Mosca, M.; Cristiani, M.; Bellelli, S.; Cortesi, P.A.; Turchetti, G. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin. Exp. Rheumatol. 2012, 30, S72–S84. [Google Scholar]
- Homik, J.E.; Suarez-Almazor, M. An economic approach to health care. Best Pract. Res. Clin. Rheumatol. 2004, 18, 203–218. [Google Scholar] [CrossRef]
- Modena, V.; Bianchi, G.; Roccatello, D. Cost-effectiveness of biologic treatment for rheumatoid arthritis in clinical practice: An achievable target? Autoimmun. Rev. 2013, 12, 835–838. [Google Scholar] [CrossRef]
- Movik, E. TNF-inhibitors for rheumatic diseases (part 3): Health economics / TNF-hemmere ved revmatiske sykdommer (del 3)/Helseøkonomi; Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH): Oslo, Norway, 2007. [Google Scholar]
- Verstappen, S.M.M. Rheumatoid arthritis and work: The impact of rheumatoid arthritis on absenteeism and presenteeism. Best Pract. Res. Clin. Rheumatol. 2015, 29, 495–511. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Appendix 4: Quality assessment for Case Series. Available online: https://www.nice.org.uk/guidance/cg3/documents/appendix-4-quality-of-case-series-form2 (accessed on 18 July 2019).
- Boers, M. The cost-utility analysis of the BeSt trial: Is a camel in fact a horse with abnormalities in the distribution of dorsal fat? Comment on the article by van den Hout et al. Arthritis Rheum. 2009, 61, 1616–1617. [Google Scholar] [CrossRef]
- Zhang, W.; Monique, A.M.; Beaton, D.; Tang, K.; Anis, A.H. Productivity Loss Due to Presenteeism Among Patients with Arthritis: Estimates from 4 Instruments. J. Rheumatol. 2010, 37, 1805–1814. [Google Scholar] [CrossRef]
- Albers, J.M.; Kuper, H.H.; van Riel, P.L.; Prevoo, M.L.; van ’t Hof, M.A.; van Gestel, A.M.; Severens, J.L. Socio-economic consequences of rheumatoid arthritis in the first years of the disease. Rheumatology (Oxford). 1999, 38, 423–430. [Google Scholar] [CrossRef]
- Lacaille, D.; Sheps, S.; Spinelli, J.J.; Chalmers, A.; Esdaile, J.M. Identification of modifiable work-related factors that influence the risk of work disability in rheumatoid arthritis. Arthritis Rheum. 2004, 51, 843–852. [Google Scholar] [CrossRef]
- Hallert, E.; Husberg, M.; Bernfort, L. The incidence of permanent work disability in patients with rheumatoid arthritis in Sweden 1990-2010: before and after introduction of biologic agents. Rheumatology 2012, 51, 338–346. [Google Scholar] [CrossRef]
- Gulácsi, L.; Brodszky, V.; Baji, P.; Kim, H.; Kim, S.Y.; Cho, Y.Y.; Péntek, M. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev. Clin. Immunol. 2015, 11, 43–52. [Google Scholar] [CrossRef]
- Manova, M.; Savova, A.; Vasileva, M.; Terezova, S.; Kamusheva, M.; Grekova, D.; Petkova, V.; Petrova, G. Comparative Price Analysis of Biological Products for Treatment of Rheumatoid Arthritis. Front. Pharmacol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- ter Wee, M.M.; Lems, W.F.; Usan, H.; Gulpen, A.; Boonen, A. The effect of biological agents on work participation in rheumatoid arthritis patients: a systematic review. Ann. Rheum. Dis. 2012, 71, 161–171. [Google Scholar] [CrossRef]
- Zhang, W.; Anis, A.H. The economic burden of rheumatoid arthritis: beyond health care costs. Clin. Rheumatol. 2011, 30, 25–32. [Google Scholar] [CrossRef]
- Franke, L.C.; Ament, A.J.H.A.; van de Laar, M.A.F.J.; Boonen, A.; Severens, J.L. Cost-of-illness of rheumatoid arthritis and ankylosing spondylitis. Clin. Exp. Rheumatol. 2009, 27, S118–S123. [Google Scholar]
- Lundkvist, J.; Kastäng, F.; Kobelt, G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur. J. Heal. Econ. 2008, 8, 49–60. [Google Scholar] [CrossRef]
- Putrik, P.; Ramiro, S.; Kvien, T.K.; Sokka, T.; Pavlova, M.; Uhlig, T.; Boonen, A. Working Group ‘Equity in access to treatment of rheumatoid arthritis in Europe’ Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann. Rheum. Dis. 2014, 73, 198–206. [Google Scholar] [CrossRef]
- Dougados, M.; Soubrier, M.; Antunez, A.; Balint, P.; Balsa, A.; Buch, M.H.; Casado, G.; Detert, J.; El-zorkany, B.; Emery, P.; et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 2014, 73, 62–68. [Google Scholar] [CrossRef]
- Batko, B.; Urbański, K.; Świerkot, J.; Wiland, P.; Raciborski, F.; Jędrzejewski, M.; Koziej, M.; Cześnikiewicz-Guzik, M.; Guzik, T.J.; Stajszczyk, M. Comorbidity burden and clinical characteristics of patients with difficult-to-control rheumatoid arthritis. Clin. Rheumatol. 2019. [Google Scholar] [CrossRef]
- Johnston, D.A.; Harvey, S.B.; Glozier, N.; Calvo, R.A.; Christensen, H.; Deady, M. The relationship between depression symptoms, absenteeism and presenteeism. J. Affect. Disord. 2019, 256, 536–540. [Google Scholar] [CrossRef]
| Author | Study Design | Treatment | n | Stage of RA | Age Range (Mean) | Disease Duration (Mean) | Absenteeism Measure | Presenteeism Measure | Method | Time Points |
|---|---|---|---|---|---|---|---|---|---|---|
| RCT Trials | ||||||||||
| Allaart 2007 [22] | RCT (BeST) | 1. Seq. monotherapy | 508 | Early RA (ACR criteria) | ≥18 (ND) | ND | Three-monthly diary on work absenteeism | - | FCM | Baseline until 2 years |
| 2. Step-up comb. Therapy (INF) c | ||||||||||
| 3. Initial comb. Therapy (INF) d | ||||||||||
| 4. MTX + INF d | ||||||||||
| Anis 2009 [23] | RCT (COMET) | MTX | 100 | Early RA (ACR criteria) | ≥18 (45.1) | 8.9 months | Number of missed work days/WPAI | Reduced working time (in days)/WLQ | HCM | 0 and 12 months (weeks 12, 24, 36, 52) |
| ETA + MTX | 105 | ≥18 (45.4) | 8.6 months | Number of stopped work days f/WLQ | ||||||
| Observational Studies | ||||||||||
| Zhang 2008 [24] | Open-label, multicenter, phase IIIb study (CanAct) | ADA | 389 | Moderate to severe active RA (ACR criteria) | (55.0) | 12.5 years | Number of absent work days multiplied by the individual’s daily wage | Number of extra work hours patients needed to catch up on tasks they were unable to complete during normal working hours multiplied by the individual’s hourly wage | HCM | Baseline and 12 months |
| Augustsson 2010 [25] | Observational (STURE register) | Anti-TNF (ETA, INF, ADA) | 594 | ND | 18–55 years (40.0) | 9.4 years | - | Hours worked/week | ND | Baseline, 6 months, 1, 2, 3, 4, and 5 years |
| Hone 2013 [26] | Prospective, observational study | ETA | 204 | Moderate to severe RA | 20–67 (46.6) | 5.1 years | WPAI measures of absenteeism – work time missed | WPAI measures of presenteeism – impairment at work | HCM | 6 months |
| Klimes 2014 [27] | Bottom-up cross-sectional cost-of-illness study | Without biologics | 137 | ND | 18–64 years (58.9) | 13.6 years | Days spent on sick leave, and the period of time spent on full disability pension or partial disability pension | - | FCM | 6 months |
| With biologics | 124 | 18–64 years (53.6) | 15.5 years | |||||||
| DMARDs | 130 | (53.7) | 10.1 months | |||||||
| Tanaka 2018 [28] | Non-interventional trial for up-verified effects and utility (ANOUVEAU) study | ADA i | 1 196 | Greater portion of the patients had established RA, with moderate disease activity | PW: (50.0) | 5.6 years | WPAI measures of absenteeism—work time missed | WPAI measures of presenteeism—impairment at work | HCM | 48 weeks |
| Author | Measure | Comparator | Difference | ∆ | 95%CI | Significant or Not | Costs |
|---|---|---|---|---|---|---|---|
| RCT Trials | |||||||
| Anis 2009 [23] | Missed work days | MTX vs. ETA+MTX | 31.9 vs. 14.2 | −17.6 | (–34.4; –2.2) | YES | –£1244 c,f |
| Reduced working time | 19.8 vs. 10.5 | –9.3 | (–21.9; 3.9) | NO | –£657 c,f | ||
| Stopped worked days | Scenario Ic: 32.9 vs.10.9 Scenario IId: 12.3 vs. 4.8 | –22.1 –7.4 | (–45.2; –0.3) (–15.9; 1.2) | YES NO | Scenario I: –£1562 c,d,f Scenario II: –£523 c,e,f | ||
| Total absenteeism | Scenario Ic: 65.6 vs.29.0 Scenario IId: 44.3 vs. 22.3 | −36.6 −22.0 | (−68.3; −5.9) (−42.6; –2.1) | YES YES | Scenario I: −£2586 c,d,f Scenario II: −£1555 c,e,f | ||
| Allaart 2007 [21] | Overall | Decrease of 0.1 on utility associated with decrease of 2 working h/week | Using the friction-cost method, overall societal costs were estimated at €19,905, €15,926, €17,810, and €28,547 (p ≤ 0.05 Group 4 vs. Groups 1–3). Indirect costs: €9113, €8638, €10,001, and €4786 (Groups 1, 2, 3, and 4 respectively) a | ||||
| 4. MTX + INF | Productivity was highest in this group | ||||||
| Observational Trials | |||||||
| Zhang 2008 [24] | Absenteeism, mean | ADA vs. baseline | ND | ND | ND | ND | Lost productivity costs, past two weeks: –$57.21 (mean) |
| Hone 2013 [26] | Hours gained/patient (absenteeism) | ETA baseline vs. 6 months | 71.1 vs. 63.5/ 9.9 ± 20.1 vs. 4.4 ± 16.1 | 7.6/ −3.5 ± 17.0 | ND/ (−6.1; −1.0) | ND/ YES | Economic gain/patient: $1794 |
| Klimes 2014 [27] | Productivity costs | Without vs. with biological treatment | ND | ND | ND | ND | Friction cost approach: €1304 vs. €2090 |
| Tanaka 2018 [28] | Absenteeism | Baseline vs. week 48 | ND | ND | ND | ND | Human capital method (cumulative reduction): PW: $9278 (mean) PTW: $6480 (mean) HM: $5449 (mean) |
| Author | Measure | Comparator | Difference | ∆ | 95%CI | Significant or Not | Costs |
|---|---|---|---|---|---|---|---|
| RCT Trial | |||||||
| Anis 2009 [23] | WPAI: Work productivity loss at work (%) | MTX vs. ETA + MTX | 23.1 vs. 15.6 | −7.5 | (−11.2; −4.2) | YES | NA |
| WPAI: Lost work days due to presenteeism | Scenario I: 34.0 vs. 28.6 Scenario II: 38.9 vs. 29.7 | −5.4 −9.3 | (−13.5; 2.8) (−16.3; −2.5) | NO YES | −£382 −£657 | ||
| WPAI: Total work productivity loss, days | Scenario I: 99.6 vs. 57.6 Scenario II: 83.3 vs. 51.9 | −42.0 −31.3 | (−69.0; −15.7) (−50.2; −12.6) | YES YES | −£2968 −£2212 | ||
| WLQ: work productivity loss at work (%) | 6.2 vs. 4.8 | −1.4 | (−2.1; −0.7) | YES | NA | ||
| WLQ: Lost work days due to presenteeism | Scenario I: 9.1 vs. 8.9 Scenario II: 10.4 vs. 9.2 | −0.3 −1.3 | (−2.3; 1.8) (−2.8; 0.3) | NO NO | −£21 −£92 | ||
| WLQ: Total work productivity loss, days | Scenario I: 74.7 vs. 37.8 Scenario II: 54.8 vs. 31.5 | −36.9 −23.3 | (−66.9; −7.6) (−43.0; −4.2) | YES YES | −£2607 −£1646 | ||
| Observational trials | |||||||
| Zhang 2008 [24] | Absenteeism, mean | ADA vs. baseline | ND | ND | ND | ND | Lost productivity costs, past two weeks: –$4.48 |
| Augustsson 2010 [25] | Overall | Unadjusted model | Improvement: first year: 4.2 h/week, thereafter: 0.5 h/week | The productivity gains for society in patients continuing treatment would total €28,000 over 5 years. | Note that these estimates only apply to patients who do not discontinue treatment, a group that may be difficult to identify before treatment initiation. | ||
| Adjusted model b | Improvement: first year: 4.1 h/week, thereafter: no change | The productivity gains for society in patients continuing treatment would total €27,000 over 5 years. This corresponds to approximately 40% of the annual anti-TNF drug cost. | |||||
| Hone 2013 [26] | Hours gained/patient (presenteeism) | ETA baseline vs. 6 months | 205.2 vs. 189.7/ 39.7 ± 24.5 vs. 24.8 ± 22.5 | 15.5/ −13.5 ± 23.3 | ND/ (−17.0; −9.9) | ND/ YES | Economic gain/patient: $5328 |
| Tanaka 2018 [28] | Presenteeism | Baseline vs. week 48 | ND | ND | ND | ND | Human capital method (cumulative reduction): PW: $5836 (mean) PTW: $2726 (mean) HM: NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batko, B.; Rolska-Wójcik, P.; Władysiuk, M. Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review. Int. J. Environ. Res. Public Health 2019, 16, 2966. https://doi.org/10.3390/ijerph16162966
Batko B, Rolska-Wójcik P, Władysiuk M. Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review. International Journal of Environmental Research and Public Health. 2019; 16(16):2966. https://doi.org/10.3390/ijerph16162966
Chicago/Turabian StyleBatko, Bogdan, Paulina Rolska-Wójcik, and Magdalena Władysiuk. 2019. "Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review" International Journal of Environmental Research and Public Health 16, no. 16: 2966. https://doi.org/10.3390/ijerph16162966
APA StyleBatko, B., Rolska-Wójcik, P., & Władysiuk, M. (2019). Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review. International Journal of Environmental Research and Public Health, 16(16), 2966. https://doi.org/10.3390/ijerph16162966




