Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review
Abstract
:1. Introduction
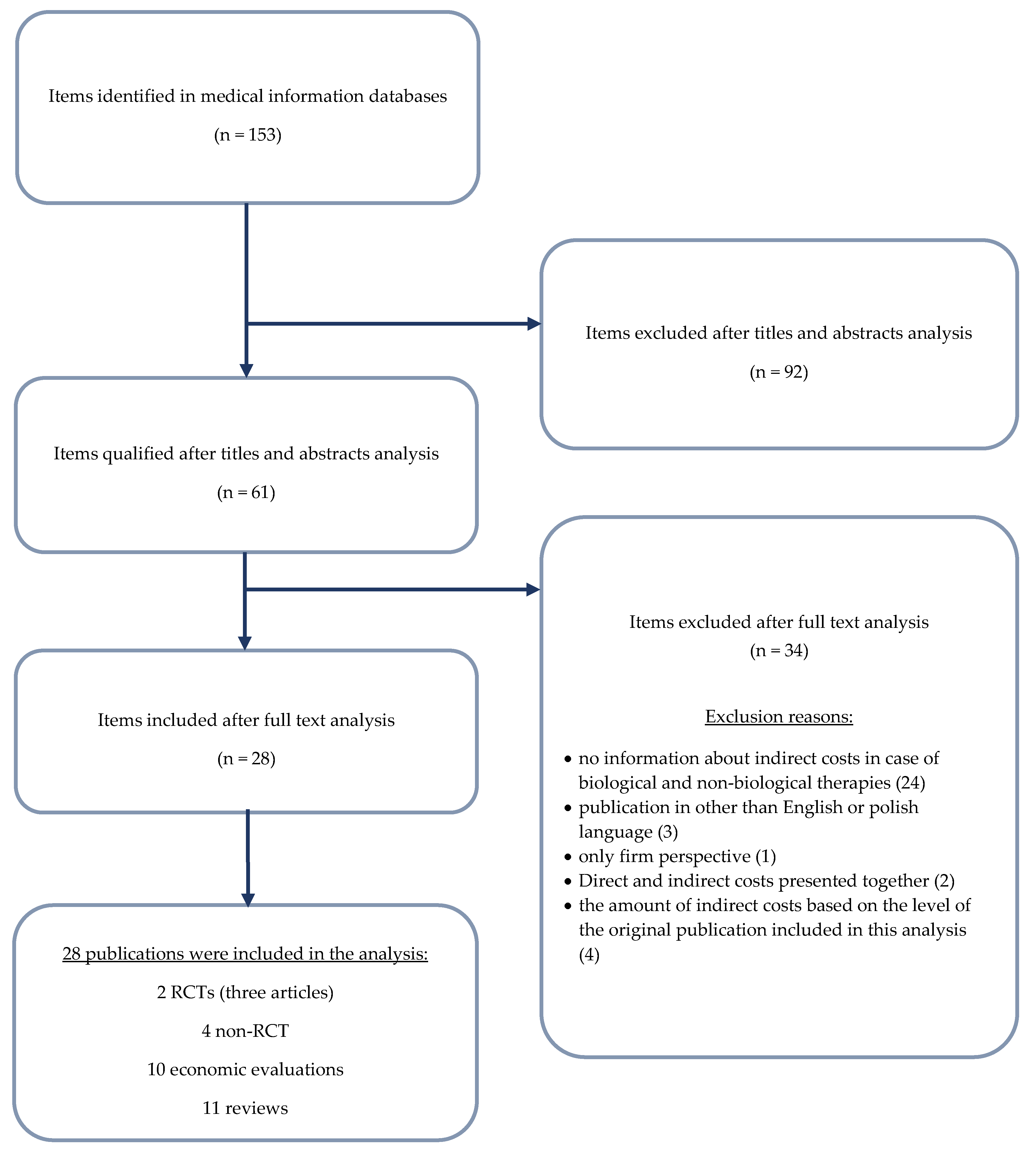
2. Materials and Methods
3. Results
3.1. Absenteeism and Presenteeism
3.2. Overview of Indirect Costs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| N. | Query | Items Found |
|---|---|---|
| #1 | adalimumab* OR Humira OR (D2E7 AND antibody) OR adalimumab [MeSH] | 6987 |
| #2 | etanercept* OR Enbrel OR Erelzi OR Benepali OR (TNR–001 AND "fusion protein") OR etanercept [MeSH] | 7804 |
| #3 | tocilizumab* OR RoActemra OR atlizumab OR tocilizumab [MeSH] | 2380 |
| #4 | certolizumab* OR Cimzia OR (CDP870 OR CDP–870) OR certolizumab [MeSH] | 1072 |
| #5 | rituximab* OR MabThera OR rituximab [MeSH] | 19,933 |
| #6 | anakinra OR Kineret OR anakinra [Mesh] | 5464 |
| #7 | abatacept OR Orencia OR abatacept [MeSH] | 3353 |
| #8 | infliximab OR Remicade OR infliximab [MeSH] | 13,165 |
| #9 | golimumab OR Simponi OR golimumab [MeSH] | 962 |
| #10 | biologic* OR bio-logic* OR "bio logic*" OR biosimilar* OR bio-similar* or "bio similar*" | 1,735,869 |
| #11 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 | 1,776,784 |
| #12 | "rheumatoid arthritis" OR RA | 159,750 |
| #13 | #11 AND #12 | 19,542 |
| #14 | (Indirect OR Productivity) AND (Cost OR Costs OR Cost* OR (Human AND Capital)) | 64,023 |
| #15 | absenteeism OR presenteeism | 11,562 |
| #16 | "human capital method"" OR HCM OR ""willingness to pay method"" OR WTP OR ""friction cost method"" OR FCM | 15,253 |
| #17 | #13 AND (#14 OR #15 OR #16) | 153 |
| Domain | BeST Allaart 2007 [22] | COMET Anis 2009 [23] |
|---|---|---|
| Random sequence generation | Unclear | Low |
| Allocation concealment | Unclear | Low |
| Blinding (participants and personnel) | High | Low |
| Blinding (outcome assessment) | Low | Low |
| Incomplete outcome data | Unclear | Unclear |
| Selective reporting | High | Low |
| Other sources of bias | Unclear | Low |
| Quality Assessment | Zhang 2008 [24] | Augustsson 2010 [25] | Hone 2013 [26] | Klimes 2014 [27] | Tanaka 2018 [28] |
|---|---|---|---|---|---|
| Case series collected in more than one centre, i.e., multi-center study | Yes | Yes | Yes | No | Yes |
| Is the hypothesis/aim/objective of the study clearly described? | Yes | Yes | Yes | No | Yes |
| Are the inclusion and exclusion criteria (case definition) clearly reported? | Yes/No | Yes/No | Yes | No | Yes |
| Is there a clear definition of the outcomes reported? | Yes | Yes | Yes | Yes | Yes |
| Were data collected prospectively? | Yes | Yes | Yes | No | Yes |
| Is there an explicit statement that patients were recruited consecutively? | Yes | Yes | Yes | Yes | Yes |
| Are the main findings of the study clearly described? | Yes | Yes | Yes | Yes | Yes |
| Are outcomes stratified? (e.g., by disease stage, abnormal test results, patient characteristics) | Yes | Yes | Yes | Yes | Yes |
References
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N.; et al. The global burden of rheumatoid arthritis: estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Batko, B.; Stajszczyk, M.; Świerkot, J.; Urbański, K.; Raciborski, F.; Jędrzejewski, M.; Wiland, P. Prevalence and clinical characteristics of rheumatoid arthritis in Poland: A nationwide study. Arch. Med. Sci. 2019, 15, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Rheumatology, 6th ed.; Hochberg, M.C. (Ed.) Mosby/Elsevier: Philadelphia, PA, USA, 2015; ISBN 978-0-323-09138-1. [Google Scholar]
- Yang, D.-H.; Huang, J.-Y.; Chiou, J.-Y.; Wei, J.C.-C. Analysis of Socioeconomic Status in the Patients with Rheumatoid Arthritis. Int. J. Environ. Res. Public Health 2018, 15, 1194. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.; Fitzpatrick, C.; O’Toole, L.; Doran, M.; O’Shea, F.; Connolly, D.; Fitzpatrick, C.; O’Toole, L.; Doran, M.; O’Shea, F. Impact of Fatigue in Rheumatic Diseases in the Work Environment: A Qualitative Study. Int. J. Environ. Res. Public Health 2015, 12, 13807–13822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokka, T. Work disability in early rheumatoid arthritis. Clin. Exp. Rheumatol. 2003, 21, S71–S74. [Google Scholar] [PubMed]
- McIntosh, E. The cost of rheumatoid arthritis. Br. J. Rheumatol. 1996, 35, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, H.; Pike, C.; Kaufman, R.; Maynchenko, M.; Kidolezi, Y.; Cifaldi, M. Societal cost of rheumatoid arthritis patients in the US. Curr. Med. Res. Opin. 2010, 26, 77–90. [Google Scholar] [CrossRef]
- Krishnan, E.; Lingala, B.; Bruce, B.; Fries, J.F. Disability in rheumatoid arthritis in the era of biological treatments. Ann. Rheum. Dis. 2012, 71, 213–218. [Google Scholar] [CrossRef]
- Pugner, K.M.; Scott, D.I.; Holmes, J.W.; Hieke, K. The costs of rheumatoid arthritis: an international long-term view. Semin. Arthritis Rheum. 2000, 29, 305–320. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lin, G.-J.; Chen, J.-W.; Wang, K.-C.; Tien, C.-H.; Hu, C.-F.; Chang, C.-N.; Hsu, W.-F.; Fan, H.-C.; Sytwu, H.-K.; et al. Immunopathogenic Mechanisms and Novel Immune-Modulated Therapies in Rheumatoid Arthritis. Int. J. Mol. Sci. 2019, 20, 1332. [Google Scholar] [CrossRef]
- Hammond, A. The use of self-management strategies by people with rheumatoid arthritis. Clin. Rehabil. 1998, 12, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jo, C. Cost-of-illness studies: concepts, scopes, and methods. Clin. Mol. Hepatol. 2014, 20, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Her, M.; Kavanaugh, A. Critical analysis of economic tools and economic measurement applied to rheumatoid arthritis. Clin. Exp. Rheumatol. 2012, 30, S107–S111. [Google Scholar] [PubMed]
- Raciborski, F.; Kłak, A.; Kwiatkowska, B. Indirect costs of rheumatoid arthritis. Reumatologia 2015, 53, 268–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolen, J.; Aletaha, D. The burden of rheumatoid arthritis and access to treatment: a medical overview. Eur. J. Heal. Econ. 2008, 8, 39–47. [Google Scholar] [CrossRef]
- Kobelt, G.; Jönsson, B. The burden of rheumatoid arthritis and access to treatment: outcome and cost-utility of treatments. Eur. J. Heal. Econ. 2008, 8, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Burton, W.; Morrison, A.; Maclean, R.; Ruderman, E. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occup. Med. (Chic. Ill). 2006, 56, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gignac, M.A.M.; Anis, A.H. The Indirect Costs of Arthritis Resulting From Unemployment, Reduced Performance, and Occupational Changes While at Work. Med. Care 2006, 44, 304–310. [Google Scholar] [CrossRef]
- Goetzel, R.Z.; Long, S.R.; Ozminkowski, R.J.; Hawkins, K.; Wang, S.; Lynch, W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J. Occup. Environ. Med. 2004, 46, 398–412. [Google Scholar] [CrossRef]
- Collins, J.J.; Baase, C.M.; Sharda, C.E.; Ozminkowski, R.J.; Nicholson, S.; Billotti, G.M.; Turpin, R.S.; Olson, M.; Berger, M.L. The assessment of chronic health conditions on work performance, absence, and total economic impact for employers. J. Occup. Environ. Med. 2005, 47, 547–557. [Google Scholar] [CrossRef]
- Allaart, C.F.; Breedveld, F.C.; Dijkmans, B.A.C. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J. Rheumatol. Suppl. 2007, 80, 25–33. [Google Scholar]
- Anis, A.; Zhang, W.; Emery, P.; Sun, H.; Singh, A.; Freundlich, B.; Sato, R. The effect of etanercept on work productivity in patients with early active rheumatoid arthritis: results from the COMET study. Rheumatology 2009, 48, 1283–1289. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Bansback, N.; Guh, D.; Li, X.; Nosyk, B.; Marra, C.A.; Anis, A.H. Short-term influence of adalimumab on work productivity outcomes in patients with rheumatoid arthritis. J. Rheumatol. 2008, 35, 1729–1736. [Google Scholar]
- Augustsson, J.; Neovius, M.; Cullinane-Carli, C.; Eksborg, S.; van Vollenhoven, R.F. Patients with rheumatoid arthritis treated with tumour necrosis factor antagonists increase their participation in the workforce: potential for significant long-term indirect cost gains (data from a population-based registry). Ann. Rheum. Dis. 2010, 69, 126–131. [Google Scholar] [CrossRef]
- Hone, D.; Cheng, A.; Watson, C.; Huang, B.; Bitman, B.; Huang, X.-Y.; Gandra, S.R. Impact of etanercept on work and activity impairment in employed moderate to severe rheumatoid arthritis patients in the United States. Arthritis Care Res. (Hoboken). 2013, 65, 1564–1572. [Google Scholar] [CrossRef]
- Klimeš, J.; Vocelka, M.; Šedová, L.; Doležal, T.; Mlčoch, T.; Petříková, A.; Vlček, J. Medical and Productivity Costs of Rheumatoid Arthritis in The Czech Republic: Cost-of-Illness Study Based on Disease Severity. Value Heal. Reg. Issues 2014, 4, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Yamazaki, K.; Nakajima, R.; Komatsu, S.; Igarashi, A.; Tango, T.; Takeuchi, T. Economic impact of adalimumab treatment in Japanese patients with rheumatoid arthritis from the adalimumab non-interventional trial for up-verified effects and utility (ANOUVEAU) study. Mod. Rheumatol. 2018, 28, 39–47. [Google Scholar] [CrossRef]
- Eriksson, J.K.; Karlsson, J.A.; Bratt, J.; Petersson, I.F.; van Vollenhoven, R.F.; Ernestam, S.; Geborek, P.; Neovius, M. Cost-effectiveness of infliximab versus conventional combination treatment in methotrexate-refractory early rheumatoid arthritis: 2-year results of the register-enriched randomised controlled SWEFOT trial. Ann. Rheum. Dis. 2015, 74, 1094–1101. [Google Scholar] [CrossRef]
- Birnbaum, H.; Pike, C.; Kaufman, R.; Cifaldi, M. Employer Model of Workplace Impacts of Anti-TNF Therapy for Rheumatoid Arthritis. J. Occup. Environ. Med. 2009, 51, 1167–1176. [Google Scholar] [CrossRef]
- DAVIES, A.; CIFALDI, M.A.; SEGURADO, O.G.; WEISMAN, M.H. Cost-Effectiveness of Sequential Therapy with Tumor Necrosis Factor Antagonists in Early Rheumatoid Arthritis. J. Rheumatol. 2009, 36, 16–26. [Google Scholar] [CrossRef]
- Gissel, C.; Götz, G.; Repp, H. Cost-effectiveness of adalimumab for rheumatoid arthritis in Germany. Z. Rheumatol. 2016, 75, 1006–1015. [Google Scholar] [CrossRef]
- Kobelt, G.; Jönsson, L.; Young, A.; Eberhardt, K. The cost-effectiveness of infliximab (Remicade®) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology 2003, 42, 326–335. [Google Scholar] [CrossRef]
- Kobelt, G. TNF inhibitors in the treatment of rheumatoid arthritis in clinical practice: costs and outcomes in a follow up study of patients with RA treated with etanercept or infliximab in southern Sweden. Ann. Rheum. Dis. 2004, 63, 4–10. [Google Scholar] [CrossRef]
- Merkesdal, S.; Kirchhoff, T.; Wolka, D.; Ladinek, G.; Kielhorn, A.; Rubbert-Roth, A. Cost-effectiveness analysis of rituximab treatment in patients in Germany with rheumatoid arthritis after etanercept-failure. Eur. J. Heal. Econ. 2010, 11, 95–104. [Google Scholar] [CrossRef]
- Soini, E.; Asseburg, C.; Taiha, M.; Puolakka, K.; Purcaru, O.; Luosujärvi, R. Modeled Health Economic Impact of a Hypothetical Certolizumab Pegol Risk-Sharing Scheme for Patients with Moderate-to-Severe Rheumatoid Arthritis in Finland. Adv. Ther. 2017, 34, 2316–2332. [Google Scholar] [CrossRef] [Green Version]
- Valle-Mercado, C.; Cubides, M.-F.; Parra-Torrado, M.; Rosselli, D. Cost-effectiveness of biological therapy compared with methotrexate in the treatment for rheumatoid arthritis in Colombia. Rheumatol. Int. 2013, 33, 2993–2997. [Google Scholar] [CrossRef]
- Van den Hout, W.B.; Goekoop-Ruiterman, Y.P.M.; Allaart, C.F.; De Vries-Bouwstra, J.K.; Hazes, J.M.; Kerstens, P.J.S.M.; Van Zeben, D.; Hulsmans, H.M.J.; De Jonge-Bok, J.M.; De Sonnaville, P.B.J.; et al. Cost-utility analysis of treatment strategies in patients with recent-onset rheumatoid arthritis. Arthritis Rheum. 2009, 61, 291–299. [Google Scholar] [CrossRef]
- Benucci, M.; Rogai, V.; Atzeni, F.; Hammen, V.; Sarzti-Puttini, P.; Migliore, A. Costs associated with rheumatoid arthritis in Italy: past, present, and future. Clinicoecon. Outcomes Res. 2016, 8, 33–41. [Google Scholar] [CrossRef]
- Fautrel, B.; Verstappen, S.M.M.; Boonen, A. Economic consequences and potential benefits. Best Pract. Res. Clin. Rheumatol. 2011, 25, 607–624. [Google Scholar] [CrossRef]
- Lapadula, G.; Marchesoni, A.; Armuzzi, A.; Blandizzi, C.; Caporali, R.; Chimenti, S.; Cimaz, R.; Cimino, L.; Gionchetti, P.; Girolomoni, G.; et al. Adalimumab in the Treatment of Immune-Mediated Diseases. Int. J. Immunopathol. Pharmacol. 2014, 27, 33–48. [Google Scholar] [CrossRef] [Green Version]
- Furneri, G.; Mantovani, L.G.; Belisari, A.; Mosca, M.; Cristiani, M.; Bellelli, S.; Cortesi, P.A.; Turchetti, G. Systematic literature review on economic implications and pharmacoeconomic issues of rheumatoid arthritis. Clin. Exp. Rheumatol. 2012, 30, S72–S84. [Google Scholar]
- Homik, J.E.; Suarez-Almazor, M. An economic approach to health care. Best Pract. Res. Clin. Rheumatol. 2004, 18, 203–218. [Google Scholar] [CrossRef]
- Modena, V.; Bianchi, G.; Roccatello, D. Cost-effectiveness of biologic treatment for rheumatoid arthritis in clinical practice: An achievable target? Autoimmun. Rev. 2013, 12, 835–838. [Google Scholar] [CrossRef] [Green Version]
- Movik, E. TNF-inhibitors for rheumatic diseases (part 3): Health economics / TNF-hemmere ved revmatiske sykdommer (del 3)/Helseøkonomi; Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH): Oslo, Norway, 2007. [Google Scholar]
- Verstappen, S.M.M. Rheumatoid arthritis and work: The impact of rheumatoid arthritis on absenteeism and presenteeism. Best Pract. Res. Clin. Rheumatol. 2015, 29, 495–511. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Appendix 4: Quality assessment for Case Series. Available online: https://www.nice.org.uk/guidance/cg3/documents/appendix-4-quality-of-case-series-form2 (accessed on 18 July 2019).
- Boers, M. The cost-utility analysis of the BeSt trial: Is a camel in fact a horse with abnormalities in the distribution of dorsal fat? Comment on the article by van den Hout et al. Arthritis Rheum. 2009, 61, 1616–1617. [Google Scholar] [CrossRef]
- Zhang, W.; Monique, A.M.; Beaton, D.; Tang, K.; Anis, A.H. Productivity Loss Due to Presenteeism Among Patients with Arthritis: Estimates from 4 Instruments. J. Rheumatol. 2010, 37, 1805–1814. [Google Scholar] [CrossRef]
- Albers, J.M.; Kuper, H.H.; van Riel, P.L.; Prevoo, M.L.; van ’t Hof, M.A.; van Gestel, A.M.; Severens, J.L. Socio-economic consequences of rheumatoid arthritis in the first years of the disease. Rheumatology (Oxford). 1999, 38, 423–430. [Google Scholar] [CrossRef]
- Lacaille, D.; Sheps, S.; Spinelli, J.J.; Chalmers, A.; Esdaile, J.M. Identification of modifiable work-related factors that influence the risk of work disability in rheumatoid arthritis. Arthritis Rheum. 2004, 51, 843–852. [Google Scholar] [CrossRef]
- Hallert, E.; Husberg, M.; Bernfort, L. The incidence of permanent work disability in patients with rheumatoid arthritis in Sweden 1990-2010: before and after introduction of biologic agents. Rheumatology 2012, 51, 338–346. [Google Scholar] [CrossRef]
- Gulácsi, L.; Brodszky, V.; Baji, P.; Kim, H.; Kim, S.Y.; Cho, Y.Y.; Péntek, M. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev. Clin. Immunol. 2015, 11, 43–52. [Google Scholar] [CrossRef]
- Manova, M.; Savova, A.; Vasileva, M.; Terezova, S.; Kamusheva, M.; Grekova, D.; Petkova, V.; Petrova, G. Comparative Price Analysis of Biological Products for Treatment of Rheumatoid Arthritis. Front. Pharmacol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- ter Wee, M.M.; Lems, W.F.; Usan, H.; Gulpen, A.; Boonen, A. The effect of biological agents on work participation in rheumatoid arthritis patients: a systematic review. Ann. Rheum. Dis. 2012, 71, 161–171. [Google Scholar] [CrossRef]
- Zhang, W.; Anis, A.H. The economic burden of rheumatoid arthritis: beyond health care costs. Clin. Rheumatol. 2011, 30, 25–32. [Google Scholar] [CrossRef]
- Franke, L.C.; Ament, A.J.H.A.; van de Laar, M.A.F.J.; Boonen, A.; Severens, J.L. Cost-of-illness of rheumatoid arthritis and ankylosing spondylitis. Clin. Exp. Rheumatol. 2009, 27, S118–S123. [Google Scholar]
- Lundkvist, J.; Kastäng, F.; Kobelt, G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur. J. Heal. Econ. 2008, 8, 49–60. [Google Scholar] [CrossRef]
- Putrik, P.; Ramiro, S.; Kvien, T.K.; Sokka, T.; Pavlova, M.; Uhlig, T.; Boonen, A. Working Group ‘Equity in access to treatment of rheumatoid arthritis in Europe’ Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann. Rheum. Dis. 2014, 73, 198–206. [Google Scholar] [CrossRef]
- Dougados, M.; Soubrier, M.; Antunez, A.; Balint, P.; Balsa, A.; Buch, M.H.; Casado, G.; Detert, J.; El-zorkany, B.; Emery, P.; et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann. Rheum. Dis. 2014, 73, 62–68. [Google Scholar] [CrossRef]
- Batko, B.; Urbański, K.; Świerkot, J.; Wiland, P.; Raciborski, F.; Jędrzejewski, M.; Koziej, M.; Cześnikiewicz-Guzik, M.; Guzik, T.J.; Stajszczyk, M. Comorbidity burden and clinical characteristics of patients with difficult-to-control rheumatoid arthritis. Clin. Rheumatol. 2019. [Google Scholar] [CrossRef]
- Johnston, D.A.; Harvey, S.B.; Glozier, N.; Calvo, R.A.; Christensen, H.; Deady, M. The relationship between depression symptoms, absenteeism and presenteeism. J. Affect. Disord. 2019, 256, 536–540. [Google Scholar] [CrossRef]
| Author | Study Design | Treatment | n | Stage of RA | Age Range (Mean) | Disease Duration (Mean) | Absenteeism Measure | Presenteeism Measure | Method | Time Points |
|---|---|---|---|---|---|---|---|---|---|---|
| RCT Trials | ||||||||||
| Allaart 2007 [22] | RCT (BeST) | 1. Seq. monotherapy | 508 | Early RA (ACR criteria) | ≥18 (ND) | ND | Three-monthly diary on work absenteeism | - | FCM | Baseline until 2 years |
| 2. Step-up comb. Therapy (INF) c | ||||||||||
| 3. Initial comb. Therapy (INF) d | ||||||||||
| 4. MTX + INF d | ||||||||||
| Anis 2009 [23] | RCT (COMET) | MTX | 100 | Early RA (ACR criteria) | ≥18 (45.1) | 8.9 months | Number of missed work days/WPAI | Reduced working time (in days)/WLQ | HCM | 0 and 12 months (weeks 12, 24, 36, 52) |
| ETA + MTX | 105 | ≥18 (45.4) | 8.6 months | Number of stopped work days f/WLQ | ||||||
| Observational Studies | ||||||||||
| Zhang 2008 [24] | Open-label, multicenter, phase IIIb study (CanAct) | ADA | 389 | Moderate to severe active RA (ACR criteria) | (55.0) | 12.5 years | Number of absent work days multiplied by the individual’s daily wage | Number of extra work hours patients needed to catch up on tasks they were unable to complete during normal working hours multiplied by the individual’s hourly wage | HCM | Baseline and 12 months |
| Augustsson 2010 [25] | Observational (STURE register) | Anti-TNF (ETA, INF, ADA) | 594 | ND | 18–55 years (40.0) | 9.4 years | - | Hours worked/week | ND | Baseline, 6 months, 1, 2, 3, 4, and 5 years |
| Hone 2013 [26] | Prospective, observational study | ETA | 204 | Moderate to severe RA | 20–67 (46.6) | 5.1 years | WPAI measures of absenteeism – work time missed | WPAI measures of presenteeism – impairment at work | HCM | 6 months |
| Klimes 2014 [27] | Bottom-up cross-sectional cost-of-illness study | Without biologics | 137 | ND | 18–64 years (58.9) | 13.6 years | Days spent on sick leave, and the period of time spent on full disability pension or partial disability pension | - | FCM | 6 months |
| With biologics | 124 | 18–64 years (53.6) | 15.5 years | |||||||
| DMARDs | 130 | (53.7) | 10.1 months | |||||||
| Tanaka 2018 [28] | Non-interventional trial for up-verified effects and utility (ANOUVEAU) study | ADA i | 1 196 | Greater portion of the patients had established RA, with moderate disease activity | PW: (50.0) | 5.6 years | WPAI measures of absenteeism—work time missed | WPAI measures of presenteeism—impairment at work | HCM | 48 weeks |
| Author | Measure | Comparator | Difference | ∆ | 95%CI | Significant or Not | Costs |
|---|---|---|---|---|---|---|---|
| RCT Trials | |||||||
| Anis 2009 [23] | Missed work days | MTX vs. ETA+MTX | 31.9 vs. 14.2 | −17.6 | (–34.4; –2.2) | YES | –£1244 c,f |
| Reduced working time | 19.8 vs. 10.5 | –9.3 | (–21.9; 3.9) | NO | –£657 c,f | ||
| Stopped worked days | Scenario Ic: 32.9 vs.10.9 Scenario IId: 12.3 vs. 4.8 | –22.1 –7.4 | (–45.2; –0.3) (–15.9; 1.2) | YES NO | Scenario I: –£1562 c,d,f Scenario II: –£523 c,e,f | ||
| Total absenteeism | Scenario Ic: 65.6 vs.29.0 Scenario IId: 44.3 vs. 22.3 | −36.6 −22.0 | (−68.3; −5.9) (−42.6; –2.1) | YES YES | Scenario I: −£2586 c,d,f Scenario II: −£1555 c,e,f | ||
| Allaart 2007 [21] | Overall | Decrease of 0.1 on utility associated with decrease of 2 working h/week | Using the friction-cost method, overall societal costs were estimated at €19,905, €15,926, €17,810, and €28,547 (p ≤ 0.05 Group 4 vs. Groups 1–3). Indirect costs: €9113, €8638, €10,001, and €4786 (Groups 1, 2, 3, and 4 respectively) a | ||||
| 4. MTX + INF | Productivity was highest in this group | ||||||
| Observational Trials | |||||||
| Zhang 2008 [24] | Absenteeism, mean | ADA vs. baseline | ND | ND | ND | ND | Lost productivity costs, past two weeks: –$57.21 (mean) |
| Hone 2013 [26] | Hours gained/patient (absenteeism) | ETA baseline vs. 6 months | 71.1 vs. 63.5/ 9.9 ± 20.1 vs. 4.4 ± 16.1 | 7.6/ −3.5 ± 17.0 | ND/ (−6.1; −1.0) | ND/ YES | Economic gain/patient: $1794 |
| Klimes 2014 [27] | Productivity costs | Without vs. with biological treatment | ND | ND | ND | ND | Friction cost approach: €1304 vs. €2090 |
| Tanaka 2018 [28] | Absenteeism | Baseline vs. week 48 | ND | ND | ND | ND | Human capital method (cumulative reduction): PW: $9278 (mean) PTW: $6480 (mean) HM: $5449 (mean) |
| Author | Measure | Comparator | Difference | ∆ | 95%CI | Significant or Not | Costs |
|---|---|---|---|---|---|---|---|
| RCT Trial | |||||||
| Anis 2009 [23] | WPAI: Work productivity loss at work (%) | MTX vs. ETA + MTX | 23.1 vs. 15.6 | −7.5 | (−11.2; −4.2) | YES | NA |
| WPAI: Lost work days due to presenteeism | Scenario I: 34.0 vs. 28.6 Scenario II: 38.9 vs. 29.7 | −5.4 −9.3 | (−13.5; 2.8) (−16.3; −2.5) | NO YES | −£382 −£657 | ||
| WPAI: Total work productivity loss, days | Scenario I: 99.6 vs. 57.6 Scenario II: 83.3 vs. 51.9 | −42.0 −31.3 | (−69.0; −15.7) (−50.2; −12.6) | YES YES | −£2968 −£2212 | ||
| WLQ: work productivity loss at work (%) | 6.2 vs. 4.8 | −1.4 | (−2.1; −0.7) | YES | NA | ||
| WLQ: Lost work days due to presenteeism | Scenario I: 9.1 vs. 8.9 Scenario II: 10.4 vs. 9.2 | −0.3 −1.3 | (−2.3; 1.8) (−2.8; 0.3) | NO NO | −£21 −£92 | ||
| WLQ: Total work productivity loss, days | Scenario I: 74.7 vs. 37.8 Scenario II: 54.8 vs. 31.5 | −36.9 −23.3 | (−66.9; −7.6) (−43.0; −4.2) | YES YES | −£2607 −£1646 | ||
| Observational trials | |||||||
| Zhang 2008 [24] | Absenteeism, mean | ADA vs. baseline | ND | ND | ND | ND | Lost productivity costs, past two weeks: –$4.48 |
| Augustsson 2010 [25] | Overall | Unadjusted model | Improvement: first year: 4.2 h/week, thereafter: 0.5 h/week | The productivity gains for society in patients continuing treatment would total €28,000 over 5 years. | Note that these estimates only apply to patients who do not discontinue treatment, a group that may be difficult to identify before treatment initiation. | ||
| Adjusted model b | Improvement: first year: 4.1 h/week, thereafter: no change | The productivity gains for society in patients continuing treatment would total €27,000 over 5 years. This corresponds to approximately 40% of the annual anti-TNF drug cost. | |||||
| Hone 2013 [26] | Hours gained/patient (presenteeism) | ETA baseline vs. 6 months | 205.2 vs. 189.7/ 39.7 ± 24.5 vs. 24.8 ± 22.5 | 15.5/ −13.5 ± 23.3 | ND/ (−17.0; −9.9) | ND/ YES | Economic gain/patient: $5328 |
| Tanaka 2018 [28] | Presenteeism | Baseline vs. week 48 | ND | ND | ND | ND | Human capital method (cumulative reduction): PW: $5836 (mean) PTW: $2726 (mean) HM: NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batko, B.; Rolska-Wójcik, P.; Władysiuk, M. Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review. Int. J. Environ. Res. Public Health 2019, 16, 2966. https://doi.org/10.3390/ijerph16162966
Batko B, Rolska-Wójcik P, Władysiuk M. Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review. International Journal of Environmental Research and Public Health. 2019; 16(16):2966. https://doi.org/10.3390/ijerph16162966
Chicago/Turabian StyleBatko, Bogdan, Paulina Rolska-Wójcik, and Magdalena Władysiuk. 2019. "Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review" International Journal of Environmental Research and Public Health 16, no. 16: 2966. https://doi.org/10.3390/ijerph16162966
APA StyleBatko, B., Rolska-Wójcik, P., & Władysiuk, M. (2019). Indirect Costs of Rheumatoid Arthritis Depending on Type of Treatment—A Systematic Literature Review. International Journal of Environmental Research and Public Health, 16(16), 2966. https://doi.org/10.3390/ijerph16162966




