Increased Incidence of Glaucoma in Sensorineural Hearing Loss: A Population-Based Cohort Study
Abstract
:1. Introduction
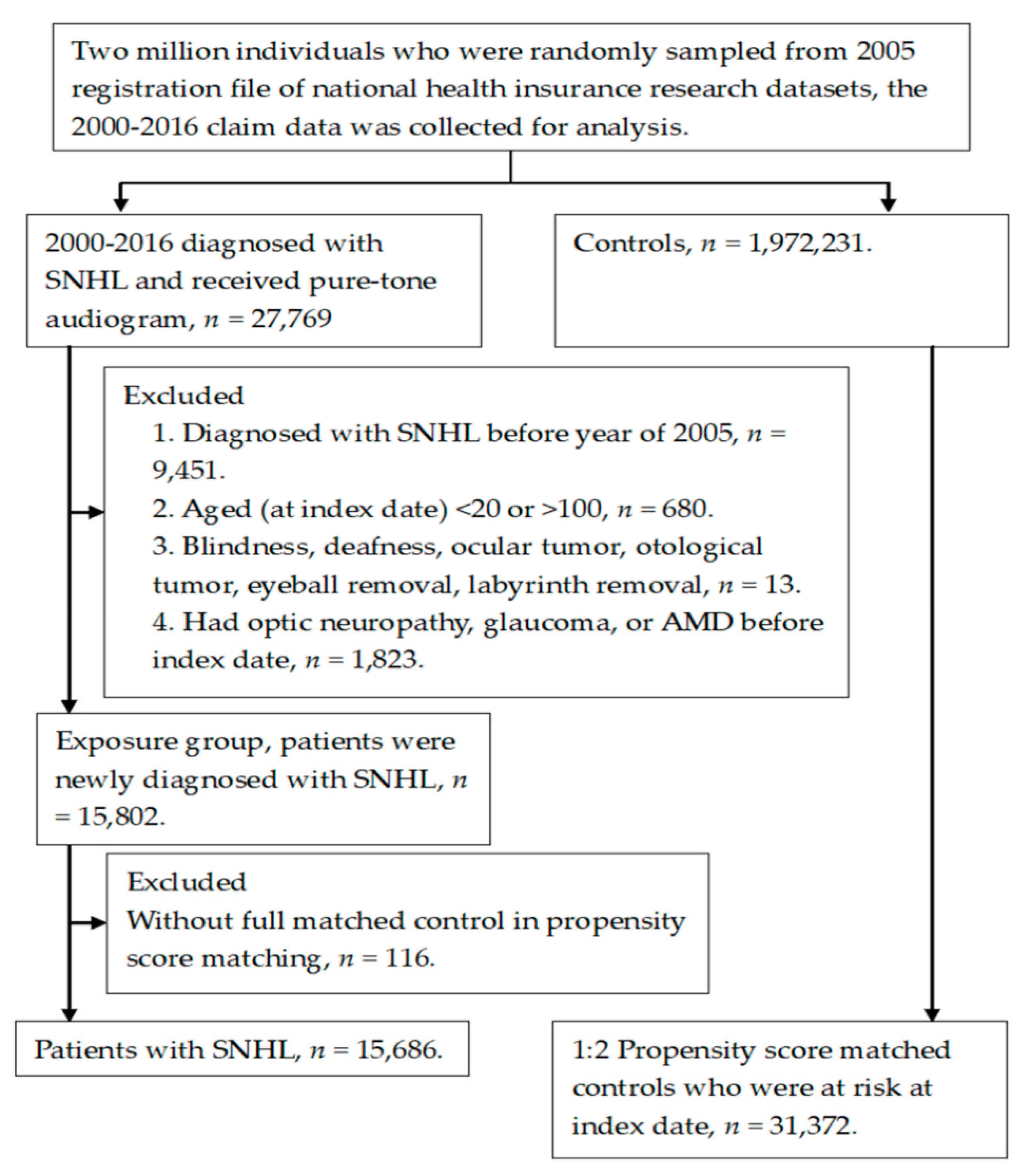
2. Materials and Methods
2.1. Data Source
2.2. Patient Selection
2.3. Main Outcome Measurement
2.4. Demographic Data and Co-Morbidities
2.5. Statistical Analysis
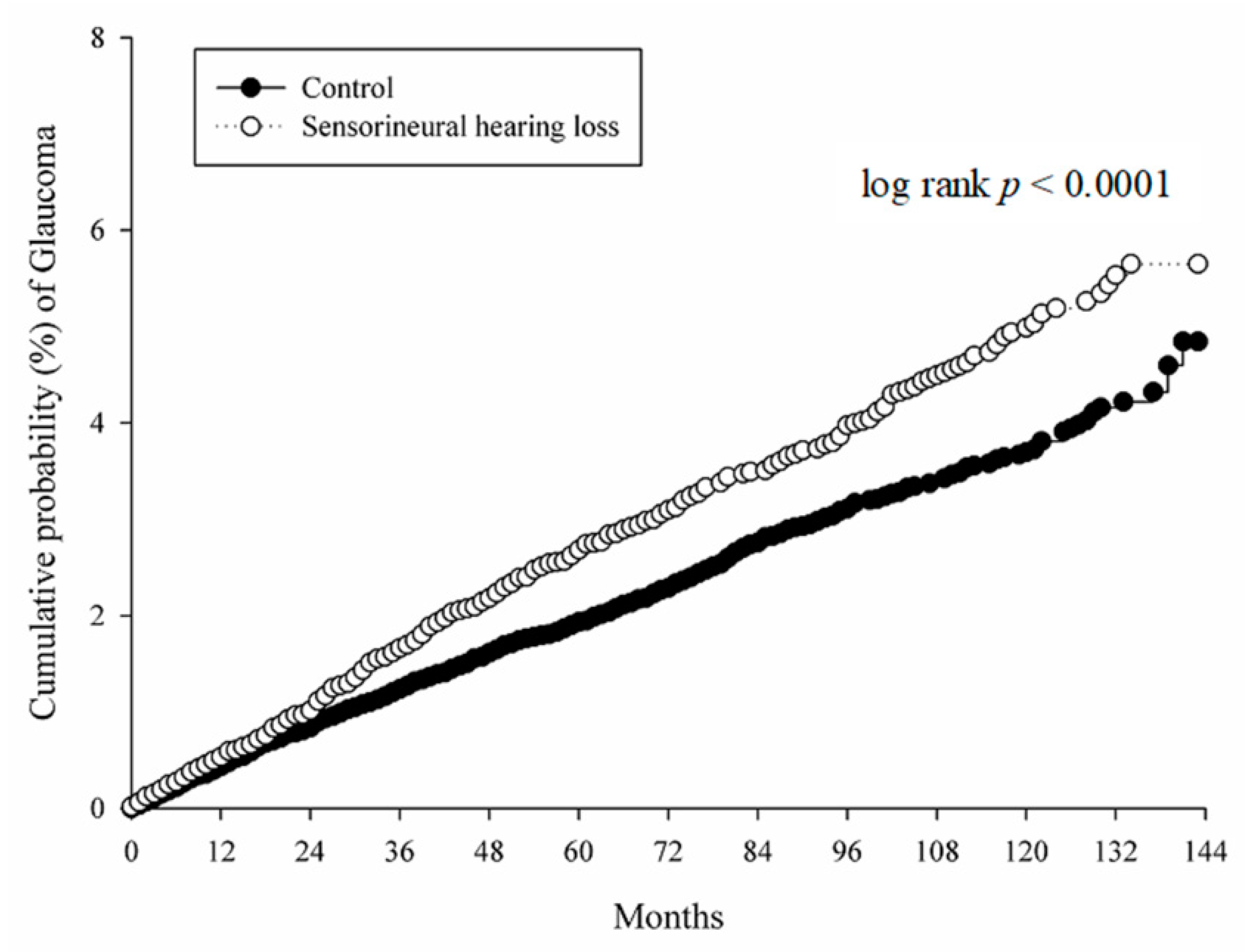
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frisina, R.D.; Ding, B.; Zhu, X.; Walton, J.P. Age-related hearing loss: Prevention of threshold declines, cell loss and apoptosis in spiral ganglion neurons. Aging 2016, 8, 2081–2099. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.K.; Cho, J.J.; Fritz, D.K. Evidence-based practice: Management of adult sensorineural hearing loss. Otolaryng Clin. N. Am. 2012, 45, 941–958. [Google Scholar] [CrossRef] [PubMed]
- Wongrakpanich, S.; Petchlorlian, A.; Rosenzweig, A. Sensorineural organs dysfunction and cognitive decline: A review article. Aging Dis. 2016, 7, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Marlin, S.; Parodi, M.; Rouillon, I.; Guerlain, J.; Pingault, V.; Couloigner, V.; Garabedian, E.N.; Denoyelle, F.; Loundon, N. Unilateral sensorineural hearing loss: Medical context and etiology. Audiol Neuro-Otol. 2017, 22, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Holy, R.; Navara, M.; Dosel, P.; Fundova, P.; Prazenica, P.; Hahn, A. Hyperbaric oxygen therapy in idiopathic sudden sensorineural hearing loss (ISSNHL) in association with combined treatment. Undersea Hyperb. Med. 2011, 38, 137–142. [Google Scholar] [PubMed]
- Di-Stadio, A.; Dipietro, L.; Ralli, M.; Meneghello, F.; Minni, A.; Greco, A.; Stabile, M.R.; Bernitsas, E. Sudden hearing loss as an early detector of multiple sclerosis: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4611–4624. [Google Scholar]
- Martini, A.; Castiglione, A.; Bovo, R.; Vallesi, A.; Gabelli, C. Aging, cognitive load, dementia and hearing loss. Audiol. Neuro-Otol. 2014, 19 (Suppl. 1), 2–5. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer's disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Quaranta, N.; Coppola, F.; Casulli, M.; Barulli, M.R.; Panza, F.; Tortelli, R.; Capozzo, R.; Leo, A.; Tursi, M.; Grasso, A.; et al. The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiol. Neuro-Otol. 2014, 19 (Suppl. 1), 10–14. [Google Scholar] [CrossRef]
- Golub, J.S. Brain changes associated with age-related hearing loss. Curr. Opin. Otolaryngo. 2017, 25, 347–352. [Google Scholar] [CrossRef]
- Jutley, G.; Luk, S.M.; Dehabadi, M.H.; Cordeiro, M.F. Management of glaucoma as a neurodegenerative disease. Neurodegener. Dis. 2017, 7, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Shi, Y.; Wang, X.; Liu, M.; Zhang, C. Interocular asymmetry of the visual field defects in newly diagnosed normal-tension glaucoma, primary open-angle glaucoma, and chronic angle-closure glaucoma. J. Glaucoma. 2014, 23, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Sanz, M.; Salinas-Navarro, M.; Nadal-Nicolas, F.M.; Alarcon-Martinez, L.; Valiente-Soriano, F.J.; de Imperial, J.M.; Aviles-Trigueros, M.; Agudo-Barriuso, M.; Villegas-Perez, M.P. Understanding glaucomatous damage: Anatomical and functional data from ocular hypertensive rodent retinas. Prog. Retin. Eye. Res. 2012, 31, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye. Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hearing Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Schreiber, B.E.; Agrup, C.; Haskard, D.O.; Luxon, L.M. Sudden sensorineural hearing loss. Lancet 2010, 375, 1203–1211. [Google Scholar] [CrossRef]
- Kuhn, M.; Heman-Ackah, S.E.; Shaikh, J.A.; Roehm, P.C. Sudden sensorineural hearing loss: A review of diagnosis, treatment, and prognosis. Trends Amplif. 2011, 15, 91–105. [Google Scholar] [CrossRef]
- Sara, S.A.; Teh, B.M.; Friedland, P. Bilateral sudden sensorineural hearing loss: Review. J. Laryngol. Otol. 2014, 128 (Suppl. 1), S8–S15. [Google Scholar] [CrossRef]
- Leske, M.C.; Wu, S.Y.; Hennis, A.; Honkanen, R.; Nemesure, B. Risk factors for incident open-angle glaucoma: The barbados eye studies. Ophthalmology 2008, 115, 85–93. [Google Scholar] [CrossRef]
- Mwanza, J.C.; Tulenko, S.E.; Barton, K.; Herndon, L.W.; Mathenge, E.; Hall, A.; Kim, H.Y.; Hay-Smith, G.; Budenz, D.L. Eight-year incidence of open-angle glaucoma in the tema eye survey. Ophthalmology 2019, 126, 372–380. [Google Scholar] [CrossRef]
- Choi, J.; Kook, M.S. Systemic and ocular hemodynamic risk factors in glaucoma. Biomed. Res. Int. 2015, 2015, 141905. [Google Scholar] [CrossRef]
- Lee, C.Y.; Liu, C.H.; Chen, H.C.; Sun, C.C.; Yao, Y.P.; Chao, S.C. Correlation between basal macular circulation and following glaucomatous damage in progressed high-tension and normal-tension glaucoma. Ophthalmic Res. 2019, 62, 1–9. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Res. 2015, 330, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Vaden, K.I., Jr.; Matthews, L.J.; Dubno, J.R. Transient-evoked otoacoustic emissions reflect audiometric patterns of age-related hearing loss. Trends Hear. 2018, 22, 2331216518797848. [Google Scholar] [CrossRef]
- Ungar, O.J.; Handzel, O.; Santos, F. Rate of spiral ganglion cell loss in idiopathic sudden sensorineural hearing loss. Hear. Res. 2018, 39, e944–e949. [Google Scholar] [CrossRef]
- Tavanai, E.; Mohammadkhani, G. Role of antioxidants in prevention of age-related hearing loss: A review of literature. Eur. Arch. Oto-Rh. 2017, 274, 1821–1834. [Google Scholar] [CrossRef]
- Ding, D.; Jiang, H.; Chen, G.D.; Longo-Guess, C.; Muthaiah, V.P.; Tian, C.; Sheppard, A.; Salvi, R.; Johnson, K.R. N-acetyl-cysteine prevents age-related hearing loss and the progressive loss of inner hair cells in gamma-glutamyl transferase 1 deficient mice. Aging 2016, 8, 730–750. [Google Scholar] [CrossRef]
- Alqawlaq, S.; Flanagan, J.G.; Sivak, J.M. All roads lead to glaucoma: Induced retinal injury cascades contribute to a common neurodegenerative outcome. Exp. Eye. Res. 2019, 183, 88–97. [Google Scholar] [CrossRef]
- Mancino, R.; Martucci, A.; Cesareo, M.; Giannini, C.; Corasaniti, M.T.; Bagetta, G.; Nucci, C. Glaucoma and alzheimer disease: One age-related neurodegenerative disease of the brain. Curr. Neuropharmacol. 2018, 16, 971–977. [Google Scholar] [CrossRef]
- Lee, C.S.; Larson, E.B.; Gibbons, L.E.; Lee, A.Y.; McCurry, S.M.; Bowen, J.D.; McCormick, W.C.; Crane, P.K. Associations between recent and established ophthalmic conditions and risk of alzheimer's disease. Alzheimers. Dement. 2019, 15, 34–41. [Google Scholar] [CrossRef]
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.H.; Goebel, J.A.; Magnusson, M.; Mandala, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic criteria for meniere's disease. J. Vestibul. Res-Equil. 2015, 25, 1–7. [Google Scholar]
- Pyykko, I.; Nakashima, T.; Yoshida, T.; Zou, J.; Naganawa, S. Meniere's disease: A reappraisal supported by a variable latency of symptoms and the mri visualisation of endolymphatic hydrops. BMJ Open 2013, 3, e001555. [Google Scholar] [CrossRef]
- Nakashima, T.; Sone, M.; Teranishi, M.; Yoshida, T.; Terasaki, H.; Kondo, M.; Yasuma, T.; Wakabayashi, T.; Nagatani, T.; Naganawa, S. A perspective from magnetic resonance imaging findings of the inner ear: Relationships among cerebrospinal, ocular and inner ear fluids. Auris Nasus Larynx 2012, 39, 345–355. [Google Scholar] [CrossRef]
- Bozkurt, M.K.; Ozturk, B.T.; Kerimoglu, H.; Ersan, I.; Arbag, H.; Bozkurt, B. Association of age-related macular degeneration with age-related hearing loss. J. Laryngol. Otol. 2011, 125, 231–235. [Google Scholar] [CrossRef]
- Hogewind, B.F.; Pennings, R.J.; Hol, F.A.; Kunst, H.P.; Hoefsloot, E.H.; Cruysberg, J.R.; Cremers, C.W. Autosomal dominant optic neuropathy and sensorineual hearing loss associated with a novel mutation of wfs1. Mol. Vis. 2010, 16, 26–35. [Google Scholar]
- Metrailer, A.M.; Babu, S.C. Management of sudden sensorineural hearing loss. Curr. Opin. Otolaryngo. 2016, 24, 403–406. [Google Scholar] [CrossRef]
- Stachler, R.J.; Chandrasekhar, S.S.; Archer, S.M.; Rosenfeld, R.M.; Schwartz, S.R.; Barrs, D.M.; Brown, S.R.; Fife, T.D.; Ford, P.; Ganiats, T.G.; et al. Clinical practice guideline: Sudden hearing loss. Otolaryng. Head. Neck. 2012, 146, S1–S35. [Google Scholar] [CrossRef]
- Fini, M.E.; Schwartz, S.G.; Gao, X.; Jeong, S.; Patel, N.; Itakura, T.; Price, M.O.; Price, F.W., Jr.; Varma, R.; Stamer, W.D. Steroid-induced ocular hypertension/glaucoma: Focus on pharmacogenomics and implications for precision medicine. Prog. Retin. Eye. Res. 2017, 56, 58–83. [Google Scholar] [CrossRef]
- Killer, H.E.; Pircher, A. Normal tension glaucoma: Review of current understanding and mechanisms of the pathogenesis. Eye (Lond). 2018, 32, 924–930. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Mallick, J.; Devi, L.; Malik, P.K.; Mallick, J. Update on normal tension glaucoma. J. Ophthalmic Vis. Res. 2016, 11, 204–208. [Google Scholar] [CrossRef]
- Evangelho, K.; Mogilevskaya, M.; Losada-Barragan, M.; Vargas-Sanchez, J.K. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: A review of the literature. Int. Ophthalmol. 2019, 39, 259–271. [Google Scholar] [CrossRef]
- Huang, J.Y.; Su, C.C.; Wang, T.H.; Tsai, I.J. Migraine and increased risk of developing open angle glaucoma: A population-based cohort study. BMC Ophthalmol. 2019, 19, 50. [Google Scholar] [CrossRef]
| Basic Characters | Study | Control | p Value |
|---|---|---|---|
| Age | 0.2296 | ||
| <40 | 1987 (12.67%) | 3839 (12.24%) | |
| 40–59 | 5338 (34.03%) | 10,518 (33.53%) | |
| 60–79 | 6542 (41.71%) | 13,362 (42.59%) | |
| ≥80 | 1819 (11.6%) | 3653 (11.64%) | |
| Sex | 0.7436 | ||
| Male | 8480 (54.06%) | 17,010 (54.22%) | |
| Female | 7206 (45.94%) | 14,362 (45.78%) | |
| Co-morbidities (before index date) | |||
| Hypertension | 7755 (49.44%) | 15,826 (50.45%) | 0.0394 |
| Diabetes mellitus | 3842 (24.49%) | 7777 (24.79%) | 0.4821 |
| Ischemic heart diseases | 3821 (24.36%) | 7807 (24.89%) | 0.2124 |
| Hyperlipidemia | 5849 (37.29%) | 11,719 (37.35%) | 0.8875 |
| Heart failure | 1487 (9.48%) | 3109 (9.91%) | 0.1383 |
| Cerebrovascular disease | 3103 (19.78%) | 6199 (19.76%) | 0.9543 |
| Dementia | 536 (3.42%) | 1003 (3.2%) | 0.2060 |
| Alzheimer's disease | 49 (0.31%) | 72 (0.23%) | 0.0942 |
| Parkinson's disease | 231 (1.47%) | 365 (1.16%) | 0.0047 |
| Liver disease | 5218 (33.27%) | 10,513 (33.51%) | 0.5947 |
| Rheumatic disease | 639 (4.07%) | 1237 (3.94%) | 0.4945 |
| Kidney disease | 6994 (44.59%) | 13,973 (44.54%) | 0.9216 |
| Hemiplegia or paraplegia | 1738 (11.08%) | 3489 (11.12%) | 0.8927 |
| Incidence | Study | Control |
|---|---|---|
| Median of follow up months | 63 | 59 |
| Follow up person months | 1,024,001 | 1,964,797 |
| New glaucoma case | 444 | 647 |
| Incidence rate * (95% CI) | 43.36 (39.51–47.84) | 32.93 (30.49–34.22) |
| Crude relative risk (95% CI) | 1.318 (1.168–1.488) | Reference |
| Variable | aHR (95% CI) |
|---|---|
| SNHL (ref: Control) | 1.318 (1.168–1.487) |
| Age (ref: 40–59) | |
| <40 | 0.298 (0.197–0.450) |
| 60–79 | 1.613 (1.387–1.877) |
| ≥80 | 1.201 (0.949–1.520) |
| Sex (ref: Female) | |
| Male | 1.044 (0.925–1.178) |
| Co-morbidities | |
| Hypertension | 1.153 (0.995–1.335) |
| Diabetes mellitus | 1.110 (0.968–1.273) |
| Ischemic heart diseases | 1.225 (1.065–1.409) |
| Hyperlipidemia | 1.346 (1.178–1.537) |
| Heart failure | 0.899 (0.739–1.093) |
| Cerebrovascular disease | 1.004 (0.866–1.164) |
| Dementia | 0.958 (0.676–1.356) |
| Alzheimer's disease | 0.646 (0.158–2.647) |
| Parkinson's disease | 1.202 (0.756–1.913) |
| Liver disease | 1.156 (0.958–1.314) |
| Rheumatic disease | 1.287 (0.990–1.674) |
| Kidney disease | 1.149 (0.867–1.302) |
| Hemiplegia or paraplegia | 0.969 (0.805–1.167) |
| Glaucoma Subtype | aHR (95% CI) for Exposure of SNHL | p Value |
|---|---|---|
| All glaucoma | 1.318 (1.168–1.487) | <0.0001 |
| OAG | 1.228 (0.943–1.599) | 0.1271 |
| NTG | 2.443 (1.561–3.825) | <0.0001 |
| ACG | 1.304 (1.053–1.614) | 0.0148 |
| Subgroups | Incidence Rate * (95% CI) of Glaucoma | aHR (95% CI) | |
|---|---|---|---|
| Study | Control | ||
| All | 43.36 (39.51–47.84) | 32.93 (30.49–34.22) | 1.318 (1.168–1.487) |
| Follow up time (Year) | |||
| <2 | 40.81 (34.52–48.25) | 33.74 (29.60–38.46) | 1.218 (0.985–1.507) |
| 2–5 | 47.80 (41.28–55.34) | 31.89 (28.02–36.29) | 1.498 (1.232–1.822) |
| ≥5 | 40.67 (34.33–48.17) | 33.39 (29.10–38.30) | 1.214 (0.976–1.511) |
| p for interaction | 0.5372 | ||
| Gender subgroups | |||
| Male | 46.69 (41.32–52.77) | 32.45 (29.18–36.09) | 1.429 (1.215–1.681) |
| Female | 39.49 (34.21–45.57) | 33.48 (29.93–37.44) | 1.191 (0.993–1.429) |
| p for interaction | 0.1342 | ||
| Age at index date | |||
| <40 | 10.44 (6.18–17.62) | 4.34 (2.41–7.84) | 2.378 (1.066–5.307) |
| 40–59 | 29.64 (24.54–35.79) | 25.05 (21.62–29.02) | 1.176 (0.925–1.495) |
| 60–79 | 62.91 (55.77–70.96) | 48.12 (43.61–53.09) | 1.306 (1.118–1.526) |
| ≥80 | 54.69 (42.18–70.9) | 34.47 (26.87–44.21) | 1.622 (1.131–2.327) |
| p for interaction | 0.2663 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chien, H.-W.; Wu, P.-H.; Wang, K.; Sun, C.-C.; Huang, J.-Y.; Yang, S.-F.; Chen, H.-C.; Lee, C.-Y. Increased Incidence of Glaucoma in Sensorineural Hearing Loss: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 2907. https://doi.org/10.3390/ijerph16162907
Chien H-W, Wu P-H, Wang K, Sun C-C, Huang J-Y, Yang S-F, Chen H-C, Lee C-Y. Increased Incidence of Glaucoma in Sensorineural Hearing Loss: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2019; 16(16):2907. https://doi.org/10.3390/ijerph16162907
Chicago/Turabian StyleChien, Hsiang-Wen, Pei-Hsuan Wu, Kai Wang, Chi-Chin Sun, Jing-Yang Huang, Shun-Fa Yang, Hung-Chi Chen, and Chia-Yi Lee. 2019. "Increased Incidence of Glaucoma in Sensorineural Hearing Loss: A Population-Based Cohort Study" International Journal of Environmental Research and Public Health 16, no. 16: 2907. https://doi.org/10.3390/ijerph16162907






