Effects of Telephone Follow-Up Intervention on %Body Fat, Inflammatory Cytokines, and Oxidative Stress in Obese Hispanic Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Summer Camp Intervention
2.3. 10 Month Telephone Intervention
2.4. Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nascimento, H.; Alves, A.I.; Medeiros, A.F.; Coimbra, S.; Catarino, C.; Bronze-da-Rocha, E.; Costa, E.; Rocha-Pereira, P.; Silva, G.; Aires, L.; et al. Impact of a School-Based Intervention Protocol-ACORDA Project-On Adipokines in an Overweight and Obese Pediatric Population. Pediatr. Exerc. Sci. 2016, 28, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Buchan, D.S.; Young, J.D.; Boddy, L.M.; Malina, R.M.; Baker, J.S. Fitness and adiposity are independently associated with cardiometabolic risk in youth. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Garanty-Bogacka, B.; Syrenicz, M.; Goral, J.; Krupa, B.; Syrenicz, J.; Walczak, M.; Syrenicz, A. Changes in inflammatory biomarkers after successful lifestyle intervention in obese children. Endokrynol. Pol. 2011, 62, 499–505. [Google Scholar] [PubMed]
- Reilly, K.C.; Briatico, D.; Irwin, J.D.; Tucker, P.; Pearson, E.S.; Burke, S.M. Participants’ Perceptions of “CHAMP Families”: A Parent-Focused Intervention Targeting Paediatric Overweight and Obesity. Int. J. Environ. Res. Public Health 2019, 16, 2171. [Google Scholar] [CrossRef] [PubMed]
- Christoffel, K.K.; Wang, X.; Binns, H.J. Early Origins of Child Obesity: Bridging Disciplines and Phases of Development-September 30–October 1, 2010. Int. J. Environ. Res. Public Health 2012, 9, 1227–1262. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.C.; Avilés-Santa, M.L.; Parrinello, C.M.; Hanna, D.B.; Jung, M.; Castañeda, S.F.; Hankinson, A.L.; Isasi, C.R.; Birnbaum-Weitzman, O.; Kim, R.S.; et al. Body mass index, sex, and cardiovascular disease risk factors among Hispanic/Latino adults: Hispanic community health study/study of Latinos. J. Am. Heart Assoc. 2014, 3, e000923. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Hashemi, M.; Mohammadifard, N.; Asgary, S.; Khavarian, N. Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clin. Chem. 2008, 54, 147–153. [Google Scholar] [CrossRef]
- Dennis, B.A.; Ergul, A.; Gower, B.A.; Allison, J.D.; Davis, C.L. Oxidative stress and cardiovascular risk in overweight children in an exercise intervention program. Child. Obes. 2013, 9, 15–21. [Google Scholar] [CrossRef]
- Zguira, M.S.; Slimani, M.; Bragazzi, N.L.; Khrouf, M.; Chaieb, F.; Saïag, B.; Tabka, Z. Effect of an 8-Week Individualized Training Program on Blood Biomarkers, Adipokines and Endothelial Function in Obese Young Adolescents with and without Metabolic Syndrome. Int. J. Environ. Res. Public Health 2019, 16, 751. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.; Roberts, L.J. Insights into oxidative stress: The isoprostanes. Curr. Med. Chem. 2007, 14, 703–717. [Google Scholar] [CrossRef]
- Squillacioti, G.; Bellisario, V.; Grignani, E.; Mengozzi, G.; Bardaglio, G.; Dalmasso, P.; Bono, R. The Asti Study: The Induction of Oxidative Stress in A Population of Children According to Their Body Composition and Passive Tobacco Smoking Exposure. Int. J. Environ. Res. Public Health 2019, 16, 490. [Google Scholar] [CrossRef] [PubMed]
- Hosick, P.A.; McMurray, R.G.; Hackney, A.C.; Battaglini, C.L.; Combs, T.P.; Harrell, J.S. Resting IL-6 and TNF-α level in children of different weight and fitness status. Pediatr. Exerc. Sci. 2013, 25, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.S.; Oliver, S.R.; Flores, R.L.; Ngo, J.; Milne, G.L.; Zaldivar, F.P.; Galassetti, P.R. Altered inflammatory, oxidative, and metabolic responses to exercise in pediatric obesity and type 1 diabetes. Pediatr. Diabetes 2011, 12, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, H.; Costa, E.; Rocha, S.; Lucena, C.; Rocha-Pereira, P.; Rêgo, C.; Mansilha, H.F.; Quintanilha, A.; Aires, L.; Mota, J.; et al. Adiponectin and markers of metabolic syndrome in obese children and adolescents: Impact of 8-mo regular physical exercise program. Pediatr. Res. 2014, 76, 159–165. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, E.; Marcovecchio, M.L.; Giannini, C.; De Giorgis, T.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. Improved oxidative stress and cardio-metabolic status in obese prepubertal children with liver steatosis treated with lifestyle combined with Vitamin E. Free Radic. Res. 2013, 47, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, M.S.; Al-Jiffri, O.; Ashmawy, E.M. Impact of weight loss on markers of systemic inflammation in obese Saudi children with asthma. Afr. Health Sci. 2013, 13, 682–688. [Google Scholar] [PubMed]
- Nemet, D.; Oren, S.; Pantanowitz, M.; Eliakim, A. Effects of a multidisciplinary childhood obesity treatment intervention on adipocytokines, inflammatory and growth mediators. Horm. Res. Paediatr. 2013, 79, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Im, J.A.; Kim, K.C.; Park, J.H.; Suh, S.H.; Kang, E.S.; Kim, S.H.; Jekal, Y.; Lee, C.W.; Yoon, Y.J.; et al. Improved insulin sensitivity and adiponectin level after exercise training in obese Korean youth. Obesity 2007, 15, 3023–3030. [Google Scholar] [CrossRef]
- Gately, P.J.; Cooke, C.B.; Butterly, R.J.; Mackreth, P.; Carroll, S. The effects of a children’s summer camp programme on weight loss, with a 10 month follow-up. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1445–1452. [Google Scholar] [CrossRef]
- Wing, R.R.; Jeffery, R.W.; Hellerstedt, W.L.; Burton, L.R. Effect of frequent phone contacts and Optional Food Provision on maintenance of weight loss. Ann. Behav. Med. 1996, 18, 172–176. [Google Scholar] [CrossRef]
- Ströbl, V.; Knisel, W.; Landgraf, U.; Faller, H. A combined planning and telephone aftercare intervention for obese patients: Effects on physical activity and body weight after one year. J. Rehabil. Med. 2013, 45, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Hyman, D.J.; Herd, J.A.; Ho, K.S.; Dunn, J.K.; Gregory, K.A. Maintenance of cholesterol reduction using automated telephone calls. Am. J. Prev. Med. 1996, 12, 129–133. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen. Available online: https://www.cdc.gov/healthyweight/bmi/calculator.html (accessed on 24 October 2018).
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohdrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; pp. 40–42. [Google Scholar]
- Mahoney, J.L. Adolescent summer care arrangements and risk for obesity the following school year. J. Adolesc. 2011, 34, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Moreno, J.P.; Johnston, C.A.; Woehler, D. Changes in weight over the school year and summer vacation: Results of a 5-year longitudinal study. J. Sch. Health 2013, 83, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.T.; Bartee, R.T.; Dorozynski, C.M.; Carr, L.J. Prevalence of overweight and influence of out-of-school seasonal periods on body mass index among American Indian schoolchildren. Prev. Chronic Dis. 2009, 6, A20. [Google Scholar] [PubMed]
- Franckle, R.; Adler, R.; Davison, K. Accelerated weight gain among children during summer versus school year and related racial/ethnic disparities: A systematic review. Prev. Chronic Dis. 2014, 11, E101. [Google Scholar] [CrossRef] [PubMed]
- Huelsing, J.; Kanafani, N.; Mao, J.; White, N.H. Camp jump start: Effects of a residential summer weight-loss camp for older children and adolescents. Pediatrics 2010, 125, e884–e890. [Google Scholar] [CrossRef]
- Gately, P.J.; Cooke, C.B.; Barth, J.H.; Bewick, B.M.; Radley, D.; Hill, A.J. Children’s residential weight-loss programs can work: A prospective cohort study of short-term outcomes for overweight and obese children. Pediatrics 2005, 116, 73–77. [Google Scholar] [CrossRef]
- Liu, F.; Kong, X.; Cao, J.; Chen, S.; Li, C.; Huang, J.; Gu, D.; Kelly, T.N. Mobile phone intervention and weight loss among overweight and obese adults: A meta-analysis of randomized controlled trials. Am. J. Epidemiol. 2015, 181, 337–348. [Google Scholar] [CrossRef]
- Hammerback, K.; Felias-Christensen, G.; Phelan, E.A. Evaluation of a telephone-based physical activity promotion program for disadvantaged older adults. Prev. Chronic Dis. 2012, 9, E62. [Google Scholar] [CrossRef]
- Herget, S.; Reichardt, S.; Grimm, A.; Petroff, D.; Käpplinger, J.; Haase, M.; Markert, J.; Blüher, S. High-intensity interval training for overweight adolescents: Program acceptance of a media supported intervention and changes in body composition. Int. J. Environ. Res. Public Health 2016, 13, 1099. [Google Scholar] [CrossRef]
- Schiel, R.; Beltschikow, W.; Radón, S.; Kramer, G.; Schmiedel, R.; Berndt, R.D. Long-term treatment of obese children and adolescents using a telemedicine support programme. J. Telemed. Telecare 2008, 14, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Cowles, M. Body image and exercise: A study of relationships and comparisons between physically active men and women. Sex Roles 1991, 25, 33–44. [Google Scholar] [CrossRef]
- Engström, G.; Hedblad, B.; Stavenow, L.; Lind, P.; Janzon, L.; Lindgӓrde, F. Inflammation-sensitive plasma proteins are associated with future weight gain. Diabetes 2003, 52, 2097–2101. [Google Scholar] [CrossRef]
- Duncan, B.B.; Schmidt, M.I.; Chambless, L.E.; Folsom, A.R.; Carpenter, M.; Heiss, G. Fibrinogen, Other Putative Markers of Inflammation, and Weight Gain in Middle-aged Adults—The ARIC Study. Obes. Res. 2000, 8, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.R.; Rosa, J.S.; Milne, G.L.; Pontello, A.M.; Borntrager, H.L.; Heydari, S.; Galassetti, P.R. Increased oxidative stress and altered substrate metabolism in obese children. Int. J. Pediatr. Obes. 2010, 5, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, R.C.; Wright, J.A.; Pepe, M.S.; Seidel, K.D.; Dietz, W.H. Predicting obesity in young adulthood from childhood and parental obesity. N. Engl. J. Med. 1997, 337, 869–873. [Google Scholar] [CrossRef]
- Gunnell, D.J.; Frankel, S.J.; Nanchahal, K.; Peters, T.J.; Davey Smith, G. Childhood obesity and adult cardiovascular mortality: A 57-y follow-up study based on the Boyd Orr cohort. Am. J. Clin. Nutr. 1998, 67, 1111–1118. [Google Scholar] [CrossRef]
- Must, A.; Jacques, P.F.; Dallal, G.E.; Bajema, C.J.; Dietz, W.H. Long-term morbidity and mortality of overweight adolescents: A follow-up of the Harvard Growth Study of 1922 to 1935. N. Engl. J. Med. 1992, 327, 1350–1355. [Google Scholar] [CrossRef]
- Mossberg, H.O. 40-year follow-up of overweight children. Lancet 1989, 334, 491–493. [Google Scholar] [CrossRef]
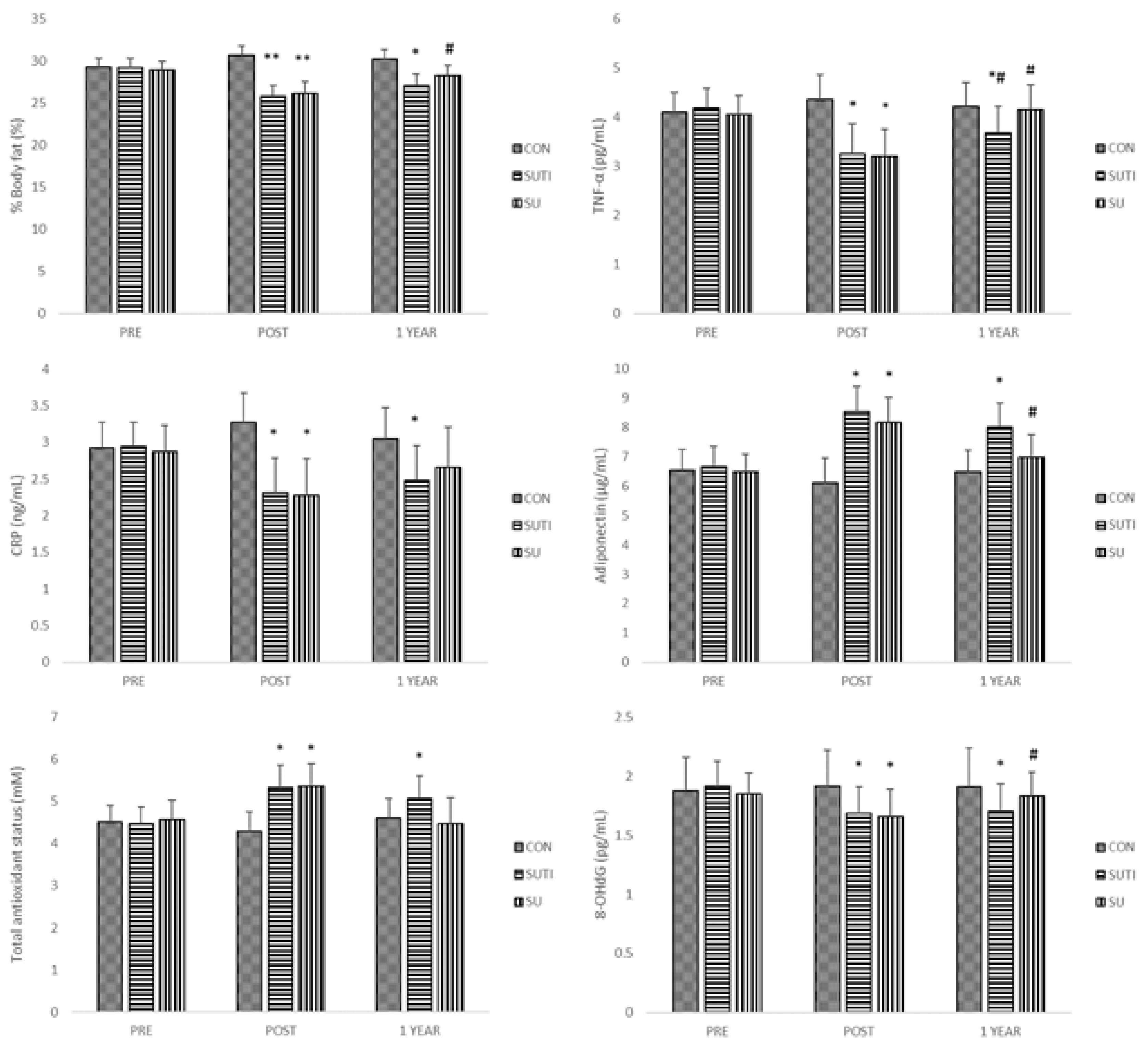
| CON (n = 19) | SUTI (n = 19) | SU (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | 1 YEAR | PRE | POST | 1 YEAR | PRE | POST | 1 YEAR | |
| Age (year) | 12.2 ± 0.34 | 12.0 ± 0.33 | 12.1 ± 0.31 | ||||||
| Height (cm) | 151.5 ± 2.4 | 151.8 ± 2.5 | ** 155.1 ± 2.8 | 152.3 ± 2.4 | 152.5 ± 2.4 | ** 156.1 ± 2.6 | 151.5 ± 2.3 | 151.7 ± 2.4 | ** 155.5 ± 2.7 |
| Weight (kg) | 62.5 ± 2.2 | * 64.7 ± 2.4 | **# 66.9 ± 2.9 | 63.0 ± 2.5 | * 60.7 ± 2.5 | # 64.4 ± 3.3 | 62.0 ± 2.5 | * 60.2 ± 2.5 | *## 65.9 ± 3.3 |
| BMI | 27.2 ± 0.5 | * 28.1 ± 0.6 | 27.8 ± 0.6 | 27.2 ± 0.5 | ** 26.1 ± 0.5 | *& 26.4 ± 0.5 | 27.0 ± 0.5 | * 26.2 ± 0.5 | 27.2 ± 0.6 |
| %Body fat | 29.3 ± 1.0 | * 30.7 ± 1.1 | 30.2 ± 1.1 | 29.2 ± 1.1 | ** 25.8 ± 1.3 | *& 27.1 ± 1.3 | 28.9 ± 1.0 | ** 26.1 ± 1.4 | # 28.3 ± 1.2 |
| CON (n = 19) | SUTI (n = 19) | SU (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | 1 YEAR | PRE | POST | 1 YEAR | PRE | POST | 1 YEAR | |
| TNF-α (pg/mL) | 4.10 ± 0.39 | 4.36 ± 0.49 | 4.21 ± 0.49 | 4.18 ± 0.40 | * 3.24 ± 0.62 | *# 3.68 ± 0.52 | 4.05 ± 0.38 | * 3.19 ± 0.56 | # 4.14 ± 0.51 |
| CRP (mg/L) | 2.92 ± 0.35 | 3.27 ± 0.41 | 3.05 ± 0.43 | 2.95 ± 0.33 | * 2.31 ± 0.47 | * 2.48 ± 0.47 | 2.87 ± 0.36 | * 2.28 ± 0.49 | 2.66 ± 0.55 |
| Adiponectin (μg/mL) | 6.54 ± 0.71 | 6.13 ± 0.84 | 6.49 ± 0.74 | 6.68 ± 0.69 | * 8.55 ± 0.85 | * 8.03 ± 0.80 | 6.48 ± 0.60 | * 8.18 ± 0.85 | # 6.98 ± 0.79 |
| TAS (mM) | 4.51 ± 0.40 | 4.29 ± 0.47 | 4.6 ± 0.47 | 4.47 ± 0.40 | * 5.33 ± 0.54 | * 5.07 ± 0.54 | 4.57 ± 0.47 | * 5.36 ± 0.54 | 4.48 ± 0.61 |
| 8-OHdG (ng/mL) | 1.88 ± 0.28 | 1.92 ± 0.30 | 1.91 ± 0.33 | 1.92 ± 0.21 | * 1.69 ± 0.22 | * 1.71 ± 0.23 | 1.85 ± 0.18 | * 1.66 ± 0.23 | # 1.83 ± 0.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhyu, H.-S.; Park, K.-S. Effects of Telephone Follow-Up Intervention on %Body Fat, Inflammatory Cytokines, and Oxidative Stress in Obese Hispanic Children. Int. J. Environ. Res. Public Health 2019, 16, 2854. https://doi.org/10.3390/ijerph16162854
Rhyu H-S, Park K-S. Effects of Telephone Follow-Up Intervention on %Body Fat, Inflammatory Cytokines, and Oxidative Stress in Obese Hispanic Children. International Journal of Environmental Research and Public Health. 2019; 16(16):2854. https://doi.org/10.3390/ijerph16162854
Chicago/Turabian StyleRhyu, Hyun-Seung, and Kyung-Shin Park. 2019. "Effects of Telephone Follow-Up Intervention on %Body Fat, Inflammatory Cytokines, and Oxidative Stress in Obese Hispanic Children" International Journal of Environmental Research and Public Health 16, no. 16: 2854. https://doi.org/10.3390/ijerph16162854
APA StyleRhyu, H.-S., & Park, K.-S. (2019). Effects of Telephone Follow-Up Intervention on %Body Fat, Inflammatory Cytokines, and Oxidative Stress in Obese Hispanic Children. International Journal of Environmental Research and Public Health, 16(16), 2854. https://doi.org/10.3390/ijerph16162854





