Blood Lactate Concentration Is Not Related to the Increase in Cardiorespiratory Fitness Induced by High Intensity Interval Training
Abstract
1. Introduction
2. Materials and Methods
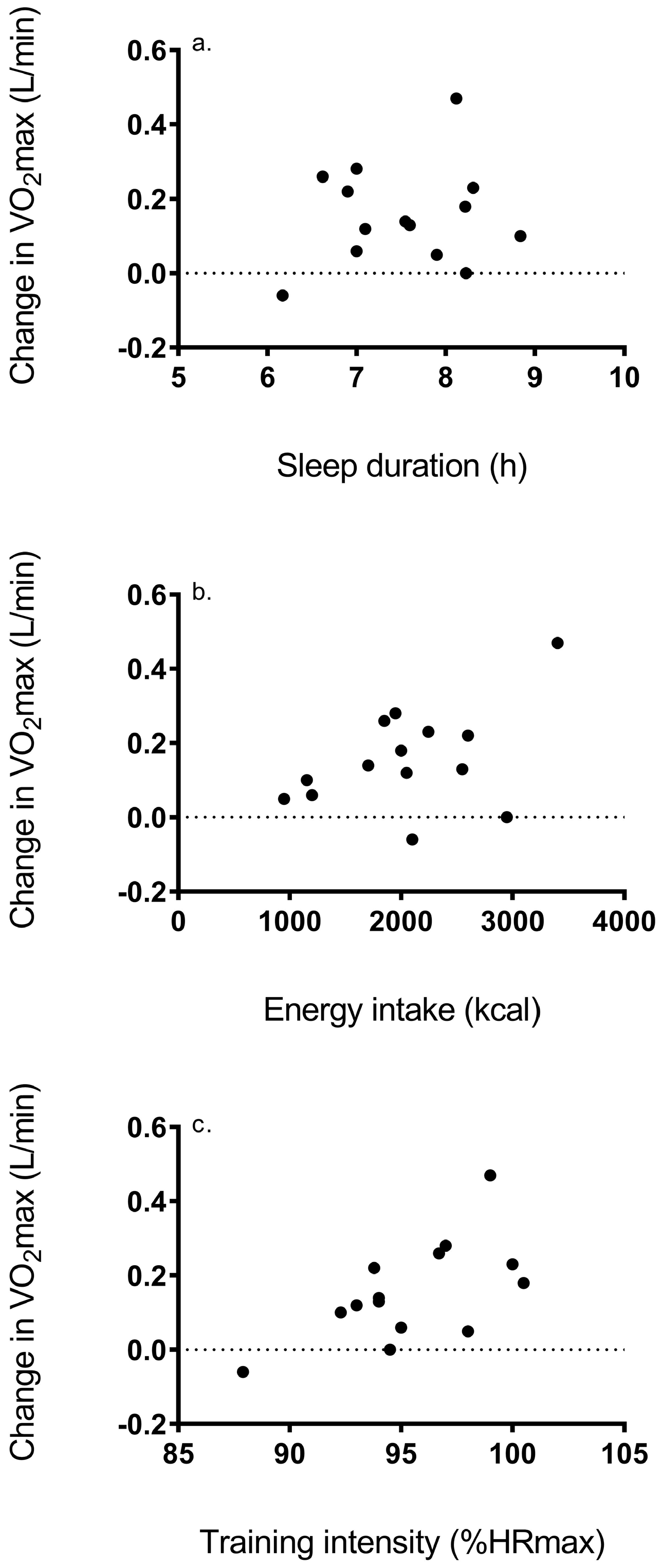
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duscha, B.D.; Slentz, C.A.; Johnson, J.L.; Houmard, J.A.; Bensimhon, D.R.; Knetzger, K.J.; Kraus, W.E. Effects of exercise training amount and intensity on peak oxygen consumption in middle-age men and women at risk for cardiovascular disease. Chest 2005, 128, 2788–2793. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Sui, X.; Artero, E.G.; Lee, I.M.; Church, T.S.; McAuley, P.A.; Stanford, F.C.; Kohl, H.W., 3rd; Blair, S.N. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: The Aerobics Center Longitudinal Study. Circulation 2011, 124, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; An, P.; Rice, T.; Skinner, J.S.; Wilmore, J.H.; Gagnon, J.; Pérusse, L.; Leon, A.S.; Rao, D.C. Familiar aggregation of VO2max response to exercise training: Results from the HERITAGE Family Study. J. Appl. Physiol. 1999, 87, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.N.; Lamberts, R.P.; Lambert, M.I. High responders and low responders: Factors associated with individual variation in response to standardized training. Sports Med. 2014, 44, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; de Lannoy, L.; Stotz, P.J. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin. Proc. 2015, 90, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, M.; Asumi, A.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. J. Am. Med. Assoc. 2009, 301, 2024–2035. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L.; Howlett, R.A.; Heigenhauser, G.J. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med. Sci. Sports Exerc. 2000, 32, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A. Invited review: Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001, 90, 1137–1157. [Google Scholar] [CrossRef]
- Brooks, G.A.; Mercier, J. Balance of carbohydrate and lipid utilization during exercise: The “crossover” concept. J. Appl. Physiol. 1994, 76, 2253–2261. [Google Scholar] [CrossRef]
- Scharhag-Rosenberger, F.; Meyer, T.; Gabler, N.; Faude, O.; Kindermann, W. Exercise at given percentages of VO2max: Heterogeneous metabolic responses between individuals. J. Sci. Med. Sport. 2010, 13, 74–79. [Google Scholar] [CrossRef]
- Preobrazenski, N.; Bonafiglia, J.T.; Nelms, M.W.; Lu, S.; Robins, L.; LeBlanc, C.; Gurd, B.J. Does blood lactate predict the chronic adaptive response to training: A comparison of traditional and talk test prescription methods. Appl. Physiol. Nutr. Metab. 2019, 44, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Milanović, Z.; Sporiš, G.; Weston, M. Effectiveness of High-intensity interval training (HIIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Tipton, K.D.; Millard-Stafford, M.L. Promoting training adaptations through nutritional interventions. J. Sports Sci. 2006, 24, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Samuels, C. Sleep, recovery, and performance: The new frontier in high-performance athletics. Phys. Med. Rehabil. Clin. N. Am. 2009, 20, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; DeRevere, J.; Anderson, T.; Kellogg, E.; Holstrom, P.; Ring, S.; Ghaseb, N. Change in VO2max and time trial performance in response to high-intensity interval training prescribed using ventilatory threshold. Eur. J. Appl. Physiol. 2018, 118, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; White, A.C.; Dalleck, L.C. Supramaximal testing to confirm attainment of VO2max in sedentary men and women. Int. J. Sports Med. 2009, 30, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Caiozzo, V.J.; Davis, J.A.; Ellis, J.F.; Azus, J.L.; Vandagriff, R.; Prietto, C.A.; McMaster, W.C. A comparison of gas exchange indices used to detect the anaerobic threshold. J. Appl. Physiol. 1982, 53, 1184–1189. [Google Scholar] [CrossRef]
- Clark, A.; De La Rosa, A.B.; DeRevere, J.L.; Astorino, T.A. Effects of various interval training regimes on changes in maximal oxygen uptake, body composition, and muscular strength in sedentary women with obesity. Eur. J. Appl. Physiol. 2019, 119, 879–888. [Google Scholar] [CrossRef]
- Billaut, F.; Bishop, D. Muscle fatigue in males and females during multiple-sprint exercise. Sports Med. 2009, 39, 257–278. [Google Scholar] [CrossRef]
- Astorino, T.A.; Sheard, A.C. Does sex mediate the affective response to high intensity interval exercise? Physiol. Behav. 2019, in press. [Google Scholar] [CrossRef]
- Chennaoui, M.; Arnal, P.J.; Sauvet, F.; Léger, D. Sleep and exercise: A reciprocal issue? Sleep Med. Rev. 2015, 20, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Azboy, O.; Kaygisiz, Z. Effects of sleep deprivation on cardiorespiratory functions of the runners and optimizing sleep in elite athletes volleyball players during rest and exercise. Acta Physiol. Hung. 2009, 96, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.; Piercy, M. The effect of partial sleep deprivation on weightlifting performance. Ergonomics 1994, 37, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.D.; Mah, K.E.; Kezirian, E.J.; Dement, W.C. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep 2011, 34, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Durrer, C.; Pistawka, K.J.; Halperin, F.A.; Chang, C.; Little, J.P. Combined interval training and post-exercise nutrition in type 2 diabetes: A randomized control trial. Front. Physiol. 2017, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Kiviniemi, A.M.; Tulppo, M.P.; Eskelinen, J.J.; Savolainen, A.M.; Kapanen, J.; Heinonen, I.H.; Hautala, A.J.; Hannukainen, J.C.; Kalliokoski, K.K. Autonomic function predicts fitness response to short-term high-intensity interval training. Int. J. Sports Med. 2015, 36, 915–921. [Google Scholar] [CrossRef]
- Hautala, A.J.; Mäkikallio, T.H.; Kiviniemi, A.; Laukkanen, R.T.; Nissilä, S.; Huikuri, H.V.; Tulppo, M.P. Cardiovascular autonomic function correlates with the response to aerobic training in healthy sedentary subjects. Am. J. Physiol. 2003, 285, 1717–1752. [Google Scholar] [CrossRef]
- Martin, L.J.; Su, W.F.; Jones, P.H.; Lockwood, G.A.; Tritchler, D.A.; Boyd, N.F. Comparison of energy intakes determined by food records and doubly labeled water in women participating in a dietary-intervention trial. Am. J. Clin. Nutr. 1996, 63, 483–490. [Google Scholar] [CrossRef]
- Claudino, J.G.; Gabbet, T.J.; de Sá Souza, H.; Simim, M.; Fowler, P.; de Alcantara Borba, D.; Melo, M.; Bottino, A.; Loturco, I.; D’Almeida, V.; et al. Which parameters to use for sleep quality monitoring in team sport athletes? A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2019, 5, e000475. [Google Scholar] [CrossRef]
- Gillen, J.B.; Percival, M.E.; Skelly, L.E.; Martin, B.J.; Tan, R.B.; Tarnopolsky, M.A.; Gibala, M.J. Three minutes of all-out intermittent exercise per week increases skeletal muscle oxidative capacity and improves cardiometabolic health. PLoS ONE 2014, 9, e111489. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astorino, T.A.; DeRevere, J.L.; Anderson, T.; Kellogg, E.; Holstrom, P.; Ring, S.; Ghaseb, N. Blood Lactate Concentration Is Not Related to the Increase in Cardiorespiratory Fitness Induced by High Intensity Interval Training. Int. J. Environ. Res. Public Health 2019, 16, 2845. https://doi.org/10.3390/ijerph16162845
Astorino TA, DeRevere JL, Anderson T, Kellogg E, Holstrom P, Ring S, Ghaseb N. Blood Lactate Concentration Is Not Related to the Increase in Cardiorespiratory Fitness Induced by High Intensity Interval Training. International Journal of Environmental Research and Public Health. 2019; 16(16):2845. https://doi.org/10.3390/ijerph16162845
Chicago/Turabian StyleAstorino, Todd A., Jamie L. DeRevere, Theodore Anderson, Erin Kellogg, Patrick Holstrom, Sebastian Ring, and Nicholas Ghaseb. 2019. "Blood Lactate Concentration Is Not Related to the Increase in Cardiorespiratory Fitness Induced by High Intensity Interval Training" International Journal of Environmental Research and Public Health 16, no. 16: 2845. https://doi.org/10.3390/ijerph16162845
APA StyleAstorino, T. A., DeRevere, J. L., Anderson, T., Kellogg, E., Holstrom, P., Ring, S., & Ghaseb, N. (2019). Blood Lactate Concentration Is Not Related to the Increase in Cardiorespiratory Fitness Induced by High Intensity Interval Training. International Journal of Environmental Research and Public Health, 16(16), 2845. https://doi.org/10.3390/ijerph16162845



