Dynamic Anemia Status from Infancy to Preschool-Age: Evidence from Rural China
Abstract
1. Introduction
2. Methods
2.1. Ethical Review
2.2. Sample Selection
2.3. Data Collection
2.3.1. Hemoglobin Concentrations
2.3.2. Anthropometric Measures
2.3.3. Socioeconomic Characteristics
2.3.4. Child Cognitive Outcomes
2.4. Statistical Analysis
3. Results
3.1. Socioeconomic and Demographic Characteristics of Participants
3.2. Hb Concentrations, Anemia, and Physical Development
3.3. Dynamic Anemia Status
3.4. Dynamic Anemia Status and Child Cognitive Outcomes
3.5. Risk Factors of Dynamic Anemia Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Characteristics. | Percentage (%)/Mean ± SD |
|---|---|
| Dietary diversity | 4.03 ± 1.56 |
| Infancy | 2.63 ± 1.57 |
| Toddlerhood | 4.26 ± 1.31 |
| Preschool-age | 4.42 ± 1.44 |
| Maternal hemoglobin values (g/L) | 127.7 ± 13.5 |
| Maternal anemia at baseline | 20.9 |
| Variable | Deteriorating (1 = Deteriorating, 0 = Never) | Improving (1 = Improving, 0 = Persistent) | ||
|---|---|---|---|---|
| Infancy to Preschool-Age | Infancy to Preschool-Age | |||
| β | ME | β | ME | |
| (1) | (2) | (3) | (4) | |
| Dietary diversity scores | 0.00 | 0.00 | −0.03 | −0.00 |
| (0.03) | (0.01) | (0.04) | (0.01) | |
| Control variables | YES | YES | YES | YES |
| County fixed effect | YES | YES | YES | YES |
| Time fixed effect | YES | YES | YES | YES |
| Observations | 1214 | 1214 | 1256 | 1256 |
| Variable | Deteriorating (1 = Deteriorating, 0 = Never) | Improving (1 = Improving, 0 = Persistent) | ||
|---|---|---|---|---|
| Infancy to Preschool-Age | Infancy to Preschool-Age | |||
| β | ME | β | ME | |
| (1) | (2) | (3) | (4) | |
| Maternal anemia | 0.34 *** | 0.09 *** | −0.07 | −0.01 |
| (1 = yes) | (0.10) | (0.03) | (0.12) | (0.02) |
| Control variables | YES | YES | YES | YES |
| County fixed effect | YES | YES | YES | YES |
| Time fixed effect | YES | YES | YES | YES |
| Observations | 1209 | 1209 | 1266 | 1266 |
Appendix B
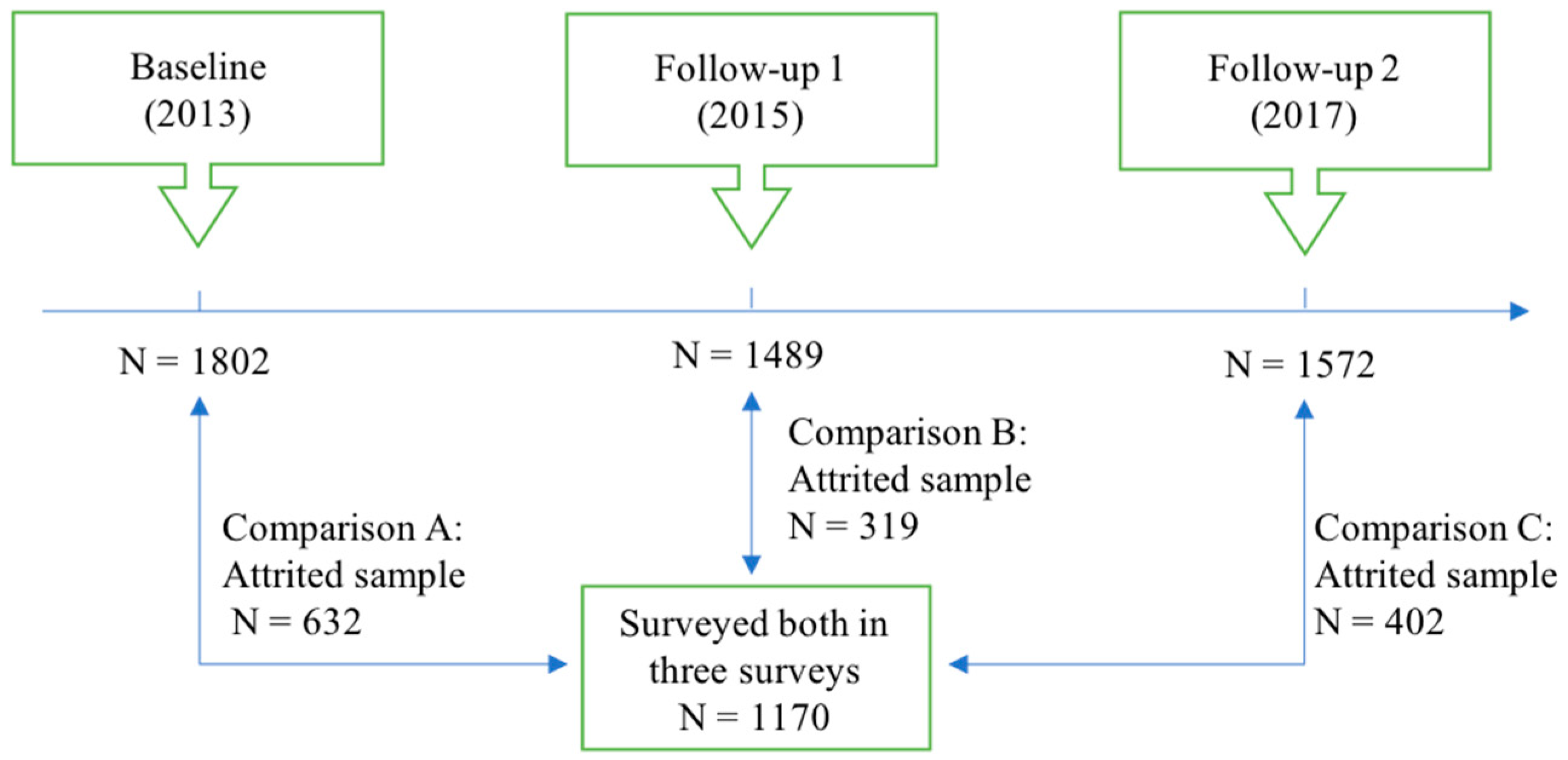
| Comparison A | Comparison B | Comparison C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (Infancy) | Follow-Up 1 (Toddlerhood) | Follow-Up 2 (Preschool-Age) | ||||||||||
| Full Sample | Attrited Sample | Non-Attrited Sample | Differences (2)–(3) | Full Sample | Attrited Sample | Non-Attrited Sample | Differences (6)–(7) | Full Sample | Attrited Sample | Non-Attrited Sample | Differences (10)–(11) | |
| Mean/SD | Mean/SD | Mean/SD | p-Value | Mean/SD | Mean/SD | Mean/SD | p-Value | Mean/SD | Mean/SD | Mean/SD | p-Value | |
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | |
| Health outcomes | ||||||||||||
| Child’s hemoglobin value | 109.19 | 110.19 | 108.66 | 0.015 | 118.37 | 119.60 | 118.12 | 0.105 | 119.58 | 120.31 | 119.39 | 0.165 |
| (g/L) | (12.58) | (12.69) | (12.49) | (13.07) | (12.73) | (13.13) | (10.49) | (10.66) | (10.44) | |||
| Anemic | 0.49 | 0.43 | 0.51 | <0.001 | 0.23 | 0.20 | 0.24 | 0.214 | 0.19 | 0.18 | 0.19 | 0.721 |
| (hb < 110 g/L) | (0.50) | (0.50) | (0.50) | (0.42) | (0.40) | (0.42) | (0.39) | (0.38) | (0.39) | |||
| Stunted | 0.04 | 0.03 | 0.04 | 0.822 | 0.03 | 0.03 | 0.03 | 0.685 | 0.02 | 0.03 | 0.02 | 0.149 |
| (LAZ ≤ −2) | (0.18) | (0.18) | (0.19) | (0.16) | (0.17) | (0.16) | (0.15) | (0.18) | (0.14) | |||
| Underweight | 0.01 | 0.01 | 0.01 | 0.846 | 0.01 | 0.01 | 0.01 | 0.292 | 0.02 | 0.02 | 0.02 | 0.673 |
| (WAZ ≤ −2) | (0.10) | (0.11) | (0.10) | (0.11) | (0.08) | (0.12) | (0.14) | (0.13) | (0.14) | |||
| Wasted | 0.02 | 0.02 | 0.02 | 0.672 | 0.02 | 0.01 | 0.02 | 0.446 | 0.04 | 0.03 | 0.04 | 0.532 |
| (WLZ ≤ −2) | (0.15) | (0.14) | (0.15) | (0.12) | (0.10) | (0.13) | (0.19) | (0.17) | (0.19) | |||
| Child’s characteristics | ||||||||||||
| Child’s age | 9.50 | 9.59 | 9.44 | 0.100 | 27.47 | 27.60 | 27.44 | 0.145 | 56.86 | 56.84 | 56.87 | 0.903 |
| (in months) | (1.83) | (1.88) | (1.81) | (1.77) | (1.79) | (1.76) | (3.42) | (3.52) | (3.38) | |||
| Male | 0.53 | 0.56 | 0.51 | 0.059 | 0.52 | 0.55 | 0.51 | 0.208 | 0.52 | 0.55 | 0.51 | 0.163 |
| (1 = yes) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | |||
| Have siblings | 0.21 | 0.13 | 0.25 | <0.001 | 0.22 | 0.13 | 0.25 | <0.001 | 0.45 | 0.38 | 0.47 | 0.002 |
| (1 = yes) | (0.41) | (0.34) | (0.43) | (0.42) | (0.34) | (0.43) | (0.50) | (0.49) | (0.50) | |||
| Low Birth Weight | 0.05 | 0.04 | 0.05 | 0.199 | 0.05 | 0.03 | 0.05 | 0.215 | 0.05 | 0.04 | 0.05 | 0.258 |
| (1 = yes) | (0.21) | (0.19) | (0.22) | (0.22) | (0.18) | (0.22) | (0.22) | (0.19) | (0.22) | |||
| Was ever breastfed | 0.58 | 0.57 | 0.59 | 0.412 | 0.58 | 0.53 | 0.59 | 0.062 | 0.59 | 0.59 | 0.59 | 0.888 |
| (1 = yes) | (0.49) | (0.50) | (0.49) | (0.49) | (0.50) | (0.49) | (0.49) | (0.49) | (0.49) | |||
| Household characteristics | ||||||||||||
| Primary caregiver | 0.82 | 0.78 | 0.85 | <0.001 | 0.60 | 0.52 | 0.62 | <0.001 | 0.61 | 0.54 | 0.63 | 0.003 |
| (1 = mother) | (0.38) | (0.41) | (0.36) | (0.49) | (0.50) | (0.48) | (0.49) | (0.50) | (0.48) | |||
| Maternal age | 0.50 | 0.48 | 0.51 | 0.280 | 0.51 | 0.52 | 0.51 | 0.688 | 0.50 | 0.49 | 0.51 | 0.434 |
| (1 = above 25 years old) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | (0.50) | |||
| Maternal education level | 0.17 | 0.21 | 0.15 | 0.003 | 0.16 | 0.18 | 0.15 | 0.194 | 0.16 | 0.18 | 0.15 | 0.165 |
| (1 = 9 years or higher) | (0.37) | (0.40) | (0.36) | (0.36) | (0.39) | (0.36) | (0.36) | (0.38) | (0.36) | |||
| Family asset index | −0.04 | 0.02 | −0.07 | 0.091 | −0.04 | 0.06 | −0.07 | 0.070 | −0.35 | −0.32 | −0.36 | 0.557 |
| (1.18) | (1.20) | (1.17) | (1.19) | (1.24) | (1.17) | (1.19) | (1.14) | (1.20) | ||||
| Observations | 1802 | 632 | 1170 | 1489 | 319 | 1170 | 1572 | 402 | 1170 | |||
Appendix C
| Variables | Infancy | Toddlerhood | Frequency (n) | Percentage (%) |
|---|---|---|---|---|
| Never | NO | NO | 454 | 39 |
| Persistent | YES | YES | 162 | 14 |
| Deteriorating | NO | YES | 114 | 10 |
| Improving | YES | NO | 440 | 38 |
| Observations | 1170 | 100 |
| Variables | Toddlerhood | Preschool-Age | Frequency (n) | Percentage (%) |
|---|---|---|---|---|
| Never | NO | NO | 746 | 64 |
| Persistent | YES | YES | 72 | 6 |
| Deteriorating | NO | YES | 148 | 13 |
| Improving | YES | NO | 204 | 17 |
| Observations | 1170 | 100 |
References
- Marotta, C.; Di Gennaro, F.; Pizzol, D.; Madeira, G.; Monno, L.; Saracino, A.; Putoto, G.; Casuccio, A.; Mazzucco, W. The at risk child clinic (ARCC): 3 years of health activities in support of the most vulnerable children in Beira, Mozambique. Int. J. Environ. Res. Public Health 2018, 15, 1350. [Google Scholar] [CrossRef] [PubMed]
- McLean, E.; Cogswell, M.; Egli, I.; Wojdyla, D.; De Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef]
- Stoltzfus, R.J.; Mullany, L.; Black, R.E. Iron deficiency anaemia. In Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2005; pp. 163–209. ISBN 9241580313. [Google Scholar]
- Greacen, J.R.; Walsh, E.J. NAPL Containment Using In Situ Solidification. In Contaminated Soils, Sediments and Water; Kluwer Academic Publishers: Boston, MA, USA, 2004; pp. 477–483. ISBN 038723036X. [Google Scholar]
- Lozoff, B.; Wolf, A.W.; Jimenez, E. Iron-deficiency anemia and infant development: Effects of extended oral iron therapy. J. Pediatr. 1996, 129, 382–389. [Google Scholar] [CrossRef]
- Idjradinata, P.; Pollitt, E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 1993, 341, 1–4. [Google Scholar] [CrossRef]
- Lozoff, B.; Brittenham, G.M.; Wolf, A.W.; McClish, D.K.; Kuhnert, P.M.; Jimenez, E.; Jimenez, R.; Mora, L.A.; Gomez, I.; Krauskoph, D. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics 1987, 79, 981–995. [Google Scholar]
- Brabin, B.J.; Premji, Z.; Verhoeff, F. Iron-Deficiency Anemia: Reexamining the Nature and Magnitude of the Public Health Problem An Analysis of Anemia and Child Mortality. J. Nutr. 2001, 131, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B.; Jimenez, E.; Hagen, J.; Mollen, E.; Wolf, A.W. Poorer Behavioral and Developmental Outcome More Than 10 Years After Treatment for Iron Deficiency in Infancy. Pediatrics 2000, 105, e51. [Google Scholar] [CrossRef]
- Lozoff, B.; Jimenez, E.; Smith, J.B. Double Burden of Iron Deficiency in Infancy and Low Socioeconomic Status. Arch. Pediatr. Adolesc. Med. 2006, 160, 1108–1113. [Google Scholar] [CrossRef]
- Maluccio, J.A.; Hoddinott, J.; Behrman, J.R.; Martorell, R.; Quisumbing, A.R.; Stein, A.D. The impact of improving nutrition during early childhood on education among Guatemalan adults. Econ. J. 2009, 119, 734–763. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T. Iron Deficiency and Reduced Work Capacity: A Critical Review of the Research to Determine a Causal Relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef]
- ChunMing, C.; Wu, H.; YuYing, W.; LiNa, D.; FengMei, J. Nutritional status of children during and post-global economic crisis in China. Biomed. Environ. Sci. 2011, 24, 321–328. [Google Scholar]
- Luo, R.; Shi, Y.; Zhou, H.; Yue, A.; Zhang, L.; Sylvia, S.; Medina, A.; Rozelle, S. Micronutrient deficiencies and developmental delays among infants: Evidence from a cross-sectional survey in rural China. BMJ Open 2015, 5, e008400. [Google Scholar] [CrossRef]
- Junqun, F.; Jiayou, L.; Kai, L.; Hua, W.; Donghua, X.; Wenzhen, Y. Analysis of anemia among children aged 6-24 months in poor rural areas of Hunan Province. Chinese J. Child Heal. Care 2017, 25, 824–826. [Google Scholar]
- WHO/UNICEF/UNU. Iron Deficiency Anaemia: Assessment, Prevention and Control. A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Zlotkin, S.; Antwi, K.Y.; Schauer, C.; Yeung, G. Use of microencapsulated iron(II) fumarate sprinkles to prevent recurrence of anaemia in infants and young children at high risk. Bull. World Health Organ. 2003. [Google Scholar]
- Baker, R.D.; Greer, F.R. American Academy of Pediatrics Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef]
- Osório, M.M.; Lira, P.I.C.; Batista-Filho, M.; Ashworth, A. Prevalence of anemia in children 6-59 months old in the state of Pernambuco, Brazil. Rev. Panam. Salud Pública 2001, 10, 101–107. [Google Scholar] [CrossRef]
- Leite, M.S.; Cardoso, A.M.; Coimbra, C.E.A.; Welch, J.R.; Gugelmin, S.A.; Lira, P.C.I.; Horta, B.L.; Santos, R.V.; Escobar, A.L. Prevalence of anemia and associated factors among indigenous children in Brazil: results from the First National Survey of Indigenous People’s Health and Nutrition. Nutr. J. 2013, 12, 69. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Y.; Liu, B.; Zheng, L.; Li, M.; Bai, Y.; Osborn, A.; Lee, M.; Rozelle, S. Is Infant/Toddler Anemia a Problem across Rural China? A Mixed-Methods Analysis. Int. J. Environ. Res. Public Health 2018, 15, 1825. [Google Scholar] [CrossRef]
- Willows, N.D.; Barbarich, B.N.; Wang, L.C.H.; Olstad, D.L.; Clandinin, M.T. Dietary inadequacy is associated with anemia and suboptimal growth among preschool-aged children in Yunnan Province, China. Nutr. Res. 2011, 31, 88–96. [Google Scholar] [CrossRef]
- Chang Zhai, Y.F.; Li, W.; Ge, K.; Jin, D.; De Onis, M. Nutritional status of preschool children in poor rural areas of China. Bull. World Health Organ. 1994, 72, 105–112. [Google Scholar]
- Binns, H.J.; Chen, T.; Kahn, J.L.; Tanz, R.R.; Listernick, R. Persistence and Emergence of Anemia in Children During Participation in the Special Supplemental Nutrition Program for Women, Infants, and Children. Arch. Pediatr. Adolesc. Med. 2013, 156, 1028. [Google Scholar]
- Cusick, S.E.; Mei, Z.; Cogswell, M.E. Continuing Anemia Prevention Strategies Are Needed Throughout Early Childhood in Low-income Preschool Children. J. Pediatr. 2007, 150, 422–428. [Google Scholar] [CrossRef]
- Walter, T.; De Andraca, I.; Chadud, P.; Perales, C.G. Iron deficiency anemia: Adverse effects on infant psychomotor development. Pediatrics 1989, 84, 7–17. [Google Scholar] [CrossRef]
- Altucher, K.; Rasmussen, K.M.; Barden, E.M.; Habicht, J.P. Predictors of improvement in hemoglobin concentration among toddlers enrolled in the massachusetts WIC program. J. Am. Diet. Assoc. 2005, 105, 709–715. [Google Scholar] [CrossRef]
- Desai, M.R.; Terlouw, D.J.; Kwena, A.M.; Phillips-Howard, P.A.; Kariuki, S.K.; Wannemuehler, K.A.; Odhacha, A.; Hawley, W.A.; Shi, Y.P.; Nahlen, B.L.; et al. Factors associated with hemoglobin concentrations in pre-school children in western Kenya: Cross-sectional studies. Am. J. Trop. Med. Hyg. 2005, 72, 47–59. [Google Scholar] [CrossRef]
- Kahigwa, E.; Schellenberg, D.; Sanz, S.; Aponte, J.J.; Wigayi, J.; Mshinda, H.; Alonso, P.; Menendez, C. Risk factors for presentation to hospital with severe anaemia in Tanzanian children: A case-control study. Trop. Med. Int. Heal. 2002, 7, 823–830. [Google Scholar] [CrossRef]
- Hassan, K.; Sullivan, K.M.; Yip, R.; Woodruff, B.A. Factors Associated with Anemia in Refugee Children. J. Nutr. 1997, 127, 2194–2198. [Google Scholar] [CrossRef]
- Ngnie-Teta, I.; Receveur, O.; Kuate-Defo, B. Risk factors for moderate to severe anemia among children in Benin and Mali: Insights from a multilevel analysis. Food Nutr. Bull. 2007, 28, 76–89. [Google Scholar] [CrossRef]
- Ai, Z.; Yumei, Z.; Ying, P.; Jiayin, L.; Titi, Y.; Zhaoyan, L.; Yanli, L.; Peiyu, W. Prevalence of Anemia and Its Risk Factors Among Children 6–36 Months Old in Burma. Am. J. Trop. Med. Hyg. 2012, 87, 306–311. [Google Scholar]
- De Pee, S.; Bloem, M.W.; Sari, M.; Kiess, L.; Yip, R.; Kosen, S. The High Prevalence of Low Hemoglobin Concentration among Indonesian Infants Aged 3–5 Months Is Related to Maternal Anemia. J. Nutr. 2002, 132, 2215–2221. [Google Scholar] [CrossRef]
- Mamiro, P.S.; Kolsteren, P.; Roberfroid, D.; Tatala, S.; Opsomer, A.S.; Va, J.H. Feeding Practices and Factors Contributing to Wasting, Stunting, and Iron-deficiency Anaemia among 3-23-month Old Children in Kilosa District, Rural Tanzan. J. Heal. Popul. Nutr. 2015, 23, 222–230. [Google Scholar]
- Osório, M.M.; Lira, P.I.C.; Ashworth, A. Factors associated with Hb concentration in children aged 6–59 months in the State of Pernambuco, Brazil. Br. J. Nutr. 2004, 91, 307. [Google Scholar] [CrossRef]
- Lehmann, F.; Gray-Donald, K.; Mongeon, M.; Di Tommaso, S. Iron deficiency anemia in 1-year-old children of disadvantaged families in Montreal. Can. Med. Assoc. J. 1992, 146, 1571–1577. [Google Scholar]
- Xiaoyong, X.; Jingsheng, L.; Jiaju, C. Patterns and Achievements of Rural Poverty Reduction and Policy Suggestions in Western Areas of China. Res. Agric. Mod. 2010, 31, 161–165. [Google Scholar]
- United Nations Development Programme in China. The Dimensions of Living Level of the Human Development Index: Applying Big Data to Measure China’s Poverty; United Nations Development Programme in China: Beijing, China, 2016. [Google Scholar]
- Zhou, H.; Sun, S.; Luo, R.; Sylvia, S.; Yue, A.; Shi, Y.; Zhang, L.; Medina, A.; Rozelle, S. Impact of text message reminders on caregivers’ adherence to a home fortification program against child anemia in rural western China: A cluster-randomized controlled trial. Am. J. Public Health 2016, 106, 1256–1262. [Google Scholar] [CrossRef]
- Von Schenck, H.; Falkensson, M.; Lundberg, B. Evaluation of “HemoCue,” a new device for determining hemoglobin. Clin. Chem. 1986. [Google Scholar]
- Sullivan, K.M.; Mei, Z.; Grummer-Strawn, L.; Parvanta, I. Haemoglobin adjustments to define anaemia. Trop. Med. Int. Heal. 2008, 13, 1267–1271. [Google Scholar] [CrossRef]
- De Onis, M.; Garza, C.; Victora, C.G.; Onyango, A.W.; Frongillo, E.A.; Martines, J. The who Multicentre Growth Reference Study: Planning, Study Design, and Methodology. Food Nutr. Bull. 2004, 25, S15–S26. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-Forheight and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- De Onis, M.; Blössner, M. WHO Global Database on Child Growth and Malnutrition; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Kolenikov, S.; Angeles, G. Socioeconomic status measurement with discrete proxy variables: Is principal component analysis a reliable answer? Rev. Income Wealth 2009, 55, 128–165. [Google Scholar] [CrossRef]
- Bayley, N. Bayley Scales of Infant Development: Manual; The Psychological Corporation: New York, NY, USA, 1969. [Google Scholar]
- Yi, S.; Luo, X.; Yang, Z.; Wan, G. The revising of Bayley Scales of Infant Development (BSID) in China. Chinese J. Clin. Psychol. 1993, 1, 71–75. [Google Scholar]
- Luiselli, J.; Happé, F.; Hurst, H.; Freeman, S.; Goldstein, G.; Mazefsky, C.; Carter, A.S.; Kaufman, A.S.; Simmons, E.S.; Eernisse, E.R.; et al. Wechsler Preschool and Primary Scale of Intelligence. In Encyclopedia of Autism Spectrum Disorders; Springer: New York, NY, USA, 2013; pp. 3351–3360. ISBN 0749123044r0158989341. [Google Scholar]
- Tung, L.-C.; Yu, T.-Y.; Chen, K.-L.; Chou, W.; Yang, S.-H.; Kung, S.-C.; Lee, Y.-C. Intelligence quotient discrepancy indicates levels of motor competence in preschool children at risk for developmental delays. Neuropsychiatr. Dis. Treat. 2016, 12, 501. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, Q.; Lee, G.T. Using Peer-Mediated LEGO® Play Intervention to Improve Social Interactions for Chinese Children with Autism in an Inclusive Setting. J. Autism Dev. Disord. 2018, 48, 2444–2457. [Google Scholar] [CrossRef]
- Chen, K.L.; Lin, C.H.; Yu, T.Y.; Huang, C.Y.; Chen, Y.D. Differences Between the Childhood Autism Rating Scale and the Social Responsiveness Scale in Assessing Symptoms of Children with Autistic Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 3191–3198. [Google Scholar] [CrossRef]
- Attanasio, O.; Cattan, S.; Fitzsimons, E.; Meghir, C.; Rubio-Codina, M. Estimating the Production Function for Human Capital: Results from A Randomized Control Trial in Colombia. Cambridge. 2017. Working Paper. Available online: http://www.nber.org/papers/w20965 (accessed on 1 August 2019).
- World Bank Prevalence of Anemia among Children (% of Children under 5). Available online: https://data.worldbank.org/indicator/SH.ANM.CHLD.ZS?end=2016&start=1990&view=chart (accessed on 14 March 2019).
- World Bank Prevalence of stunting, height for age (% of children under 5). Available online: https://data.worldbank.org/indicator/SH.STA.STNT.ZS (accessed on 24 May 2019).
- World Bank Prevalence of Underweight, Weight for Age (% of children under 5). Available online: https://data.worldbank.org/indicator/SH.STA.MALN.ZS?end=2016&locations=ZA&start=1990&view=chart (accessed on 24 May 2019).
- World Bank Prevalence of Wasting, Weight for Height (% of children under 5). Available online: https://data.worldbank.org/indicator/SH.STA.WAST.ZS?end=2016&start=1990&view=chart (accessed on 24 May 2019).
- China Development Research Fundation. Third Party Evaluation Report of Child Nutrition Improvement Project in Poor Areas. Available online: http://www.cdrf.org.cn/jjh/pdf/pinkun.pdf (accessed on 1 August 2019).
- Zhou, H.; Mo, D.; Luo, R.; Yue, A.; Rozelle, S. Are Children with Siblings Really More Vulnerable Than Only Children in Health, Cognition and Non-cognitive Outcomes? Evidence from a Multi-province Dataset in China. China World Econ. 2016, 24, 3–17. [Google Scholar] [CrossRef]
- ShuFa, D.; FengYing, Z.; YouFa, W.; Popkin, B.M. Current methods for estimating dietary iron bioavailability do not work in China. J. Nutr. 2000, 130, 193–198. [Google Scholar]
- Woldie, H.; Kebede, Y.; Tariku, A. Factors Associated with Anemia among Children Aged 6–23 Months Attending Growth Monitoring at Tsitsika Health Center, Wag-Himra Zone, Northeast Ethiopia. J. Nutr. Metab. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Balarajan, Y.; Ramakrishnan, U.; Özaltin, E.; Shankar, A.H.; Subramanian, S.V. Anaemia in low-income and middle-income countries. Lancet 2011, 378, 2123–2135. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Chang, S.; Zhao, L.; Fu, P.; Yu, W.; Man, Q.; Scherpbier, R.; Pan, L.; Duan, Y.; et al. The Influence of Malnutrition and Micronutrient Status on Anemic Risk in Children under 3 Years Old in Poor Areas in China. PLoS ONE 2015, 10, e0140840. [Google Scholar] [CrossRef]
- Meinzen-Derr, J.K.; Guerrero, M.L.; Altaye, M.; Ortega-Gallegos, H.; Ruiz-Palacios, G.M.; Morrow, A.L. Risk of Infant Anemia Is Associated with Exclusive Breast-Feeding and Maternal Anemia in a Mexican Cohort. J. Nutr. 2006, 136, 452–458. [Google Scholar] [CrossRef]
- World Health Organization. Indicators for assessing infant and young child feeding practices Part 1 Definitions. In Proceedings of the World Health Organization, Washington, DC, USA, 6–8 November 2007; WHO Press: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Available online: http://www.who.int/vmnis/indicators/haemoglobin. pdf (accessed on 27 July 2019).
- Mikki, N.; Abdul Rahim, H.F.; Stigum, H.; Holmboe Ottesen, G. Anaemia prevalence and associated sociodemographic and dietary factors among Palestinian adolescents in the West Bank. East. Mediterr. Heal. J. 2018, 17, 208–217. [Google Scholar] [CrossRef]
- Saaka, M.; Zakaria Galaa, S. How is dietary diversity related to haematological status of preschool children in Ghana? Food Nutr. Res. 2017, 61, 1333389. [Google Scholar] [CrossRef]
- Yue, A.; Zhang, N.; Liu, X.; Tang, L.; Luo, R.; Yang, M.; Rozelle, S.; Medina, A. Do Infant Feeding Practices Differ Between Grandmothers and Mothers in Rural China? Evidence From Rural Shaanxi Province. Fam. Community Health 2018, 41, 233–243. [Google Scholar] [CrossRef]

| Characteristics | Frequency (n) | Percentage (%)/Mean ± SD |
|---|---|---|
| (1) | (2) | |
| Child characteristics | ||
| Gender | ||
| Male | 599 | 51.2 |
| Female | 571 | 48.8 |
| Has siblings | ||
| Yes | 289 | 24.7 |
| No | 881 | 75.3 |
| Low birth weight | ||
| Yes | 61 | 5.2 |
| No | 1109 | 94.8 |
| Was ever breastfed | ||
| Yes | 688 | 58.8 |
| No | 482 | 41.2 |
| Household characteristics | ||
| Mother is primary caregiver | ||
| Yes | 730 | 62.4 |
| No | 440 | 37.6 |
| Maternal age | ||
| Age ≤ 25 | 576 | 49.2 |
| Age > 25 | 594 | 50.8 |
| Maternal education level (years) | ||
| ≤9 | 995 | 85.0 |
| >9 | 175 | 15.0 |
| Family asset index | 1170 | −0.07 ± 1.17 |
| Variables | Infancy | Toddlerhood | Preschool-Age | Differences (1)–(2) | Differences (1)–(3) | Differences (2)–(3) |
|---|---|---|---|---|---|---|
| Mean/SD | Mean/SD | Mean/SD | p-Value | p-Value | p-Value | |
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Child’s hemoglobin value | 108.7 | 118.1 | 119.4 | 0.000 | 0.000 | 0.010 |
| (g/L) | (12.49) | (13.13) | (10.44) | |||
| Anemic | 0.51 | 0.24 | 0.19 | 0.000 | 0.000 | 0.005 |
| (0.50) | (0.42) | (0.39) | ||||
| Stunted | 0.04 | 0.03 | 0.02 | 0.187 | 0.024 | 0.340 |
| (LAZ ≤ −2) | (0.19) | (0.16) | (0.14) | |||
| Underweight | 0.01 | 0.01 | 0.02 | 0.344 | 0.043 | 0.274 |
| (WAZ ≤ −2) | (0.10) | (0.12) | (0.14) | |||
| Wasted | 0.02 | 0.02 | 0.04 | 0.165 | 0.053 | 0.001 |
| (WLZ ≤ −2) | (0.15) | (0.13) | (0.19) | |||
| Observations | 1170 | 1170 | 1170 |
| Variables | Infancy | Toddlerhood | Preschool-Age | Frequency (n) | Percentage (%) |
|---|---|---|---|---|---|
| Never | NO | NO | NO | 380 | 33 |
| Persistent | YES | YES | YES | 46 | 4 |
| Deteriorating | NO | NO | YES | 74 | 6 |
| NO | YES | YES | 26 | 2 | |
| Improving | YES | NO | NO | 366 | 31 |
| YES | YES | NO | 116 | 10 | |
| Fluctuating | YES | NO | YES | 74 | 6 |
| NO | YES | NO | 88 | 8 | |
| Observations | 1170 | 100 | |||
| Variable | Standardized FSIQ (Preschool-Age) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Infancy to Preschool-Age | Infancy to Toddlerhood | Toddlerhood to Preschool-Age | |||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |
| Improving | 0.36 ** | 0.22 *** | 0.29 ** | ||||||
| (1 = Improving, 0 = Persistent) | (0.14) | (0.09) | (0.13) | ||||||
| Deteriorating | 0.02 | -0.03 | 0.01 | ||||||
| (1 = Deteriorating, 0 = Never) | (0.11) | (0.10) | (0.08) | ||||||
| Persistent | −0.33 ** | −0.23 *** | −0.31 *** | ||||||
| (1 = Persistent, 0 = Never) | (0.15) | (0.09) | (0.12) | ||||||
| Baseline MDI scores | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| Control variables | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| County fixed effect | YES | YES | YES | YES | YES | YES | YES | YES | YES |
| Observations | 528 | 480 | 426 | 602 | 568 | 616 | 276 | 894 | 818 |
| Variable | Deteriorating (1 = Deteriorating, 0 = Never) | Improving (1 = Improving, 0 = Persistent) | ||
|---|---|---|---|---|
| Infancy to Preschool-Age | Infancy to Preschool-Age | |||
| β | ME | β | ME | |
| (1) | (2) | (3) | (4) | |
| Child’s characteristics | ||||
| Male | 0.13 | 0.03 | −0.10 | −0.02 |
| (1 = yes) | (0.08) | (0.02) | (0.10) | (0.01) |
| Has siblings | 0.12 | 0.03 | 0.35 *** | 0.05 *** |
| (1 = yes) | (0.09) | (0.02) | (0.12) | (0.02) |
| Low birth weight | 0.42 *** | 0.11 *** | 0.02 | 0.00 |
| (1 = yes) | (0.16) | (0.04) | (0.21) | (0.03) |
| Was ever breastfed | −0.24 *** | −0.06 *** | 0.16 | 0.02 |
| (1 = yes) | (0.09) | (0.02) | (0.11) | (0.02) |
| Household characteristics | ||||
| Primary caregiver | −0.05 | −0.01 | 0.00 | 0.00 |
| (1 = mother) | (0.09) | (0.02) | (0.12) | (0.02) |
| Maternal age | 0.04 | 0.01 | 0.08 | 0.01 |
| (1 = above 25 years old) | (0.08) | (0.02) | (0.10) | (0.01) |
| Maternal education level | −0.07 | −0.02 | 0.04 | 0.01 |
| (1 = 9 years or higher) | (0.11) | (0.03) | (0.14) | (0.02) |
| Family asset index | 0.03 | 0.01 | 0.08 | 0.01 |
| (0.04) | (0.01) | (0.04) | (0.01) | |
| County fixed effect | YES | YES | YES | YES |
| Time fixed effect | YES | YES | YES | YES |
| Observations | 1440 | 1440 | 1584 | 1584 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, M.; Dill, S.-E.; Hu, Y.; Rozelle, S. Dynamic Anemia Status from Infancy to Preschool-Age: Evidence from Rural China. Int. J. Environ. Res. Public Health 2019, 16, 2761. https://doi.org/10.3390/ijerph16152761
Wang L, Li M, Dill S-E, Hu Y, Rozelle S. Dynamic Anemia Status from Infancy to Preschool-Age: Evidence from Rural China. International Journal of Environmental Research and Public Health. 2019; 16(15):2761. https://doi.org/10.3390/ijerph16152761
Chicago/Turabian StyleWang, Lei, Mengjie Li, Sarah-Eve Dill, Yiwei Hu, and Scott Rozelle. 2019. "Dynamic Anemia Status from Infancy to Preschool-Age: Evidence from Rural China" International Journal of Environmental Research and Public Health 16, no. 15: 2761. https://doi.org/10.3390/ijerph16152761
APA StyleWang, L., Li, M., Dill, S.-E., Hu, Y., & Rozelle, S. (2019). Dynamic Anemia Status from Infancy to Preschool-Age: Evidence from Rural China. International Journal of Environmental Research and Public Health, 16(15), 2761. https://doi.org/10.3390/ijerph16152761






