Benthic Diatom Communities in Korean Estuaries: Species Appearances in Relation to Environmental Variables
Abstract
1. Introduction
2. Materials and Methods
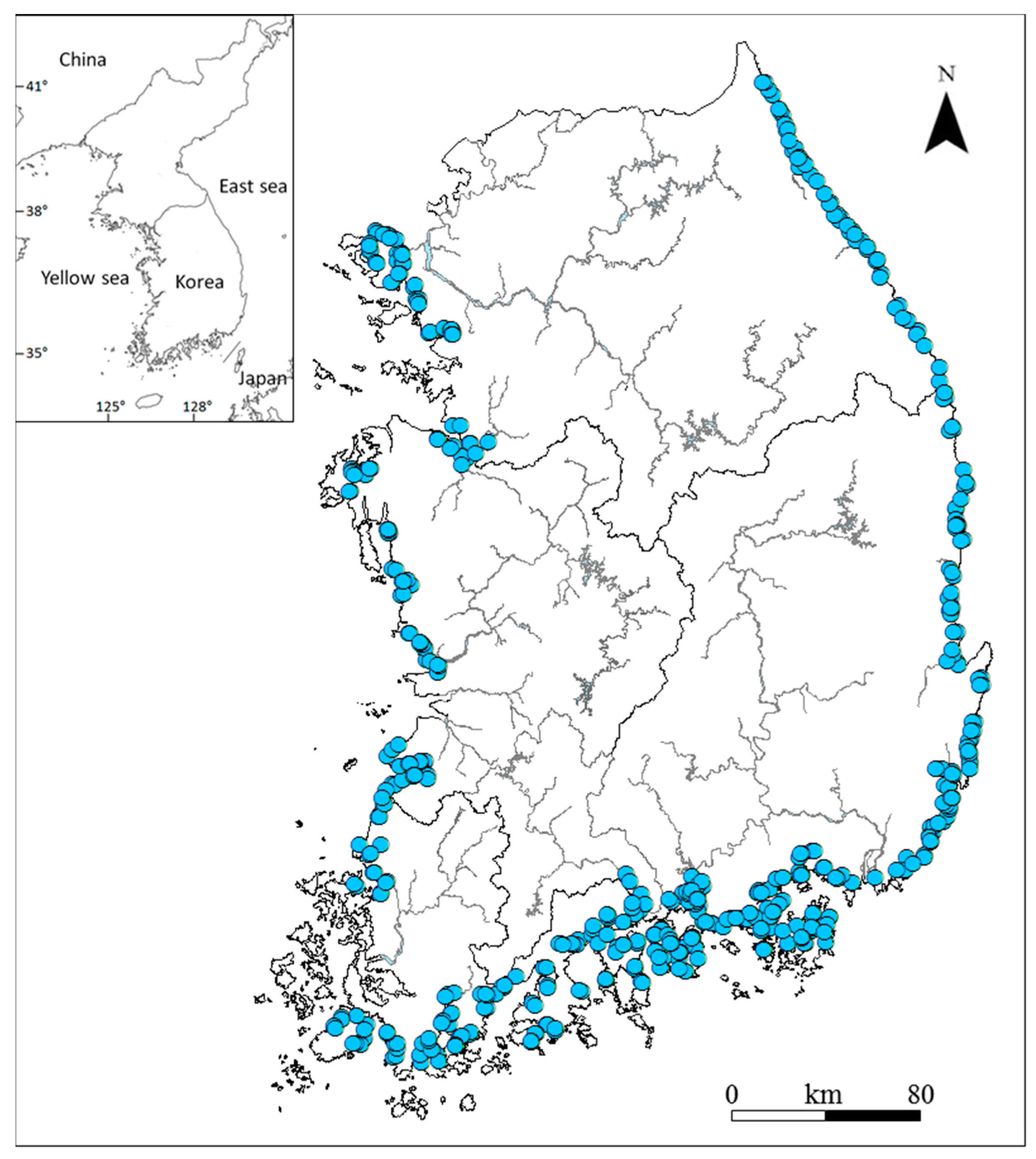
2.1. Study Area
2.2. Data Collection
2.2.1. Water Quality
2.2.2. Epilithic Diatom Community
2.3. Data Analysis
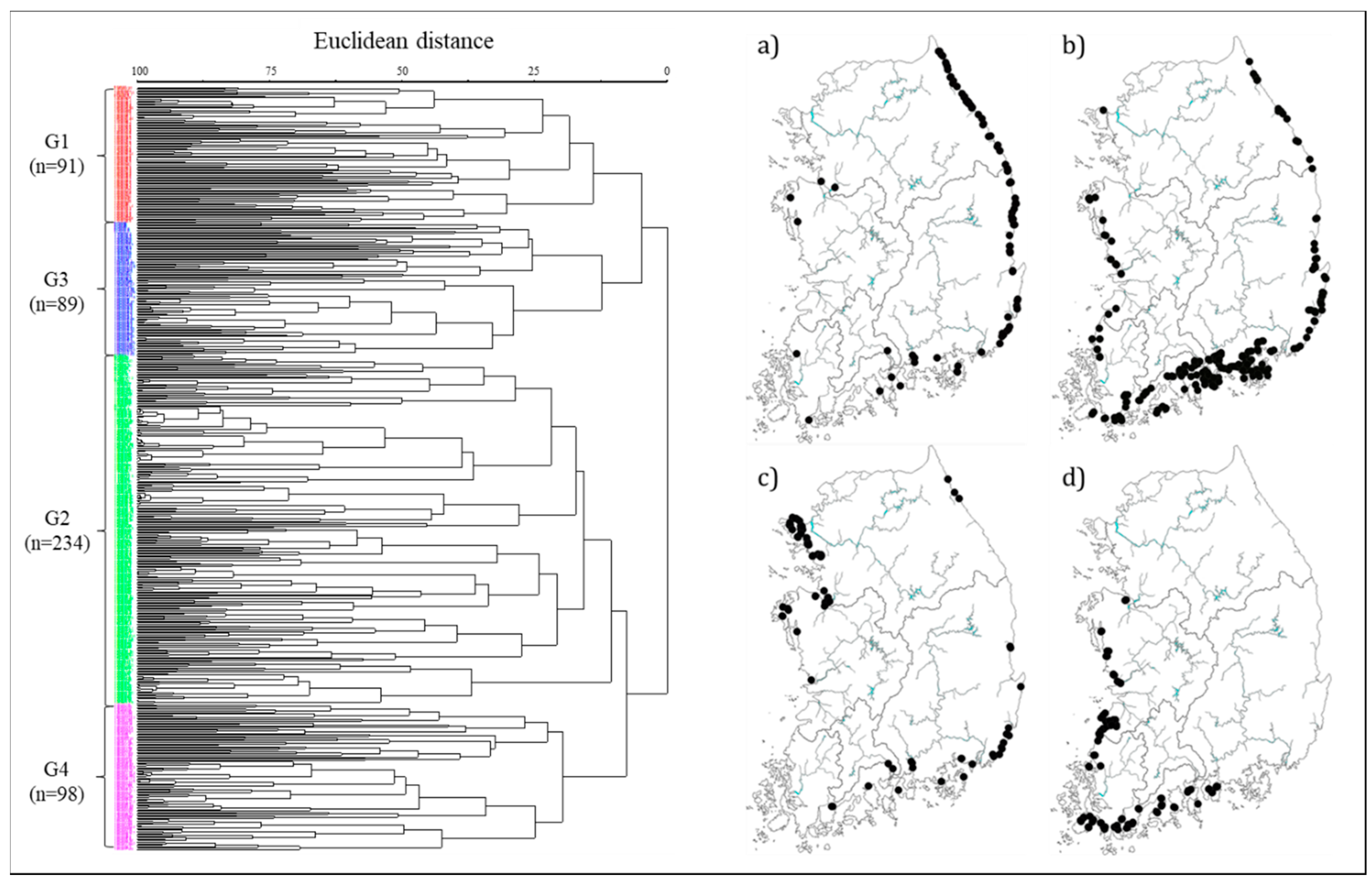
2.3.1. Cluster Analysis
2.3.2. Indicator Species Analysis
2.3.3. Random Forest
2.4. Biological Integrity Assessment
3. Results
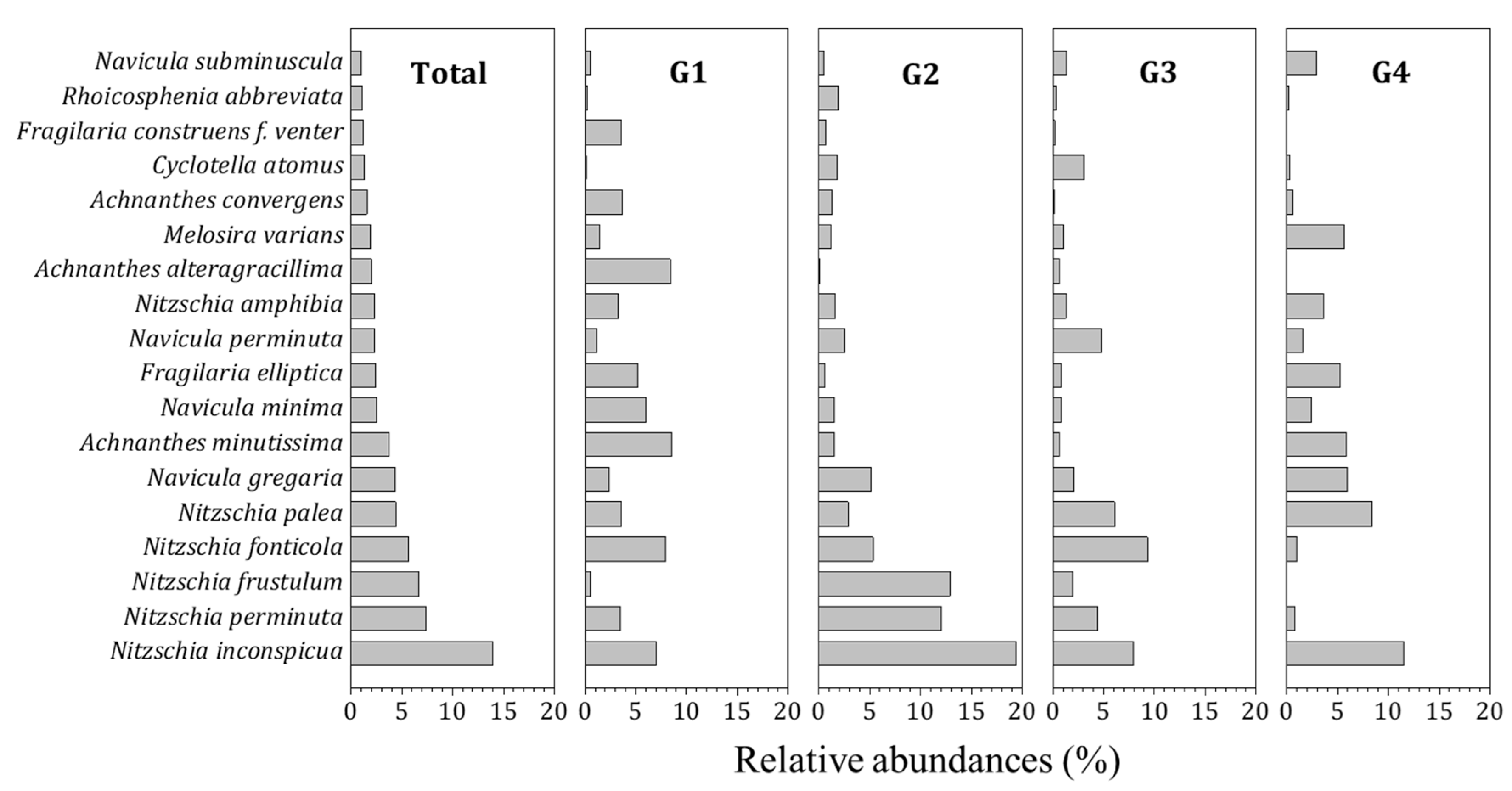
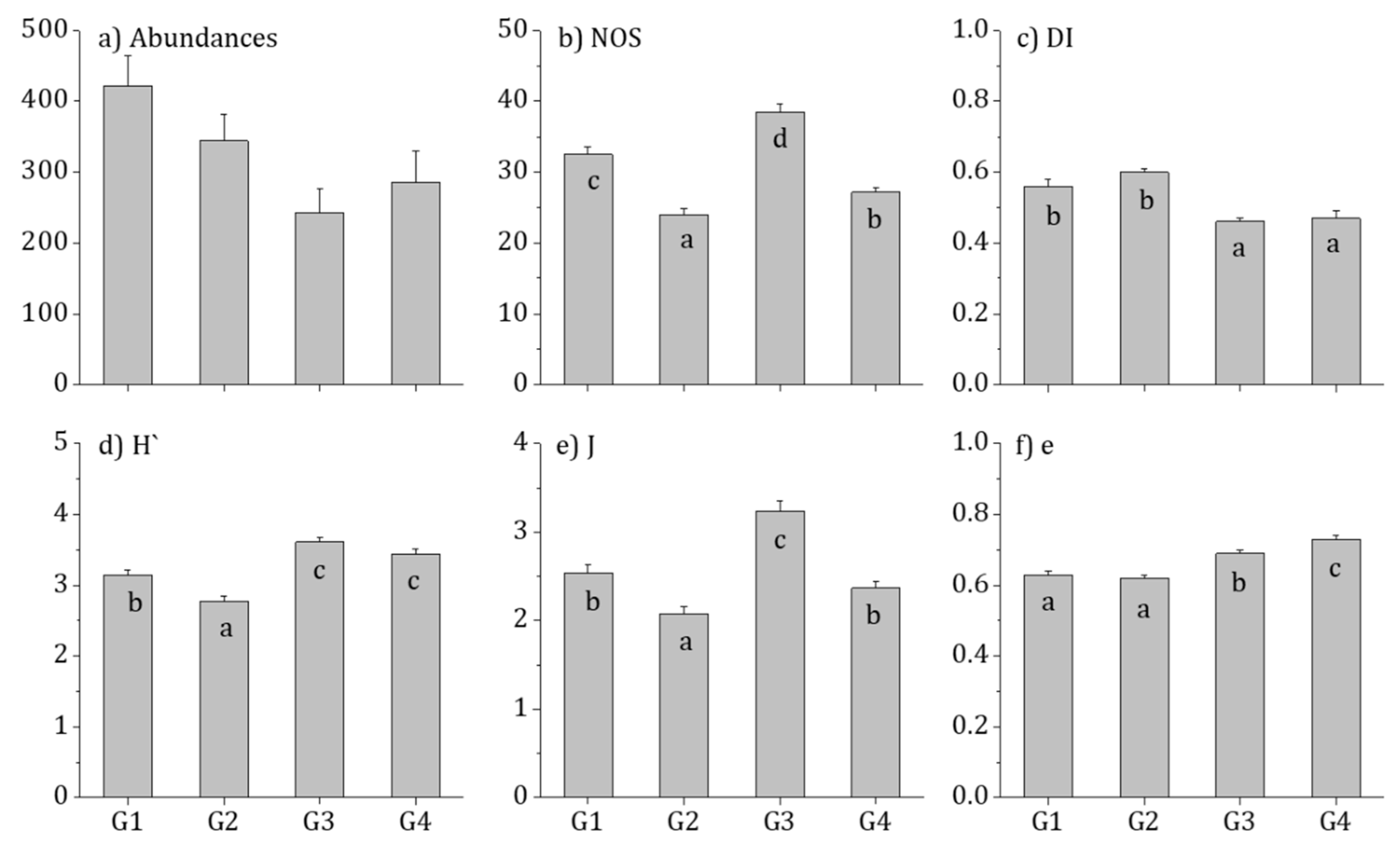
3.1. Diatom Distribution and Community Characteristics
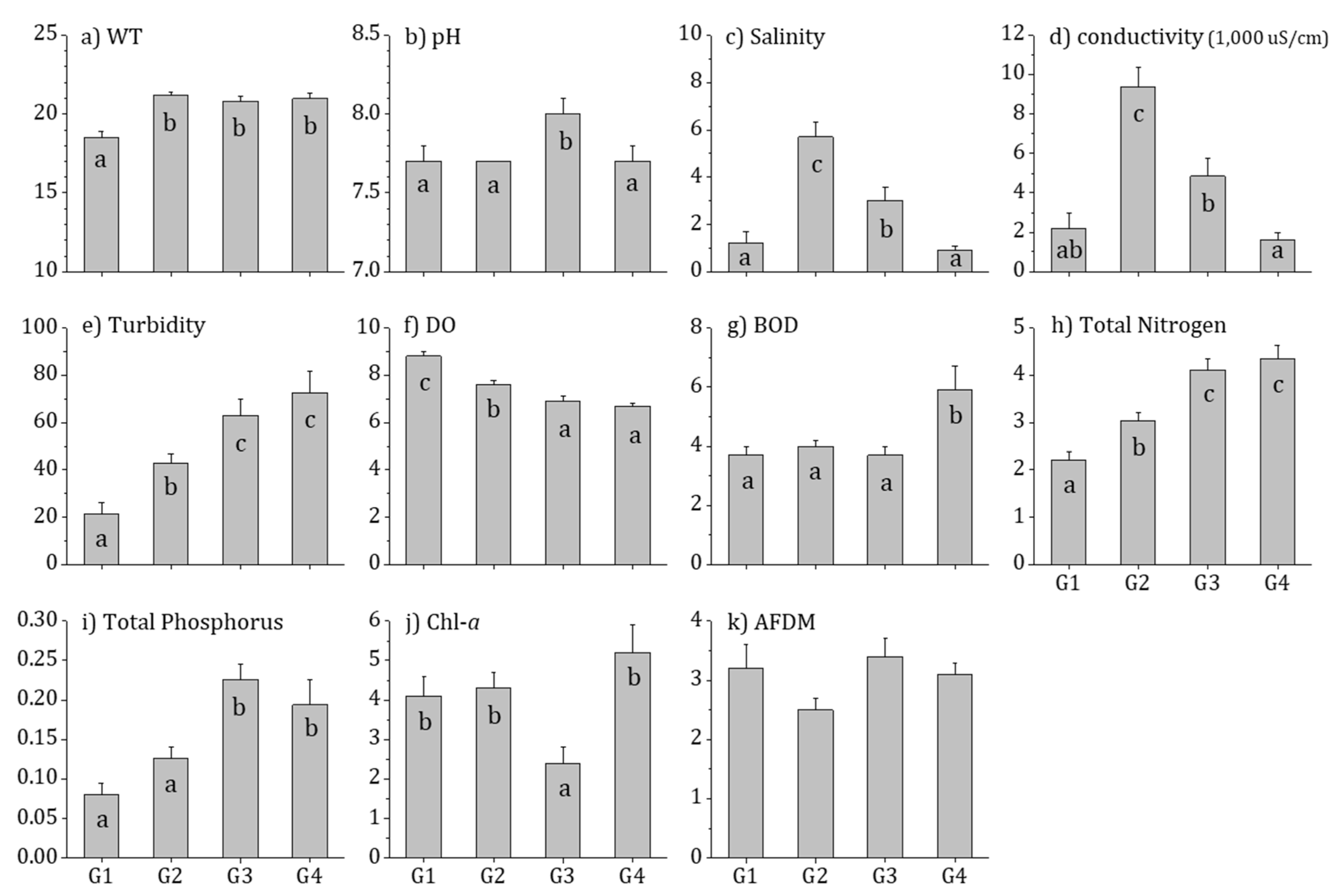
3.2. Physiochemical Water Quality
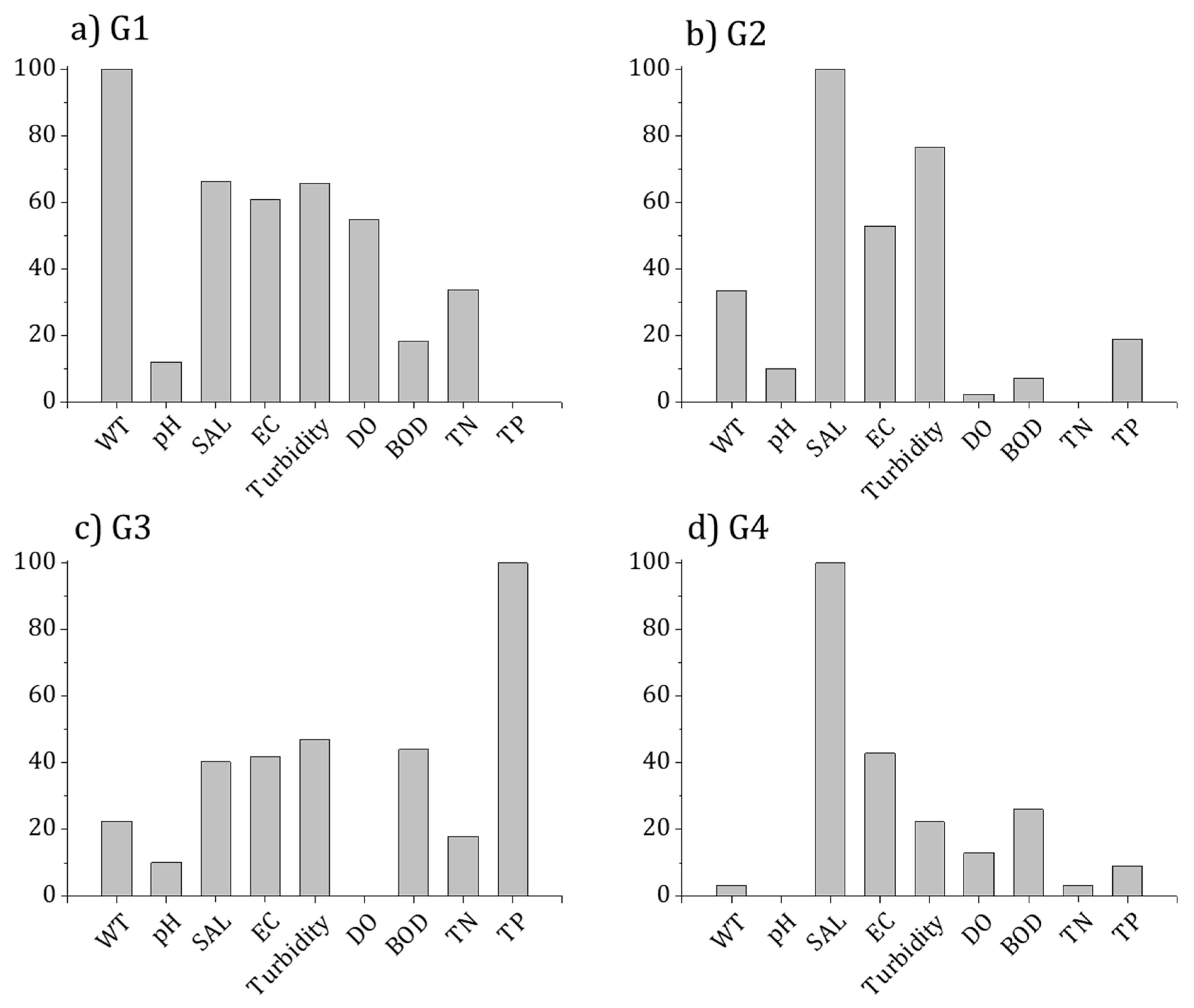
3.3. Relationship between Diatom Distribution and the Environment
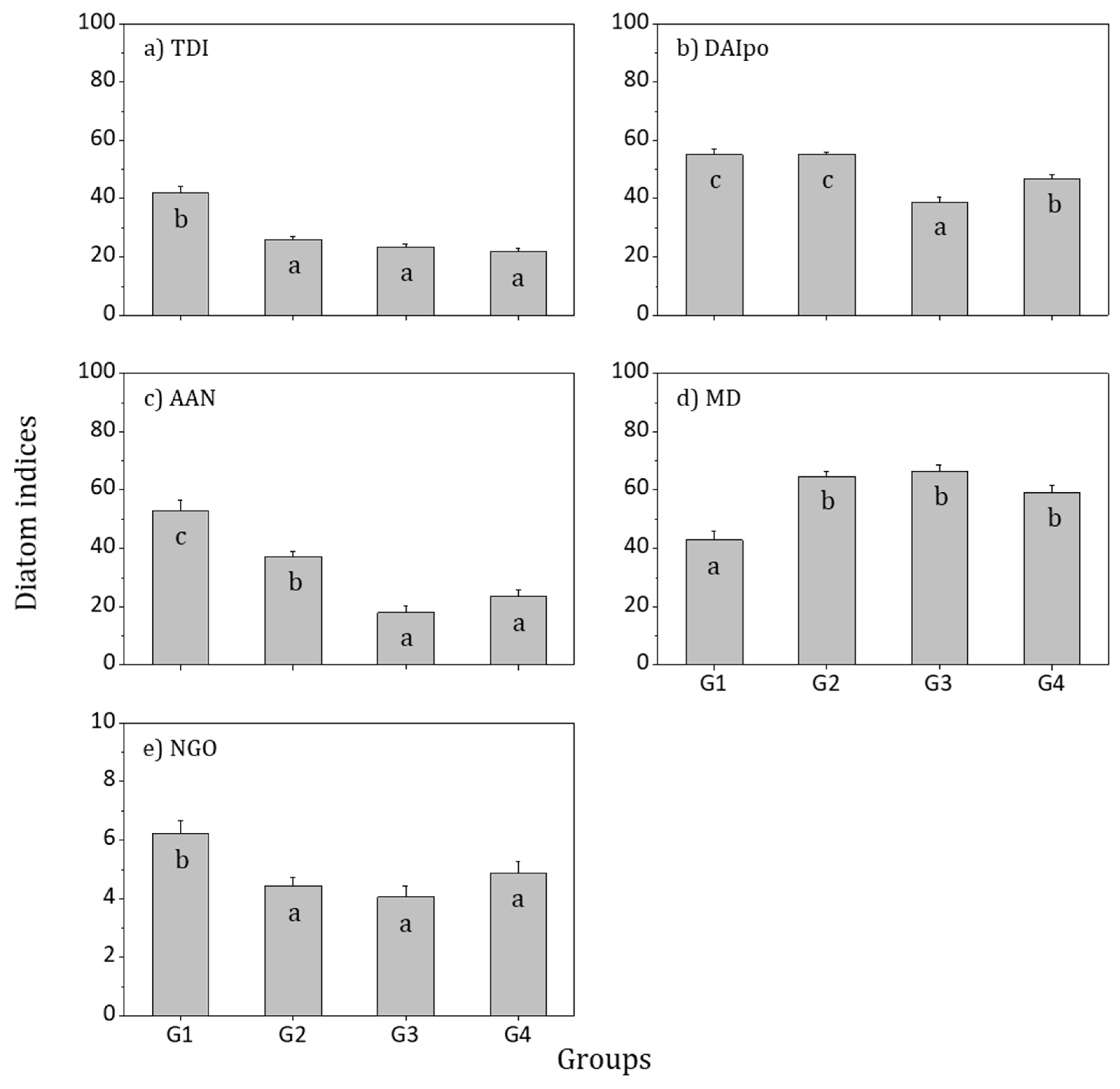
3.4. Biological Integrity and Water Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Westen, C.J.; Scheele, R.J. Characteristics of Estuaries. In Planning Estuaries; Springer: New York, NY, USA, 1996; pp. 9–60. [Google Scholar]
- Flemer, D.A.; Champ, M.A. What is the future fate of estuaries given nutrient over-enrichment, freshwater diversion and low flows? Mar. Pollut. Bull. 2006, 52, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.M.; Eça, G.F.; Felix, D.F.; Santos, D.F.; Guimarães, A.G.; Lima, M.C.; de Souza, M.F.L. Dissolved inorganic nutrients and chlorophyll a in an estuary receiving sewage treatment plant effluents: Cachoeira River estuary (NE Brazil). Environ. Monit. Assess. 2013, 185, 5387–5399. [Google Scholar] [CrossRef] [PubMed]
- Rho, P.H.; Lee, C.H. Spatial Distribution and Temporal Variation of Estuarine Wetlands by Estuary Type. J. Korean Geogr. Soc. 2014, 49, 321–338. [Google Scholar]
- Ohrel, R.L.; Register, K.M. (Eds.) Voluntary Estuary Monitoring Manual; Environmental Protection Agency: Washington, DC, USA, 2006. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/2007_04_09_estuaries_monitoruments_manual.pdf (accessed on 26 July 2019).
- Lee, K.H.; Rho, B.H.; Cho, H.J.; Lee, C.H. Estuary Classification Based on the Characteristics of Geomorphological Features, Natural Habitat Distributions and Land Uses. J. Korean Soc. Oceanogr. 2011, 16, 53–69. [Google Scholar]
- Cloern, J.E.; Powell, T.M.; Huzzey, L.M. Spatial and temporal variability in South San-Francisco Bay (USA). Temporal changes in salinity, suspended sediments, and phytoplankton biomass and productivity over tidal time scales. Estuar. Coast. Shelf Sci. 1989, 28, 599–613. [Google Scholar] [CrossRef]
- Webster, I.T.; Parslow, J.S.; Smith, S.V. Implications of spatial and temporal variation for biogeochemical budgets of estuaries. Estuarine 2000, 23, 341–350. [Google Scholar] [CrossRef]
- Rovira, L.; Trobajo, R.; Ibáñez, C. Periphytic diatom community in a Mediterranean salt wedge estuary: The Ebro Estuary (NE Iberian Peninsula). Acta Bot. Croat. 2009, 68, 285–300. [Google Scholar]
- Smol, J.P.; Stoermer, E.F. The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; University Press: Cambridge, UK, 2010. [Google Scholar]
- Meleder, V.; Rincé, Y.; Barillé, L.; Gaudin, P.; Rosa, P. Spatiotemporal changes in microphytobenthos assemblages in a macrotidal flat (Bourgneuf Bay, France). J. Phycol. 2007, 43, 1177–1190. [Google Scholar] [CrossRef]
- Biggs, B.J.; Goring, D.G.; Nikora, V.I. Subsidy and stress responses of stream periphyton to gradients in water velocity as a function of community growth form. J. Phycol. 1998, 34, 598–607. [Google Scholar] [CrossRef]
- Ewe, S.M.L.; Gaiser, E.E.; Childers, D.L.; Rivera-Monroy, V.H.; Iwaniec, D.; Fourquerean, J.; Twilley, R.R. Spatial and temporal patterns of aboveground net primary productivity (ANPP) in the Florida Coastal Everglades LTER (2001–2004). Hydrobiologia 2006, 569, 459–474. [Google Scholar] [CrossRef]
- Evelyn, G. Periphyton as an indicator of restoration in the Florida Everglades. J. Ecol. Indic. 2009, 9, 37–45. [Google Scholar]
- Lamberti, G.A. Grazing experiments in artificial streams. J. N. Am. Benthol. Soc. 1993, 12, 337–343. [Google Scholar]
- Leland, H.V.; Porter, S.D. Distribution of benthic algae in the upper Illinois River basin in relation to geology and land use. Freshw. Biol. 2000, 44, 279–301. [Google Scholar] [CrossRef]
- McCormick, P.V.; Stevenson, R.J. Periphyton as a tool for ecological assessment and management in the Florida Everglades. J. Phycol. 1998, 34, 726–733. [Google Scholar] [CrossRef]
- Maarten, D.J.; Vijverb, B.V.; Blusta, R.; Bervoets, L. Responses of aquatic organisms to metal pollution in a lowland river in Flanders: A comparison of diatoms and macroinvertebrates. Sci. Total Environ. 2008, 407, 615–629. [Google Scholar]
- Kim, N.; Thomas, I.; Murphy, P. Assessment of eutrophication and phytoplankton community impairment in the Buffalo River area of concern. J. Great Lakes Res. 2009, 35, 83–93. [Google Scholar]
- Maria, J.F.; Almeida, S.F.P.; Craveiro, S.C.; Calado, A.J. A comparison between biotic indices and predictive models in stream water quality assessment based on benthic diatom communities. J. Ecol. Indic. 2009, 9, 497–507. [Google Scholar]
- Watanabe, T.; Asai, K.; Houki, A. Numerical estimation of organic pollution of flowing water by using the epilithic diatom assemblage—Diatom Assemblage Index (DAIpo). Sci. Total Environ. 1986, 55, 209–218. [Google Scholar] [CrossRef]
- Coste, M.; Boutry, S.; Tison-Rosebery, J.; Delmas, F. Improvements of the Biological Diatom Index (BDI): Description and efficiency of the new version (BDI-2006). J. Ecol. Indic. 2009, 9, 621–650. [Google Scholar] [CrossRef]
- Kelly, M.G.; Adam, C.; Graves, A.C.; Jamieson, J.; Krokowski, J.; Lycett, E.B.; Murray-Bligh, J.; Pritchard, S.; Wilkins, C. The Trophic Diatom Index: A User’s Manual, R&D Technical Report E2/TR2, Revised ed.; Environment Agency: Bristol, UK, 2001.
- Cemagref. Etude des Methodes Biologiques Quantitative D’Appreciation de la Qualite des Eaux; Rapport Q.E. Lyon- A.F. Bassin; Rhône-Méditerranée-Corse: Lyon, France, 1982; p. 218. [Google Scholar]
- Stevenson, R.J.; Smol, J.P. Use of Algae in Environmental Assessments. In Freshwater Algae in North America: Classification and Ecology; Academic Press: New York, NY, USA, 2003. [Google Scholar]
- The Ministry of Environment/National Institute of Environmental Research (MOE/NIER). Nationwide Aquatic Ecological Monitoring Program; MOE/NIER: Incheon, Korea, 2008–2018.
- Bogaczewicz-Adamczak, B.; Dziengo, M. Using benthic diatom communities and diatom indices to assess water pollution in the Puck Bay (southern Baltic Sea) littoral zone. Oceanol. Hydrobiol. Stud. 2003, 32, 131–157. [Google Scholar]
- Zgrundo, A.; Bogaczewicz-Adamczak, B. Applicability of diatom indices for monitoring water quality in coastal streams in the Gulf of Gdańsk region northern Poland. Oceanol. Hydrobiol. Stud. 2004, 33, 31–46. [Google Scholar]
- Della Bella, V.; Puccinelli, C.; Marcheggiani, S.; Mancini, L. Benthic diatom communities and their relationship to water chemistry in wetlands of central Italy. Annales De Limnologie. Int. J. Limnol. 2007, 43, 89–99. [Google Scholar] [CrossRef][Green Version]
- Kim, H.K.; Kim, Y.J.; Won, D.H.; Hwang, S.J.; Hwang, S.O.; Kim, B.H. Spatial and temporal distribution of epilithic diatom communities in major harbors of Korean Peninsula. J. Korean Soc. Water Environ. 2013, 29, 598–609. [Google Scholar]
- Kim, H.K.; Kwon, Y.S.; Kim, Y.J.; Kim, B.H. Distribution of epilithic diatoms in estuaries of the Korean Peninsula in relation to environmental variables. Water 2015, 7, 6702–6718. [Google Scholar] [CrossRef]
- Yoon, K.T.; Park, H.S.; Chang, M. Implication to ecosystem assessment from distribution pattern of subtidal macrobenthic communities in Nakdong River Estuary. J. Korean Soc. Oceanogr. 2011, 16, 246–253. [Google Scholar]
- Kim, C.H.; Kang, E.J.; Yang, H.; Kim, K.S.; Chol, W.S. Characteristics of fish fauna collected from Near Estuary of Seomjin River and population ecology. Korean J. Environ. Biol. 2012, 30, 319–327. [Google Scholar] [CrossRef]
- Lee, Y.K.; Ahn, K.H. Actual vegetation and vegetation structure at the coastal sand bars in the Nakdong Estuary, South Korea. Korean J. Environ. Ecol. 2012, 26, 911–922. [Google Scholar]
- Shin, Y.K. An ecological study of phytoplankton community in the Geum River Estuary. Korean J. Ecol. Environ. 2013, 46, 524–540. [Google Scholar]
- The Ministry of Environment/National Institute of Environmental Research (MOE/NIER). Survey and Evaluation of Aquatic Ecosystem Health in Korea; MOE/NIER: Incheon, Korea, 2008.
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Waste Water; APHA: Washington, DC, USA, 2001. [Google Scholar]
- Hendey, N.I. The permanganate method for cleaning freshly gathered diatoms. Microscopy 1974, 32, 423–426. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süsβwasserflora von Mitteleuropa, Band 2/1: Bacillariophyceae 1. Teil: Naviculaceae; Ettl, H., Gerloff, J., Heying, H., Mollenhauer, D., Eds.; Elsevier Book Co.: Berlin, Germany, 2007. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süsβwasserflora von Mitteleuropa, Band 2/1: Bacillariophyceae 1. Teil: Basillariaceae, Epithemiaceae, Surirellaceae; Ettl, H., Gerloff, J., Heying, H., Mollenhauer, D., Eds.; Elsevier Book Co.: Berlin, Germany, 2007. [Google Scholar]
- Simonsen, R. The diatom system: Ideas on phylogeny. Bacillaria 1979, 2, 9–71. [Google Scholar]
- McNaughton, S.J. Relationships among functional properties of Californian grassland. Nature 1967, 216, 168–169. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champagne, IL, USA, 1959. [Google Scholar]
- Margalef, R. Information Theory in Ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Pielou, E.C. Ecological Diversity; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Dufrene, M.; Legendre, P. Species assemblages and indicator species: The need for flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Peterson, W.T.; Keister, J.E. Interannual variability in copepod community composition at a coastal station in the northern California Current: A multivariate approach. Deep Sea Res. Part. II Top. Stud. Oceanogr. 2003, 50, 2499–2517. [Google Scholar] [CrossRef]
- Keister, J.E.; Peterson, W.T. Zonal and seasonal variations in zooplankton community structure off the central Oregon coast, 1998–2000. Prog. Oceanogr. 2003, 57, 341–361. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Robnik-Šikonja, M. Improving random forests. In Machine Learning: ECML 2004; Springer: Berlin/Heidelberg, Germany, 2004; pp. 359–370. [Google Scholar]
- The Ministry of Environment/National Institute of Environmental Research (MOE/NIER). Biomonitoring Survey and Assessment Manual; MOE/NIER: Incheon, Korea, 2017.
- Wang, Y.K.; Stevenson, R.J.; Metzmeier, L. Development and evaluation of a diatom-based Index of Biotic Integrity for the Interior Plateau Ecoregion, USA. J. N. Am. Benthol. Soc. 2005, 24, 990–1008. [Google Scholar] [CrossRef]
- Passy, S.I. Spatial paradigms of lotic diatom distribution: A landscape ecology perspective. J. Phycol. 2001, 37, 370–378. [Google Scholar] [CrossRef]
- Hill, B.H.; Herlihy, A.T.; Kaufmann, P.R.; DeCelles, S.J.; Vander Borgh, M.A. Assessment of streams of the eastern United States using a periphyton index of biotic integrity. Ecol. Indic. 2003, 2, 325–338. [Google Scholar] [CrossRef]
- Rovira, L.; Trobajo, R.; Leira, M.; Ibáñez, C. The effects of hydrological dynamics on benthic diatom community structure in a highly stratified estuary: The case of the Ebro Estuary (Catalonia, Spain). Estuar. Coast. Shelf Sci. 2012, 101, 1–14. [Google Scholar] [CrossRef]
- Kovács, C.; Kahlert, M.; Padisák, J. Benthic diatom communities along pH and TP gradients in Hungarian and Swedish streams. J. Appl. Phycol. 2006, 18, 105–117. [Google Scholar] [CrossRef]
- Potapova, M.G.; Charles, D.F. Benthic diatoms in USA rivers: Distributions along spatial and environmental gradients. J. Biogeogr. 2002, 29, 167–187. [Google Scholar] [CrossRef]
- Licursi, M.; Sierra, M.V.; Gómez, N. Diatom assemblages from a turbid coastal plain estuary: Río de la Plata (South America). J. Mar. Syst. 2006, 62, 35–45. [Google Scholar] [CrossRef]
- Underwood, G.; Phillips, J.; Saunders, K. Distribution of estuarine benthic diatom species along salinity and nutrient gradients. Eur. J. Phycol. 1998, 33, 173–183. [Google Scholar] [CrossRef]
- Tong, S.T.; Chen, W. Modeling the relationship between land use and surface water quality. J. Environ. Manag. 2002, 66, 377–393. [Google Scholar] [CrossRef]
- Lee, S.W.; Hwang, S.J. Investigation on the relationship between land use and water quality with spatial dimension, reservoir type and shape complexity. J. Korean Inst. Landsc. Archit. 2007, 34, 1–9. [Google Scholar]
- Hwang, S.I.; Yoon, S.O. Geomorphic characteristics of coastal lagoons and river basins, and sedimentary environment at river mouths along the Middle East Coast in the Korean Peninsula. J. Korean Geomorphol. Assoc. 2008, 15, 17–33. [Google Scholar]
- Ha, K.H. Geomorphological Characteristics and Salinity Distribution of Natural Estuaries in the East Sea (Yeongokcheon Stream, Gangneung-si and Namdea Stream, Yangyang-Gun); Seoul National University: Seoul, Korea, 2009. [Google Scholar]
- Allan, J.D.; Erickson, D.L.; Fay, J. The influence of catchment land use on stream integrity across multiple spatial scales. Freshw. Biol. 1997, 37, 149–161. [Google Scholar] [CrossRef]
- Johnson, L.B.; Richards, C.; Host, G.E.; Arthur, J.W. Landscape influences on water chemistry in midwestern stream ecosystems. Freshw. Biol. 1997, 37, 193–208. [Google Scholar] [CrossRef]
- Azim, M.E.; Milstein, A.; Wahab, M.A.; Verdegam, M.C.J. Periphyton-water quality relationships in fertilized fishponds with artificial substrates. Aquaculture 2003, 228, 169–187. [Google Scholar] [CrossRef]
- Park, C.G.; Kang, M.A. Impact assessment of turbidity water caused clays on algae growth. J. Eng. Geol. 2006, 16, 403–409. [Google Scholar]
- Watanabe, T.; Ohtsuka, T.; Tuji, A.; Houki, A. Picture Book and Ecology of the Freshwater Diatoms; Uchida-Rokakuho: Tokyo, Japan, 2005. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 4. Teil: Achnanthaceae, Kritische Erganzungen zu Navicula (Lineolate) und Gomphonema Gesammliteraturverzeichnis; Gustav Fischer Verlag: Jena, Germany, 1991. [Google Scholar]
- Kobayasi, H.; Mayama, S. Evaluation of river water quality by diatoms. Korean J. Phycol. 1989, 4, 121–133. [Google Scholar]
- Lange-Bertalot, H. Navicula sensu stricto 10 genera separated from Navicula sensu lato Frustulia. In Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats; Gantner Verlag: Ruggell, Germany, 2001; Volume 2, p. 526. [Google Scholar]
- Potapova, M.; Charles, D.F. Distribution of benthic diatoms in US rivers in relation to conductivity and ionic composition. Freshw. Biol. 2003, 48, 1311–1328. [Google Scholar] [CrossRef]
- Soininen, J.; Paavola, R.; Muotka, T. Benthic diatom communities in boreal streams: Community structure in relation to environmental and spatial gradients. Ecography 2004, 27, 330–342. [Google Scholar] [CrossRef]
- Kolbe, R.W. Zur Okologie, Morphologie und Systematik der Brackwasser-Diatomeen. Pflanzenforschung 1927, 7, 1–146. [Google Scholar]
- Kolbe, R.W. Grundlinien einer allgemeinen Ökologie der Diatomeen. Ergeb. Biol. 1932, 8, 221–348. [Google Scholar]
- Hustedt, F. Die Diatomeenflora des Fluss-systems der Weser in Gebiet der Hansestadt Bremen. Abh. Nat. Ver. Brem. 1957, 34, 181–440. [Google Scholar]
- Cleave, M.L.; Porcella, D.B.; Adams, V.D. The application of batch bioassay techniques to the study of salinity toxicity to freshwater phytoplankton. Water Res. 1981, 15, 573–584. [Google Scholar] [CrossRef]
- Tuchman, M.L.; Theriot, E.; Stoermer, E.F. Effects of low-level salinity concentrations on the growth of Cyclotella meneghiniana Kütz (Bacillariophyta). Arch. Protistenkd. 1984, 128, 319–326. [Google Scholar] [CrossRef]
- Admiraal, W.; Riaux-Gobin, C.; Laane, R.W. Interactions of ammonium, nitrate, and D-and L-amino acids in the nitrogen assimilation of two species of estuarine benthic diatoms. Mar. Ecol. Prog. Ser. 1987, 40, 267–273. [Google Scholar] [CrossRef]
- Rovira, L.; Trobajo, R.; Ibáñez, C. The use of diatom assemblages as ecological indicators in highly stratified estuaries and evaluation of existing diatom indices. Mar. Pollut. Bull. 2012, 64, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Leira, M.; Sabater, S. Diatom assemblages distribution in Catalan Rivers, NE Spain, in relation to chemical and physiographical factors. Water Res. 2005, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from The Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar]
- Rimet, F. Benthic diatom assemblages and their correspondence with ecoregional classifications: Case study of rivers in north-eastern France. Hydrobiologia 2009, 636, 137–151. [Google Scholar] [CrossRef]
- Underwood, G.J.; Provot, L. Determining the environmental preferences of four estuarine epipelic diatom taxa: Growth across a range of salinity, nitrate and ammonium conditions. Eur. J. Phycol. 2000, 35, 173–182. [Google Scholar] [CrossRef]
| Locations | Open Stream * | Closed Stream ** | Total |
|---|---|---|---|
| East sea | 124 | 11 | 135 |
| South sea | 149 | 81 | 230 |
| West sea | 23 | 124 | 147 |
| Locations | Dominant Species (%) | Subdominant Species (%) | No. Species |
|---|---|---|---|
| East sea | Nitzschia inconspicua (8.8) | Nitzschia fonticola (8.3) | 354 |
| South sea | Nitzschia inconspicua (18.9) | Nitzschia perminuta (11.2) | 457 |
| West sea | Nitzschia inconspicua (10.2) | Nitzschia palea (6.8) | 346 |
| Total | Nitzschia inconspicua (13.9) | Nitzschia perminuta (7.4) | 566 |
| Indicator Species | CODE | G1 | G2 | G3 | G4 | p |
|---|---|---|---|---|---|---|
| Cymbella silesiaca | CYSI | 39 | 5 | 5 | 2 | <0.001 |
| Fragilaria rumpens var. fragilarioides | FRRV | 30 | 0 | 5 | 0 | <0.001 |
| Fragilaria capucina var. gracilis | FRVG | 28 | 0 | 5 | 0 | <0.001 |
| Fragilaria construens f. venter | FRCO | 27 | 4 | 0 | 0 | <0.001 |
| Reimeria sinuata | RESI | 27 | 1 | 2 | 0 | <0.001 |
| Stephanodiscus invisitatus | STIN | 0 | 0 | 46 | 0 | <0.001 |
| Cyclotella atomus | CYAT | 0 | 1 | 39 | 6 | <0.001 |
| Stephanodiscus hantzschii | STHA | 0 | 0 | 38 | 3 | <0.001 |
| Nitzschia constricta | NICN | 0 | 1 | 35 | 0 | <0.001 |
| Navicula atomus | NAAT | 1 | 0 | 34 | 0 | <0.001 |
| Navicula veneta | NAVE | 5 | 0 | 33 | 2 | <0.001 |
| Navicula halophila | NAHA | 1 | 0 | 32 | 0 | <0.001 |
| Navicula accomoda | NAAC | 1 | 1 | 25 | 1 | <0.001 |
| Bacillaria paradoxa | BAPA | 1 | 2 | 6 | 37 | <0.001 |
| Navicula capitata | NACA | 0 | 0 | 3 | 30 | <0.001 |
| Nitzschia calida | NICA | 0 | 1 | 1 | 25 | <0.001 |
| CODE | WT | pH | SAL | EC | TURB | DO | BOD | TN | TP | CHL | AFDM | G |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYSI | −0.306 ** | −0.130 ** | −0.212 ** | −0.270 ** | −0.319 ** | 0.274 ** | −0.137 ** | −0.225 ** | −0.194 ** | 0.064 | −0.160 ** | 1 |
| FRRV | −0.225 ** | −0.021 | −0.175 ** | −0.185 ** | −0.071 | 0.162 ** | −0.039 | −0.018 | −0.035 | −0.061 | −0.054 | 1 |
| FRVG | −0.217 ** | −0.013 | −0.196 ** | −0.235 ** | −0.097 * | 0.159 ** | −0.016 | −0.022 | −0.084 | −0.088 * | −0.105 * | 1 |
| FRCO | −0.149 ** | −0.074 | −0.106 * | −0.056 | −0.164 ** | 0.199 ** | −0.031 | −0.152 ** | −0.078 | 0.074 | 0.127 ** | 1 |
| RESI | −0.106 * | −0.074 | −0.143 ** | −0.210 ** | −0.145 ** | 0.183 ** | −0.130 ** | −0.114 ** | −0.124 ** | −0.007 | −0.094 * | 1 |
| STIN | 0.018 | 0.160 ** | −0.005 | 0.053 | 0.196 ** | −0.062 | −0.018 | 0.170 ** | 0.183 ** | −0.203 ** | 0.141 ** | 3 |
| CYAT | 0.130 ** | 0.211 ** | 0.019 | 0.059 | 0.319 ** | −0.226 ** | −0.068 | 0.188 ** | 0.162 ** | −0.275 ** | 0.230 ** | 3 |
| STHA | 0.009 | 0.174 ** | −0.033 | 0.025 | 0.261 ** | −0.123 ** | 0.073 | 0.208 ** | 0.198 ** | −0.214 ** | 0.164 ** | 3 |
| NICN | 0.039 | 0.055 | 0.123 ** | 0.147 ** | 0.082 | −0.005 | −0.014 | −0.032 | 0.039 | −0.086 | 0.053 | 3 |
| NAAT | −0.018 | 0.158 ** | −0.095 * | −0.049 | 0.189 ** | −0.179 ** | −0.075 | 0.187 ** | 0.217 ** | −0.202 ** | 0.157 ** | 3 |
| NAVE | −0.023 | 0.089 * | −0.157 ** | −0.082 | 0.151 ** | −0.016 | 0.042 | 0.172 ** | 0.109 * | −0.185 ** | 0.075 | 3 |
| NAHA | 0.002 | 0.088 * | 0.007 | 0.068 | 0.147 ** | −0.091 * | −0.016 | 0.126 ** | 0.158 ** | −0.187 ** | 0.147 ** | 3 |
| NAAC | −0.042 | 0.100 * | 0.025 | −0.015 | 0.154 ** | −0.021 | −0.022 | −0.018 | 0.112 * | −0.011 | 0.066 | 3 |
| BAPA | 0.087 | 0.042 | −0.024 | 0.060 | 0.137 ** | −0.042 | 0.144 ** | 0.024 | 0.005 | 0.061 | 0.093 * | 4 |
| NACA | 0.065 | 0.062 | −0.102 * | −0.074 | 0.196 ** | −0.072 | 0.135 ** | 0.107 * | 0.079 | 0.055 | 0.044 | 4 |
| NICA | 0.090 * | 0.006 | 0.058 | 0.073 | 0.106 * | −0.160 ** | 0.101 * | 0.084 | 0.074 | 0.077 | 0.070 | 4 |
| Species | Ar | AUC | Important Variables | GI | |
|---|---|---|---|---|---|
| 1st | 2nd | ||||
| Achnanthes alteragracillima Lange-Bertalot | 0.88 | 0.99 | TN (100) | TURB (94) | |
| Achnanthes brevipes Agardh | 0.88 | 0.98 | SAL (100) | EC (75) | |
| Achnanthes brevipes var. intermedia (Kützing) Cleve | 0.93 | 0.98 | EC (100) | TURB (61) | |
| Achnanthes clevei Grunow | 0.94 | 0.99 | TN (100) | EC (90) | |
| Achnanthes conspicua A. Mayer | 0.95 | 0.99 | TP (100) | TN (93) | |
| Achnanthes convergens Kobayasi, Nagumo & Mayama | 0.85 | 0.94 | TN (100) | SAL (97) | |
| Achnanthes delicatula (Kützing) Grunow | 0.83 | 0.99 | WT (100) | TP (96) | |
| Achnanthes exigua Grunow | 0.84 | 0.96 | DO (100) | TURB (99) | |
| Achnanthes hungarica (Grunow) Grunow | 0.93 | 0.99 | SAL (100) | EC (88) | |
| Achnanthes inflata (Kützing) Grunow | 0.94 | 1.00 | SAL (100) | WT (84) | |
| Achnanthes lanceolata (Brébisson) Grunow | 0.90 | 0.96 | EC (100) | TURB (97) | |
| Achnanthes laterostrata Hustedt | 0.93 | 0.99 | pH (100) | TN (92) | |
| Achnanthes minutissima Kützing | 0.86 | 0.95 | EC (100) | TP (51) | |
| Achnanthes subhudsonis Hustedt | 0.91 | 0.96 | TURB (100) | EC (95) | |
| Amphora sp. | 0.93 | 0.99 | TN (100) | EC (97) | |
| Amphora coffeaeformis (Agardh) Kützing | 0.90 | 0.97 | SAL (100) | EC (54) | |
| Amphora copulate (Kützing) Schoeman & Archibald | 0.90 | 0.98 | EC (100) | TP (65) | |
| Amphora montana Krasske | 0.93 | 0.99 | BOD (100) | TURB (78) | |
| Amphora pediculus (Kützing) Grunow | 0.90 | 0.96 | BOD (100) | TP (48) | |
| Amphora veneta Kützing | 0.92 | 0.99 | EC (100) | SAL (71) | |
| Aulacoseira alpigena (Grunow) Krammer | 0.93 | 0.99 | EC (100) | SAL (65) | |
| Aulacoseira ambigua (Grunow) Simonsen | 0.90 | 0.97 | TP (100) | pH (42) | |
| Aulacoseira granulata (Ehrenberg) Simonsen | 0.92 | 0.98 | EC (100) | pH (85) | |
| Bacillaria paradoxa Gmelin | 0.85 | 0.99 | BOD (100) | EC (92) | 4 |
| Cocconeis placentula Ehrenberg | 0.86 | 0.99 | SAL (100) | EC (76) | |
| Cocconeis placentula var. euglypta (Ehrenberg) Grunow | 0.91 | 0.97 | TN (100) | SAL (99) | |
| Cocconeis placentula var. lineata (Ehrenberg) Van Heurck | 0.88 | 0.97 | EC (100) | TURB (77) | |
| Cyclostephanos dubius (Hustedt) Round | 0.95 | 0.99 | pH (100) | EC (52) | |
| Cyclotella atomus Hustedt | 0.92 | 0.98 | TURB (100) | WT (40) | 3 |
| Cyclotella meneghiniana Kützing | 0.83 | 0.96 | TURB (100) | TP (77) | |
| Cyclotella pseudostelligera Hustedt | 0.84 | 0.98 | TP (100) | WT (80) | |
| Cyclotella stelligera (Cleve & Grunow) Van Heurck | 0.92 | 0.99 | TURB (100) | TP (58) | |
| Cymbella affinis Kützing | 0.88 | 0.97 | EC (100) | SAL (74) | |
| Cymbella minuta Hilse | 0.86 | 0.97 | EC (100) | SAL (80) | |
| Cymbella silesiaca Bleisch | 0.87 | 0.96 | EC (100) | TURB (97) | 1 |
| Cymbella tumida (Brébisson) Van Heurck | 0.91 | 0.96 | TURB (100) | TP (59) | |
| Diatoma vulgaris Bory | 0.95 | 0.98 | EC (100) | SAL (87) | |
| Diploneis oblongella (Nägeli ex Kützing) Cleve-Euler | 0.95 | 0.99 | SAL (100) | BOD (88) | |
| Diploneis subovalis Cleve | 0.87 | 0.98 | SAL (100) | EC (75) | |
| Entomoneis alata (Ehrenberg) Ehrenberg | 0.94 | 0.98 | EC (100) | SAL (99) | |
| Eunotia minor (Kützing) Grunow | 0.95 | 1.00 | EC (100) | TN (94) | |
| Fragilaria capitellata (Grunow) J.B. Petersen | 0.90 | 0.96 | WT (100) | TURB (92) | |
| Fragilaria capucina Desmazières | 0.83 | 0.95 | EC (100) | WT (59) | |
| Fragilaria capucina var. gracilis (Østrup) Hustedt | 0.90 | 0.96 | SAL (100) | TN (48) | 1 |
| Fragilaria capucina var. vaucheriae (Kützing) Lange-Bertalot | 0.98 | 0.99 | TURB (100) | TP (40) | |
| Fragilaria construens f. venter (Ehrenberg) Hustedt | 0.89 | 0.98 | SAL (100) | TURB (74) | 1 |
| Fragilaria elliptica Schumann | 0.90 | 0.97 | TURB (100) | WT (76) | |
| Fragilaria fasciculata (Agardh) Lange-Bertalot | 0.93 | 0.99 | SAL (100) | EC (83) | |
| Fragilaria parva (Grunow) A. Tuji & D.M. Williams | 0.93 | 0.99 | SAL (100) | DO (95) | |
| Fragilaria pinnata Ehrenberg | 0.86 | 0.98 | WT (100) | SAL (85) | |
| Fragilaria rumpens (Kützing) G.W.F. Carlson | 0.88 | 0.97 | SAL (100) | WT (68) | |
| Fragilaria rumpens var. familiaris (Kützing) Grunow | 0.87 | 0.97 | SAL (100) | EC (63) | |
| Fragilaria rumpens var. fragilarioides (Grunow) Cleve | 0.91 | 0.97 | WT (100) | SAL (51) | 1 |
| Frustulia vulgaris (Thwaites) De Toni | 0.94 | 0.99 | EC (100) | BOD (73) | |
| Gomphonema angustum Agardh | 0.94 | 0.98 | WT (100) | BOD (91) | |
| Gomphonema clevei Fricke | 0.87 | 0.96 | EC (100) | DO (25) | |
| Gomphonema lagenula Kützing | 0.82 | 0.98 | EC (100) | SAL (72) | |
| Gomphonema minutum (Agardh) Agardh | 0.94 | 0.99 | WT (100) | TP (78) | |
| Gomphonema parvulum (Kützing) Kützing | 0.86 | 0.98 | TN (100) | SAL (66) | |
| Gomphonema pseudoaugur Lange-Bertalot | 0.88 | 0.97 | TP (100) | SAL (99) | |
| Gomphonema quadripunctatum (Østrup) Wislouch | 0.92 | 0.96 | pH (100) | BOD (81) | |
| Gomphonema truncatum Ehrenberg | 0.94 | 0.99 | SAL (100) | EC (86) | |
| Gyrosigma acuminatum (Kützing) Rabenhorst | 0.91 | 0.99 | EC (100) | DO (84) | |
| Hantzschia amphioxys (Ehrenberg) Grunow | 0.91 | 0.98 | SAL (100) | EC (68) | |
| Melosira nummuloides Agardh | 0.94 | 0.99 | TURB (100) | TP (69) | |
| Melosira varians Agardh | 0.91 | 0.98 | EC (100) | TP (92) | |
| Meridion circulare var. constrictum (Ralfs) Van Heurck | 0.92 | 0.98 | EC (100) | BOD (81) | |
| Navicula accomoda Hustedt | 0.88 | 0.98 | TURB (100) | WT (71) | 3 |
| Navicula atomus (Kützing) Grunow | 0.96 | 0.99 | SAL (100) | BOD (71) | 3 |
| Navicula atomus var. permitis (Hustedt) Lange-Bertalot | 0.98 | 1.00 | TURB (100) | SAL (99) | |
| Navicula bacillum Ehrenberg | 0.95 | 0.99 | pH (100) | SAL (67) | |
| Navicula capitate (Ehrenberg) R. Ross | 0.87 | 0.97 | SAL (100) | DO (97) | 4 |
| Navicula capitatoradiata H. Germain | 0.92 | 0.97 | SAL (100) | EC (84) | |
| Navicula cincta (Ehrenberg) Ralfs | 0.91 | 1.00 | SAL (100) | EC (93) | |
| Navicula clementis Grunow | 0.93 | 0.98 | TN (100) | TP (58) | |
| Navicula contenta Grunow | 0.90 | 0.98 | SAL (100) | EC (74) | |
| Navicula cryptocephala Kützing | 0.87 | 0.96 | EC (100) | SAL (61) | |
| Navicula cryptotenella Lange-Bertalot | 0.83 | 0.97 | EC (100) | WT (70) | |
| Navicula decussis Østrup | 0.91 | 0.97 | EC (100) | SAL (48) | |
| Navicula goeppertiana (Bleisch) H.L. Smith | 0.88 | 0.98 | EC (100) | TURB (74) | |
| Navicula gregaria Donkin | 0.82 | 0.97 | SAL (100) | EC (77) | |
| Navicula halophile (Grunow) Cleve | 0.95 | 0.98 | EC (100) | SAL (91) | 3 |
| Navicula menisculus Schumann | 0.93 | 1.00 | EC (100) | SAL (86) | |
| Navicula minima Grunow | 0.88 | 0.96 | EC (100) | SAL (71) | |
| Navicula minuscula Grunow | 0.88 | 0.98 | TP (100) | SAL (98) | |
| Navicula mutica (Kützing) Frenguelli | 0.92 | 0.99 | EC (100) | SAL (84) | |
| Navicula mutica var. ventricosa (Kützing) Cleve & Grunow | 0.92 | 0.99 | SAL (100) | EC (54) | |
| Navicula peregrine (Ehrenberg) Kützing | 0.95 | 1.00 | EC (100) | SAL (87) | |
| Navicula perminuta Grunow | 0.92 | 0.98 | WT (100) | pH (40) | |
| Navicula phyllepta Kützing | 0.94 | 0.99 | WT (100) | BOD (87) | |
| Navicula pupula Kützing | 0.83 | 0.97 | TN (100) | SAL (84) | |
| Navicula radiosa Kützing | 0.89 | 0.98 | TN (100) | pH (54) | |
| Navicula recens (Lange-Bertalot) Lange-Bertalot | 0.84 | 0.98 | EC (100) | SAL (87) | |
| Navicula rhynchocephala Kützing | 0.91 | 1.00 | BOD (100) | EC (66) | |
| Navicula salinarum Grunow | 0.86 | 0.97 | TURB (100) | TP (73) | |
| Navicula saprophila Lange-Bertalot & Bonik | 0.90 | 0.98 | TP (100) | WT (88) | |
| Navicula schroeteri F. Meister | 0.89 | 0.98 | TN (100) | SAL (99) | |
| Navicula seminuloides Hustedt | 0.95 | 0.99 | TP (100) | pH (60) | |
| Navicula seminulum Grunow | 0.93 | 0.97 | EC (100) | SAL (83) | |
| Navicula subatomoides Hustedt | 0.92 | 0.98 | TN (100) | TP (86) | |
| Navicula subminuscula Manguin | 0.85 | 0.95 | SAL (100) | TP (68) | |
| Navicula tenera Hustedt | 0.93 | 1.00 | SAL (100) | EC (89) | |
| Navicula tripunctata (O.F. Müller) Bory | 0.91 | 0.97 | SAL (100) | EC (58) | |
| Navicula trivialis Lange-Bertalot | 0.87 | 0.98 | SAL (100) | EC (49) | |
| Navicula veneta Kützing | 0.88 | 0.97 | TP (100) | EC (56) | 3 |
| Navicula viridula (Kützing) Ehrenberg | 0.95 | 0.98 | EC (100) | SAL (73) | |
| Navicula viridula var. rostellata (Kützing) Cleve | 0.86 | 0.98 | SAL (100) | EC (56) | |
| Nitzschia acicularis (Kützing) W. Smith | 0.94 | 0.98 | DO (100) | WT (94) | |
| Nitzschia amphibia Grunow | 0.90 | 0.97 | WT (100) | SAL (96) | |
| Nitzschia calida Grunow | 0.89 | 0.99 | DO (100) | BOD (85) | 4 |
| Nitzschia capitellata Hustedt | 0.87 | 0.99 | BOD (100) | TP (75) | |
| Nitzschia communis Rabenhorst | 0.91 | 0.99 | EC (100) | SAL (72) | |
| Nitzschia constricta (Kützing) Ralfs | 0.88 | 0.97 | EC (100) | SAL (54) | 3 |
| Nitzschia dissipata (Kützing) Rabenhorst | 0.82 | 0.99 | SAL (100) | TP (58) | |
| Nitzschia filiformis (W. Smith) Van Heurck | 0.86 | 0.98 | SAL (100) | pH (92) | |
| Nitzschia fonticola (Grunow) Grunow | 0.88 | 0.97 | SAL (100) | DO (54) | |
| Nitzschia frustulum (Kützing) Grunow | 0.89 | 0.99 | SAL (100) | EC (91) | |
| Nitzschia gracilis Hantzsch | 0.87 | 0.96 | TN (100) | BOD (75) | |
| Nitzschia inconspicua Grunow | 0.83 | 0.97 | EC (100) | TN (91) | |
| Nitzschia linearis W. Smith | 0.89 | 0.98 | WT (100) | TP (68) | |
| Nitzschia littoralis Grunow | 0.91 | 0.99 | WT (100) | EC (77) | |
| Nitzschia nana Grunow | 0.94 | 0.99 | EC (100) | BOD (90) | |
| Nitzschia palea (Kützing) W. Smith | 0.86 | 0.97 | SAL (100) | EC (95) | |
| Nitzschia paleacea (Grunow) Grunow | 0.84 | 0.98 | BOD (100) | SAL (44) | |
| Nitzschia pellucida Grunow | 0.95 | 0.99 | EC (100) | SAL (89) | |
| Nitzschia perminuta (Grunow) M. Peragallo | 0.92 | 0.98 | TURB (100) | pH (98) | |
| Nitzschia tryblionella Hantzsch | 0.95 | 0.99 | TN (100) | DO (92) | |
| Reimeria sinuata (W. Gregory) Kociolek & Stoermer | 0.92 | 0.97 | EC (100) | TURB (45) | 1 |
| Rhoicosphenia abbreviate (Agardh) Lange-Bertalot | 0.92 | 0.97 | TURB (100) | TP (21) | |
| Stephanodiscus hantzschii Grunow | 0.94 | 0.98 | pH (100) | TURB (63) | 3 |
| Stephanodiscus invisitatus Hohn & Hellermann | 0.94 | 0.98 | pH (100) | TP (64) | 3 |
| Surirella angusta Kützing | 0.90 | 0.97 | EC (100) | SAL (78) | |
| Surirella minuta Brébisson ex Kützing | 0.83 | 0.97 | SAL (100) | EC (82) | |
| Surirella ovalis Brébisson | 0.94 | 0.99 | TP (100) | WT (65) | |
| Surirella ovata Kützing | 0.96 | 0.99 | TP (100) | TURB (90) | |
| Synedra acus Kützing | 0.86 | 0.99 | SAL (100) | BOD (79) | |
| Synedra pulchella (Ralfs ex Kützing) Kützing | 0.90 | 0.99 | SAL (100) | EC (95) | |
| Synedra ulna (Nitzsch) Ehrenberg | 0.86 | 0.96 | SAL (100) | EC (83) | |
| Thalassiosira bramaputrae (Ehrenberg) Håkansson & Locker | 0.91 | 0.99 | EC (100) | TURB (91) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-K.; Cho, I.-H.; Hwang, E.-A.; Kim, Y.-J.; Kim, B.-H. Benthic Diatom Communities in Korean Estuaries: Species Appearances in Relation to Environmental Variables. Int. J. Environ. Res. Public Health 2019, 16, 2681. https://doi.org/10.3390/ijerph16152681
Kim H-K, Cho I-H, Hwang E-A, Kim Y-J, Kim B-H. Benthic Diatom Communities in Korean Estuaries: Species Appearances in Relation to Environmental Variables. International Journal of Environmental Research and Public Health. 2019; 16(15):2681. https://doi.org/10.3390/ijerph16152681
Chicago/Turabian StyleKim, Ha-Kyung, In-Hwan Cho, Eun-A Hwang, Yong-Jae Kim, and Baik-Ho Kim. 2019. "Benthic Diatom Communities in Korean Estuaries: Species Appearances in Relation to Environmental Variables" International Journal of Environmental Research and Public Health 16, no. 15: 2681. https://doi.org/10.3390/ijerph16152681
APA StyleKim, H.-K., Cho, I.-H., Hwang, E.-A., Kim, Y.-J., & Kim, B.-H. (2019). Benthic Diatom Communities in Korean Estuaries: Species Appearances in Relation to Environmental Variables. International Journal of Environmental Research and Public Health, 16(15), 2681. https://doi.org/10.3390/ijerph16152681






