Knowledge, Attitudes and Intentions to Prescribe Antibiotics: A Structural Equation Modeling Study of Primary Care Institutions in Hubei, China
Abstract
1. Introduction
2. Participants and Methods
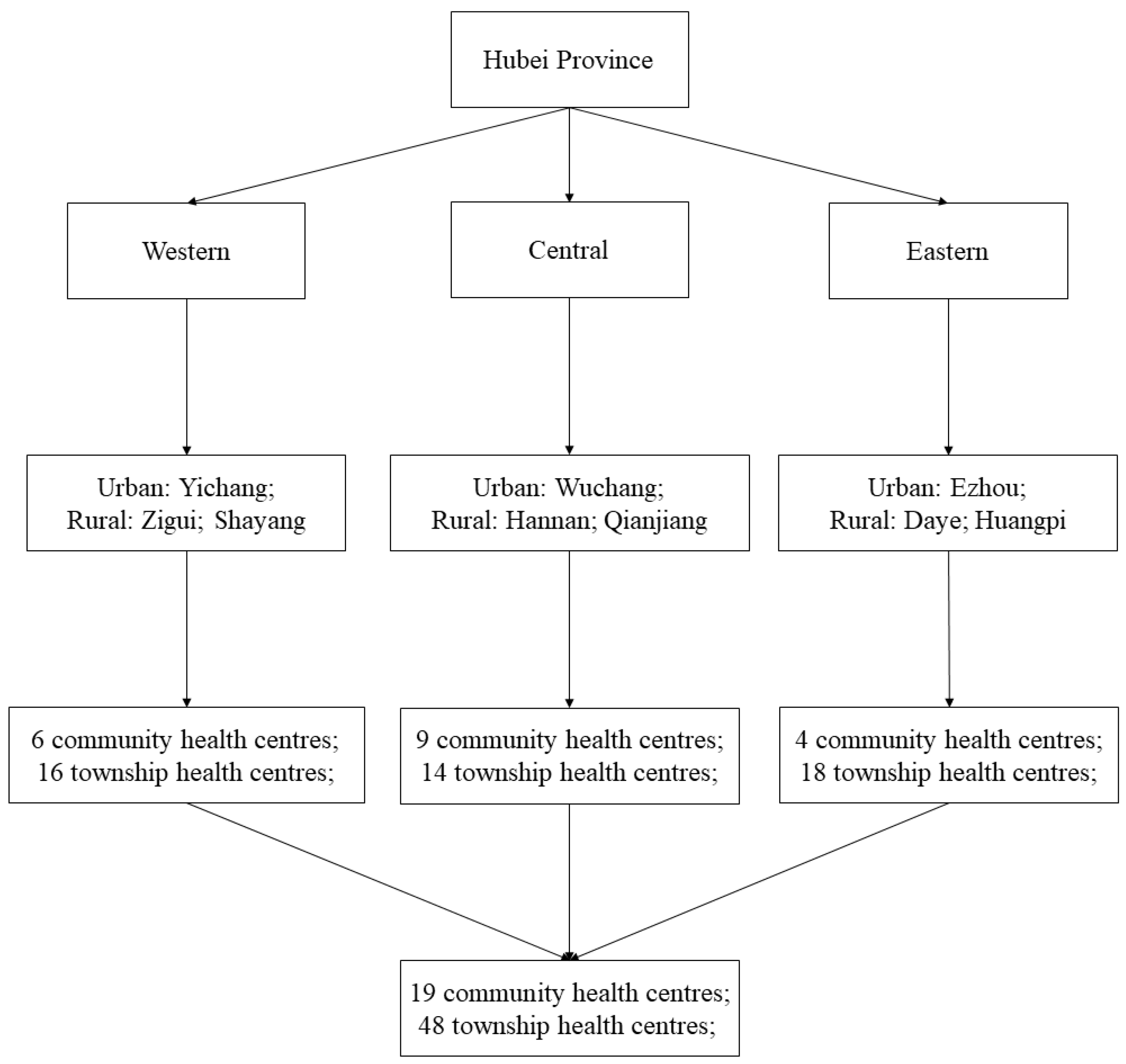
2.1. Settings
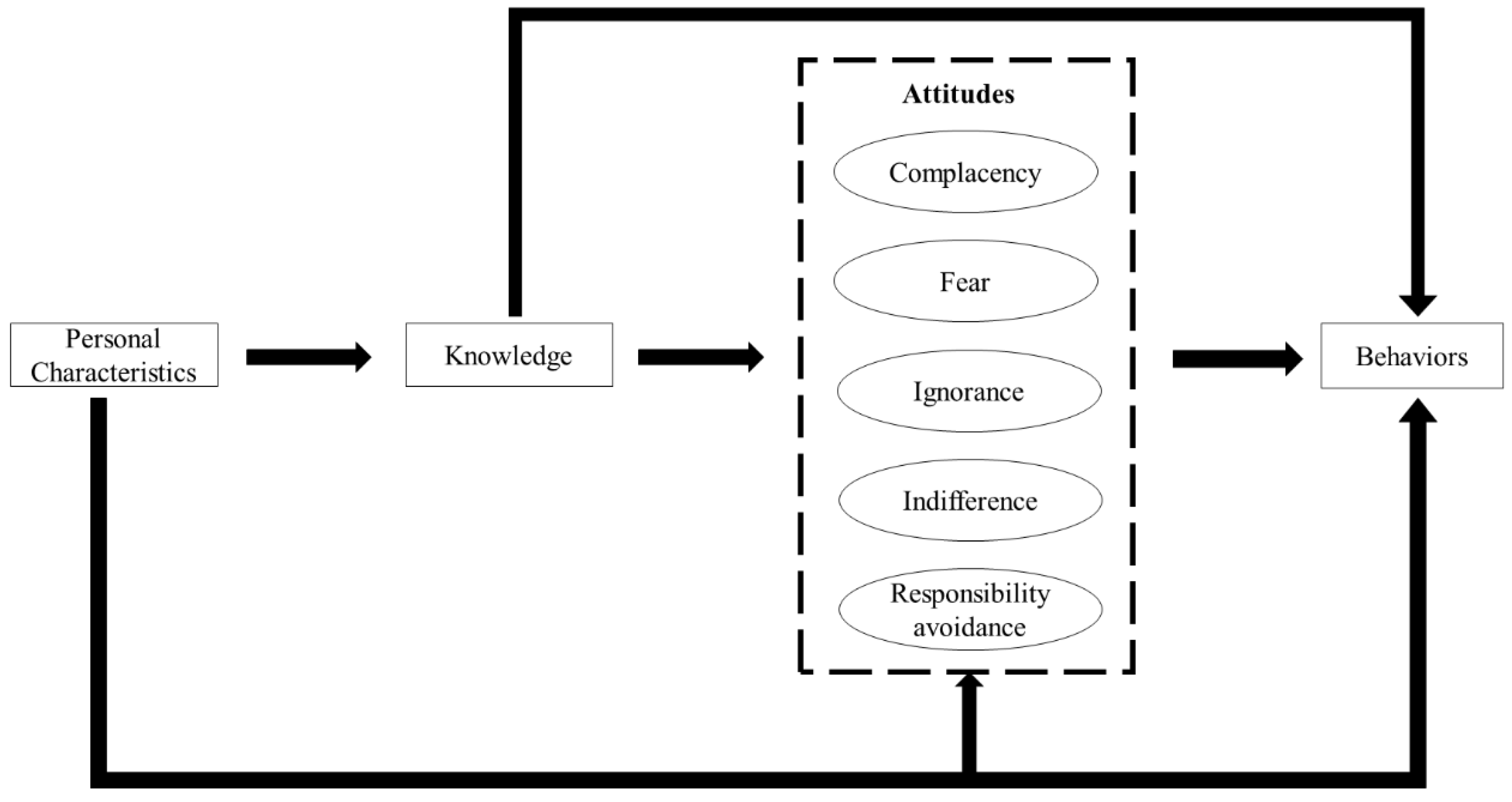
2.2. Theoretical Framework
- Complacency: prescribing antibiotics to satisfy patient demands and expectations;
- Fear: prescribing antibiotics for fear of losing patients or losing in potential disputes with patients;
- Ignorance: a lack of concern in relation to antibiotic resistance resulting from over-prescriptions of antibiotics;
- Indifference: a lack of motivation to change antibiotic prescribing practices; and
- Responsibility avoidance: a belief that others (patients, governments and other professionals) are responsible for the problem of antibiotic resistance.
2.3. Survey Instruments
2.4. Sampling and Data Collection
2.5. Data Analysis
3. Results
3.1. Characteristics of Respondents
3.2. Knowledge, Attitudes, and Behavioral Intentions Toward Antibiotic Prescriptions
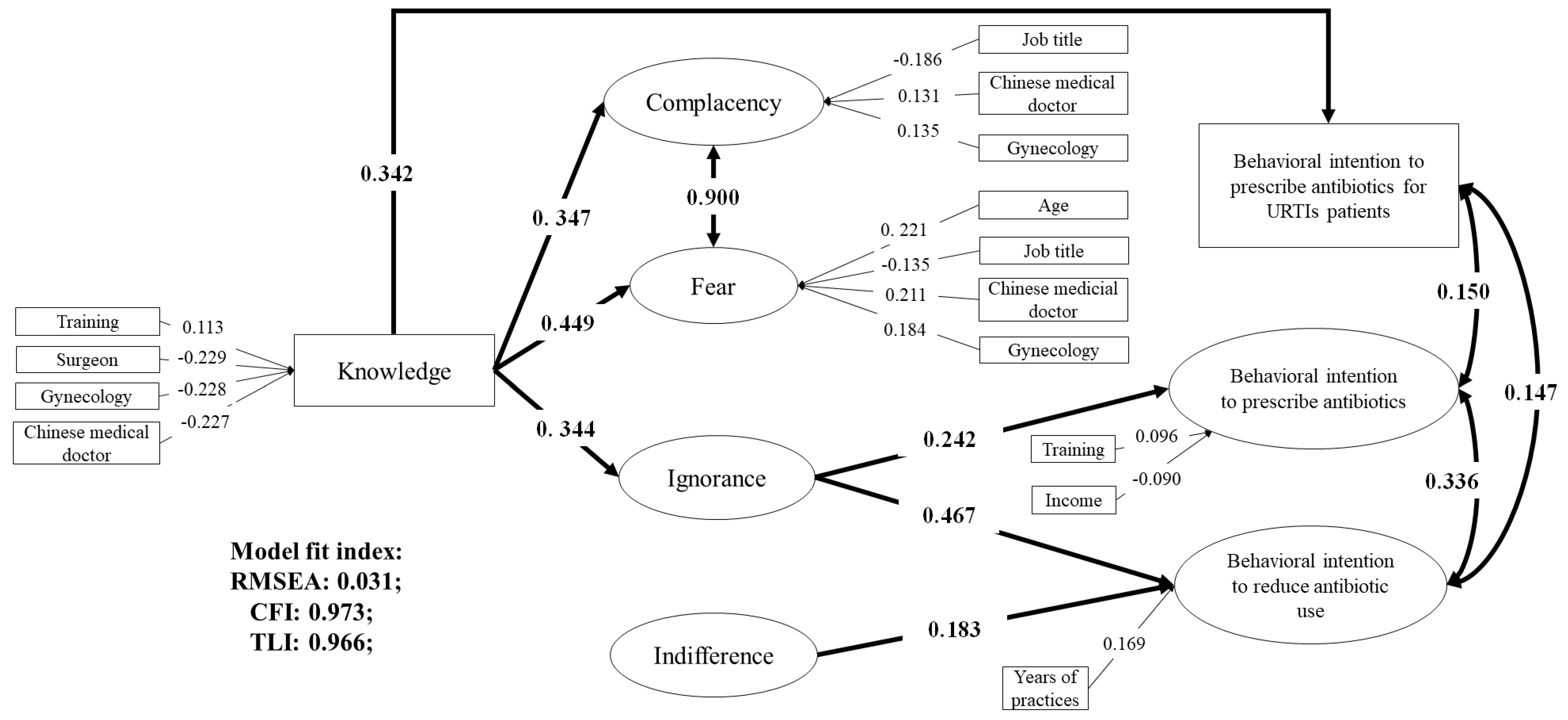
3.3. Associations between Knowledge, Attitudes, and Behavioral Intentions
4. Discussion
4.1. Knowledge
4.2. Attitudes
4.3. Policy Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
References
- WHA Resolution. WHA68.7—Global Action Plan on Antimicrobial Resistance; Sixty-Eighth World Health Assembly; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- WHO. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, 1120. [Google Scholar] [CrossRef] [PubMed]
- Van De Sande-Bruinsma, N.; Grundmann, H.; Verloo, D.; Tiemersma, E.; Monen, J.; Goossens, H.; Ferech, M.; Mittermayer, H.; Metz, S.; Koller, W.; et al. Antimicrobial drug use and resistance in Europe. Emerging Infect. Dis. 2008, 14, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- WHO. Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Fleming-Dutra, K.E.; Hersh, A.L.; Shapiro, D.J.; Bartoces, M.; Enns, E.A.; File, T.M.J.; Finkelstein, J.A.; Gerber, J.S.; Hyun, D.Y.; Linder, J.A.; et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016, 315, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E. Dynamics of antibiotic prescribing for children. JAMA 2002, 287, 3133–3135. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, D.J.; Hicks, L.A.; Pavia, A.T.; Hersh, A.L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J. Antimicrob. Chemother. 2014, 69, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Vu, H.; Xie, Z.; Chen, W.; Tang, S. Systematic review on irrational use of medicines in China and Vietnam. PLoS ONE 2015, 10, e0117710. [Google Scholar] [CrossRef]
- Teixeira Rodrigues, A.; Roque, F.; Falcão, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef]
- Lopez-Vazquez, P.; Vazquez-Lago, J.M.; Figueiras, A. Misprescription of antibiotics in primary care: A critical systematic review of its determinants. J. Eval. Clin. Pract. 2012, 18, 473–484. [Google Scholar] [CrossRef]
- Livermore, D.M. Minimising antibiotic resistance. Lancet Infect. Dis. 2005, 5, 450–459. [Google Scholar] [CrossRef]
- McCullough, A.R.; Rathbone, J.; Parekh, S.; Hoffmann, T.C.; Del Mar, C.B. Not in my backyard: A systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2015, 70, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, A.E.; Kuyvenhoven, M.M.; van der Wouden, J.C.; Verheij, T.J. Determinants of antibiotic overprescribing in respiratory tract infections in general practice. J. Antimicrob. Chemother. 2005, 56, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, A.E.; Kuyvenhoven, M.M.; van der Wouden, J.C.; Verheij, T.J. Analysis of under- and overprescribing of antibiotics in acute otitis media in general practice. J. Antimicrob. Chemother. 2005, 56, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Mangione-Smith, R.; McGlynn, E.A.; Elliott, M.N.; Krogstad, P.; Brook, R.H. The relationship between perceived parental expectations and pediatrician antimicrobial prescribing behavior. Pediatrics 1999, 103, 711–718. [Google Scholar] [CrossRef] [PubMed]
- De Sutter, A.I.; De Meyere, M.J.; De Maeseneer, J.M.; Peersman, W.P. Antibiotic prescribing in acute infections of the nose or sinuses: A matter of personal habit? Fam. Pract. 2001, 18, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.L.; Achike, F.I.; Phua, K.L.; Norhayati, Y.; Nurjahan, M.I.; Nor, A.H.; Koh, C.N. General and URTI-specific antibiotic prescription rates in a Malaysian primary care setting. Int. J. Antimicrob. Agents 2004, 24, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Liabsuetrakul, T.; Islam, M. Evidence on antibiotic prophylaxis for cesarean section alone is not sufficient to change the practices of doctors in a teaching hospital. J. Obstet. Gynaecol. Res. 2005, 31, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Liabsuetrakul, T.; Chongsuvivatwong, V.; Lumbiganon, P.; Lindmark, G. Obstetricians’ attitudes, subjective norms, perceived controls, and intentions on antibiotic prophylaxis in caesarean section. Soc. Sci. Med. 2003, 57, 1665–1674. [Google Scholar] [CrossRef]
- Huang, N.; Chou, Y.J.; Chang, H.J.; Ho, M.; Morlock, L. Antibiotic prescribing by ambulatory care physicians for adults with nasopharyngitis, URIs, and acute bronchitis in Taiwan: A multi-level modeling approach. Fam. Pract. 2005, 22, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, R.; Sridharan, S.; Chelliah, L.R.; Suresh, S.; Senguttuvan, M. Factors affecting antibiotic prescribing pattern in pediatric practice. Indian J. Pediat. 2005, 72, 877–879. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. National Data; National Bureau of Statistics of China: Beijing, China, 2018.
- Wang, J.; Wang, P.; Wang, X.; Zheng, Y.; Xiao, Y. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern. Med. 2014, 174, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, J.; Wang, F.; Wang, B.; Liu, L.; Hou, W.; Fan, H.; Tong, Y.; Zhang, J.; Lu, Z. Overprescribing in China, driven by financial incentives, results in very high use of antibiotics, injections, and corticosteroids. Health Aff. 2012, 31, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Song, F.; Gong, Y.; Tu, X.; Wang, Y.; Cao, S.; Liu, J.; Lu, Z. A systematic review of antibiotic utilization in China. J. Antimicrob. Chemother. 2013, 68, 2445–2452. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, X.; Wan, J. Public reporting influences antibiotic and injection prescription in primary care: A segmented regression analysis. J. Eval. Clin. Pract. 2015, 21, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Harvard Medical School; Harvard Pilgrim Health. Using Indicators to Measure Country Pharmaceutical Situations: Fact Book on Who Level One and Level Two Monitoring Indicators; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- WHO. Advocacy, Communication and Social Mobilization for TB Control: A Guide to Developing Knowledge, Attitude and Practice Surveys; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Quet, F.; Vlieghe, E.; Leyer, C.; Buisson, Y.; Newton, P.N.; Naphayvong, P.; Keoluangkhot, V.; Chomarat, M.; Longuet, C.; Steenkeste, N.; et al. Antibiotic prescription behaviours in Lao People’s Democratic Republic: A knowledge, attitude and practice survey. Bull. World Health Organ. 2015, 93, 219–227. [Google Scholar] [CrossRef]
- Thriemer, K.; Katuala, Y.; Batoko, B.; Alworonga, J.P.; Devlieger, H.; Van Geet, C.; Ngbonda, D.; Jacobs, J. Antibiotic prescribing in DR Congo: A knowledge, attitude and practice survey among medical doctors and students. PLoS ONE 2013, 8, e55495. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Llamocca, L.P.; Garcia, K.; Jimenez, A.; Samalvides, F.; Gotuzzo, E.; Jacobs, J. Knowledge, attitudes and practice survey about antimicrobial resistance and prescribing among physicians in a hospital setting in Lima, Peru. BMC Clin. Pharmacol. 2011, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Hassali, M.A.; Kamil, T.K.; Md Yusof, F.A.; Alrasheedy, A.A.; Yusoff, Z.M.; Saleem, F.; Al-Tamimi, S.K.; Wong, Z.Y.; Aljadhey, H.; Godman, B. General practitioners’ knowledge, attitude and prescribing of antibiotics for upper respiratory tract infections in Selangor, Malaysia: Findings and implications. Expert Rev. Anti. Infect. Ther. 2015, 13, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Hoa, N.Q.; Larson, M.; Kim Chuc, N.T.; Eriksson, B.; Trung, N.V.; Stalsby, C.L. Antibiotics and paediatric acute respiratory infections in rural Vietnam: Health-care providers’ knowledge, practical competence and reported practice. Trop. Med. Int. Health 2009, 14, 546–655. [Google Scholar] [CrossRef]
- Teixeira Rodrigues, A.; Ferreira, M.; Roque, F.; Falcao, A.; Ramalheira, E.; Figueiras, A.; Herdeiro, M.T. Physicians’ attitudes and knowledge concerning antibiotic prescription and resistance: Questionnaire development and reliability. BMC Infect. Dis. 2016, 16, 7. [Google Scholar] [CrossRef]
- Lopez-Vazquez, P.; Vazquez-Lago, J.M.; Gonzalez-Gonzalez, C.; Pineiro-Lamas, M.; Lopez-Duran, A.; Herdeiro, M.T.; Figueiras, A. Development and validation of the knowledge and attitudes regarding antibiotics and resistance (KAAR-11) questionnaire for primary care physicians. J. Antimicrob. Chemother. 2016, 71, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.; McKee, M. Factors influencing antibiotic prescribing in China: An exploratory analysis. Health Policy 2009, 90, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Lambert, B.L.; Salmon, J.W.; Stubbings, J.; Gilomen-Study, G.; Valuck, R.J.; Kezlarian, K. Factors associated with antibiotic prescribing in a managed care setting: An exploratory investigation. Soc. Sci. Med. 1997, 45, 1767–1779. [Google Scholar] [CrossRef]
- Walker, A.E.; Grimshaw, J.M.; Armstrong, E.M. Salient beliefs and intentions to prescribe antibiotics for patients with a sore throat. Br. J. Health Psychol. 2001, 6, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Limbert, C.; Lamb, R. Doctors’ use of clinical guidelines: Two applications of the Theory of Planned Behaviour. Psychol. Health Med. 2002, 7, 301–310. [Google Scholar] [CrossRef]
- Perkins, M.B.; Jensen, P.S.; Jaccard, J.; Gollwitzer, P.; Oettingen, G.; Pappadopulos, E.; Hoagwood, K.E. Applying theory-driven approaches to understanding and modifying clinicians’ behavior: What do we know? Psychiatr. Serv. 2007, 58, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C.J.; Conner, M. Efficacy of the theory of planned behaviour: A meta-analytic review. Br. J. Soc. Psychol. 2001, 40, 471–499. [Google Scholar] [CrossRef]
- Francis, J.; Eccles, M.P.; Johnston, M.; Walker, A.E.; Grimshaw, J.M.; Foy, R.; Kaner, E.F.S.; Smith, L.; Bonetti, D. Constructing Questionnaires Based on the Theory of Planned Behaviour: A Manual for Health Services Researchers; Centre for Health Services Research, University of Newcastle upon Tyne: Newcastle upon Tyne, UK, 2004. [Google Scholar]
- Barkan, S.E. Social Science Research: Principles, Methods and Practices; University of South Florida: Tampa, FL, USA, 2012. [Google Scholar]
- Hinkin, T.R.; Tracey, J.B.; Enz, C.A. Scale cnstruction: Developing reliable and valid measurement instruments. J. Hospitality Tour. Res. 1997, 21, 100–120. [Google Scholar] [CrossRef]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equation Model. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Bentler, P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990, 107, 238–246. [Google Scholar] [CrossRef]
- Statulator. Sample Size Calculator for Estimating a Single Mean. Available online: http://statulator.com/SampleSize/ss1M.html (accessed on 29 July 2018).
- Beauducel, A.; Herzberg, P.Y. On the performance of maximum likelihood versus means and variance adjusted weighted least squares estimation in CFA. Struct. Equation Model. 2006, 13, 186–203. [Google Scholar] [CrossRef]
- Pratter, M.R. Cough and the common cold: ACCP evidence-based clinical practice guidelines. Chest 2006, 129, 72S–74S. [Google Scholar] [CrossRef] [PubMed]
- Centre for Clinical Practice at NICE. Respiratory Tract Infections—Antibiotic Prescribing: Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care; National Institute for Health and Clinical Excellence: London, UK, 2008. [Google Scholar]
- Order of the Ministry of Health. Administrative Measures for the Clinical Use of Antibacterial Drugs; Order of the Ministry of Health, Ed.; Ministry of Health: Beijing, China, 2012. [Google Scholar]
- Nanshan, Z.; Xirun, W.; Xiaojun, M.; Chen, W.; Rui, W.; Dayou, W.; Minggui, W.; Xuanding, W.; Xiaoyang, L.; Kunling, S.; et al. Guiding Principles for Clinical Application of Antimicrobial Agents (2015 Version); National Health and Family Planning Commission People’s Republic of China: Beijing, China, 2015. [Google Scholar]
- Arroll, B.; Kenealy, T. Are antibiotics effective for acute purulent rhinitis? Systematic review and meta-analysis of placebo controlled randomised trials. BMJ 2006, 333, 279. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Dyar, O.J.; Zhao, L.; Tomson, G.; Nilsson, L.E.; Grape, M.; Song, Y.; Yan, L.; Lundborg, C.S. Overuse of antibiotics for the common cold—Attitudes and behaviors among doctors in rural areas of Shandong Province, China. BMC Pharmacol. Toxicol. 2015, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, J.; Holmes, W.; Macfarlane, R.; Britten, N. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: Questionnaire study. BMJ 1997, 315, 1211–1214. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, J.; Pit, S. Prescribing behaviour in clinical practice: Patients’ expectations and doctors’ perceptions of patients’ expectations—A questionnaire study. BMJ 1997, 315, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Fang, H. International Profiles of Health Care Systems-Section of China; The Commonwealth Fund: New York, NY, USA, 2017. [Google Scholar]
- Li, X.; Lu, J.; Hu, S.; Cheng, K.K.; De Maeseneer, J.; Meng, Q.; Mossialos, E.; Xu, D.R.; Yip, W.; Zhang, H.; et al. The primary health-care system in China. Lancet 2017, 390, 2584–2594. [Google Scholar] [CrossRef]
- Jin, W.; Tianyou, H.; Xiuying, H. Doctors’ workload in China: A status-quo study. Chin. J. Evidence-Based Med. 2015, 133–136. [Google Scholar] [CrossRef]
- Teixeira Rodrigues, A.; Ferreira, M.; Pineiro-Lamas, M.; Falcao, A.; Figueiras, A.; Herdeiro, M.T. Determinants of physician antibiotic prescribing behavior: A 3 year cohort study in Portugal. Curr. Med. Res. Opin. 2016, 32, 949–957. [Google Scholar] [CrossRef]
- Labricciosa, F.M.; Sartelli, M.; Correia, S.; Abbo, L.M.; Severo, M.; Ansaloni, L.; Coccolini, F.; Alves, C.; Melo, R.B.; Baiocchi, G.L.; et al. Emergency surgeons’ perceptions and attitudes towards antibiotic prescribing and resistance: A worldwide cross-sectional survey. World J. Emerg. Surg. 2018, 13, 27. [Google Scholar] [CrossRef]
- Yuqin, L. Current status and progress of antibiotic use in obstetrics and gynecology [Chinese]. Guide China Med. 2014, 152–153. Available online: http://www.cqvip.com/qk/86373x/201409/49103270.html (accessed on 4 July 2019).
- Fung, C.H.; Lim, Y.W.; Mattke, S.; Damberg, C.; Shekelle, P.G. Systematic review: The evidence that publishing patient care performance data improves quality of care. Ann. Intern. Med. 2008, 148, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Bou-Antoun, S.; Costelloe, C.; Honeyford, K.; Mazidi, M.; Hayhoe, B.W.J.; Holmes, A.; Johnson, A.P.; Aylin, P. Age-related decline in antibiotic prescribing for uncomplicated respiratory tract infections in primary care in England following the introduction of a national financial incentive (the Quality Premium) for health commissioners to reduce use of antibiotics in the community: An interrupted time series analysis. J. Antimicrob. Chemother. 2018, 73, 2883–2892. [Google Scholar] [PubMed]
- Ouldali, N.; Bellettre, X.; Milcent, K.; Guedj, R.; de Pontual, L.; Cojocaru, B.; Soussan-Banini, V.; Craiu, I.; Skurnik, D.; Gajdos, V.; et al. Impact of implementing national guidelines on antibiotic prescriptions for acute respiratory tract infections in pediatric Emergency departments: An interrupted time series analysis. Clin. Infect. Dis. 2017, 65, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Charani, E.; Edwards, R.; Sevdalis, N.; Alexandrou, B.; Sibley, E.; Mullett, D.; Franklin, B.D.; Holmes, A. Behavior Change Strategies to Influence Antimicrobial Prescribing in Acute Care: A Systematic Review. Clin. Infect. Dis. 2011, 53, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Currie, J.; Lin, W.; Zhang, W. Patient knowledge and antibiotic abuse: Evidence from an audit study in China. J. Health Econ. 2011, 30, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Alumran, A.; Hou, X.-Y.; Hurst, C. Validity and reliability of instruments designed to measure factors influencing the overuse of antibiotics. J. Infect. Public Health 2012, 5, 221–232. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean ± SD */N (%) |
|---|---|
| Age (years) | 43.27 ± 10.43 |
| Gender | |
| Male | 436 (69.76) |
| Female | 189 (30.24) |
| Facility | |
| Urban community health center | 137 (21.92) |
| Rural township health center | 488 (78.08) |
| Medical sub-specialization | |
| General practitioner | 264 (42.24) |
| Internist/pediatrician | 154 (24.64) |
| Surgeon | 77 (12.32) |
| Gynecologist | 87 (13.92) |
| Chinese medical practitioner | 43 (6.88) |
| Professional title | |
| Junior doctor | 324 (51.84) |
| Attending doctor | 236 (37.76) |
| Associate senior or senior consultant | 65 (10.40) |
| Level of education | |
| Vocational training | 51 (8.16) |
| Associate degree | 329 (52.64) |
| University degree | 245 (39.20) |
| Annual household income (Chinese RMB ¥) | |
| <40,000 | 169 (27.04) |
| 40,000~ | 305 (48.80) |
| 80,000~ | 107 (17.12) |
| ≥120,000 | 44 (7.04) |
| Clinical experience (years) | 16.64 ± 11.11 |
| Training about antibiotics over the last year | |
| Yes | 477 (76.32) |
| No/Not aware | 148 (23.68) |
| Knowledge Questions | Number (Percentage) of Respondents Giving a Correct Answer | p *-Value | |||||
|---|---|---|---|---|---|---|---|
| Total n = 625 | General Practitioner n = 264 | Internist/Pediatrician n = 154 | Surgeon n = 77 | Gynecologist n = 87 | Chinese Medical Practitioner n = 43 | ||
| Antibiotics should not be prescribed for non-febrile diarrhea | 591 (94.56) | 253 (95.83) | 149 (96.75) | 70 (90.91) | 84 (96.55) | 39 (90.70) | 0.171 |
| Antibiotics should not be prescribed for upper respiratory tract infections | 36 (5.76) | 15 (5.68) | 9 (5.84) | 5 (6.49) | 5 (5.75) | 2 (4.65) | 0.998 |
| Dosage reduction of antibiotics is needed for renal failure | 64 (10.24) | 21 (7.95) | 11 (7.14) | 12 (15.58) | 17 (19.54) | 3 (6.98) | 0.011 |
| Amoxicillin is a safe antibiotic product for pregnant patients | 596 (95.36) | 254 (96.21) | 148 (96.10) | 72 (93.51) | 87 (100.00) | 35 (81.40) | <0.001 |
| Metronidazole has the best activity against anaerobes | 601 (96.16) | 261 (98.86) | 150 (97.40) | 73 (94.81) | 80 (91.95) | 37 (86.05) | <0.001 |
| Methicillin resistant staphylococcus aureus is resistant to beta- lactam antibiotics | 182 (29.12) | 86 (32.58) | 49 (31.81) | 13 (16.88) | 19 (21.84) | 15 (34.88) | 0.027 |
| Ceftriaxone most effectively crosses the blood-brain barrier | 246 (39.36) | 120 (45.45) | 53 (34.41) | 27 (35.07) | 33 (37.93) | 13 (30.23) | 0.102 |
| Aminoglycosides are very active if they are administered as parenteral once daily | 286 (45.76) | 126 (47.73) | 66 (42.85) | 36 (46.75) | 43 (49.43) | 15 (34.88) | 0.483 |
| Bacterial pneumonia (including one of the following symptoms: fast breathing, chest in-drawing or stridor) requires antibiotic treatment | 311 (49.76) | 145 (54.92) | 83 (53.89) | 33 (42.86) | 33 (37.93) | 17 (39.53) | 0.017 |
| Antibiotics do not reduce the duration and the occurrence of complications of upper respiratory tract infections | 380 (60.80) | 177 (67.05) | 113 (73.37) | 35 (45.45) | 36 (41.38) | 19 (44.19) | <0.001 |
| The average number of patients taking antibiotics should be below 30 per 100 in a primary care facility | 478 (76.48) | 218 (82.58) | 119 (77.27) | 51 (66.23) | 57 (65.52) | 33 (76.74) | <0.001 |
| Overall score (mean ± SD) | 6.04 ± 1.46 | 6.34 ± 1.36 | 6.16 ± 1.43 | 5.55 ± 1.53 | 5.68 ± 1.34 | 5.30 ± 1.70 | <0.001 |
| Measurement | Scores (Mean ± SD) | p * | Cronbach’s Alpha | |||||
|---|---|---|---|---|---|---|---|---|
| Total n = 625 | General Practitioner n = 264 | Internist/Pediatrician n = 154 | Surgeon n = 77 | Gynecologist n = 8 7 | Chinese Medical Practitioner n = 43 | |||
| Attitude | ||||||||
| Complacency | 1.29 ± 0.65 | 1.26 ± 0.65 | 1.30 ± 0.68 | 1.22 ± 0.69 | 1.41 ± 0.56 | 1.36 ± 0.69 | 0.173 | 0.912 |
| Fear | 1.11 ± 0.63 | 1.07 ± 0.64 | 1.09 ± 0.64 | 1.00 ± 0.62 | 1.31 ± 0.54 | 1.27 ± 0.67 | 0.002 | 0.797 |
| Ignorance | 1.28 ± 0.43 | 1.32 ± 0.44 | 1.24 ± 0.42 | 1.21 ± 0.41 | 1.26 ± 0.38 | 1.27 ± 0.57 | 0.140 | 0.694 |
| Indifference | −0.29 ± 0.70 | −0.29 ± 0.70 | −0.27 ± 0.67 | −0.32 ± 0.77 | −0.36 ± 0.64 | −0.22 ± 0.74 | 0.775 | 0.669 |
| Responsibility avoidance | −1.15 ± 0.45 | −1.22 ± 0.45 | −1.19 ± 0.46 | −1.15 ± 0.46 | −1.14 ± 0.39 | −1.17 ± 0.48 | 0.286 | 0.385 |
| Behavioral intention | ||||||||
| Prescribe antibiotics for upper respiratory tract infections | 3.98 ± 2.21 | 3.94 ± 2.09 | 3.86 ± 2.28 | 4.58 ± 2.57 | 3.92 ± 2.14 | 3.65 ± 2.02 | 0.221 | N/A |
| Prescribe antibiotics | 0.86 ± 0.63 | 0.84 ± 0.61 | 0.83 ± 0.62 | 0.86 ± 0.73 | 0.95 ± 0.59 | 0.86 ± 0.67 | 0.761 | 0.898 |
| Reduce antibiotic prescriptions | 1.29 ± 0.54 | 1.31 ± 0.52 | 1.24 ± 0.55 | 1.30 ± 0.54 | 1.36 ± 0.50 | 1.22 ± 0.64 | 0.694 | 0.893 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, C.; Wang, D.; Zhang, X. Knowledge, Attitudes and Intentions to Prescribe Antibiotics: A Structural Equation Modeling Study of Primary Care Institutions in Hubei, China. Int. J. Environ. Res. Public Health 2019, 16, 2385. https://doi.org/10.3390/ijerph16132385
Liu C, Liu C, Wang D, Zhang X. Knowledge, Attitudes and Intentions to Prescribe Antibiotics: A Structural Equation Modeling Study of Primary Care Institutions in Hubei, China. International Journal of Environmental Research and Public Health. 2019; 16(13):2385. https://doi.org/10.3390/ijerph16132385
Chicago/Turabian StyleLiu, Chenxi, Chaojie Liu, Dan Wang, and Xinping Zhang. 2019. "Knowledge, Attitudes and Intentions to Prescribe Antibiotics: A Structural Equation Modeling Study of Primary Care Institutions in Hubei, China" International Journal of Environmental Research and Public Health 16, no. 13: 2385. https://doi.org/10.3390/ijerph16132385
APA StyleLiu, C., Liu, C., Wang, D., & Zhang, X. (2019). Knowledge, Attitudes and Intentions to Prescribe Antibiotics: A Structural Equation Modeling Study of Primary Care Institutions in Hubei, China. International Journal of Environmental Research and Public Health, 16(13), 2385. https://doi.org/10.3390/ijerph16132385






