Antibiotic Consumption and Resistance during a 3-Year Period in Sicily, Southern Italy
Abstract
1. Introduction
2. Materials and Methods
3. Results
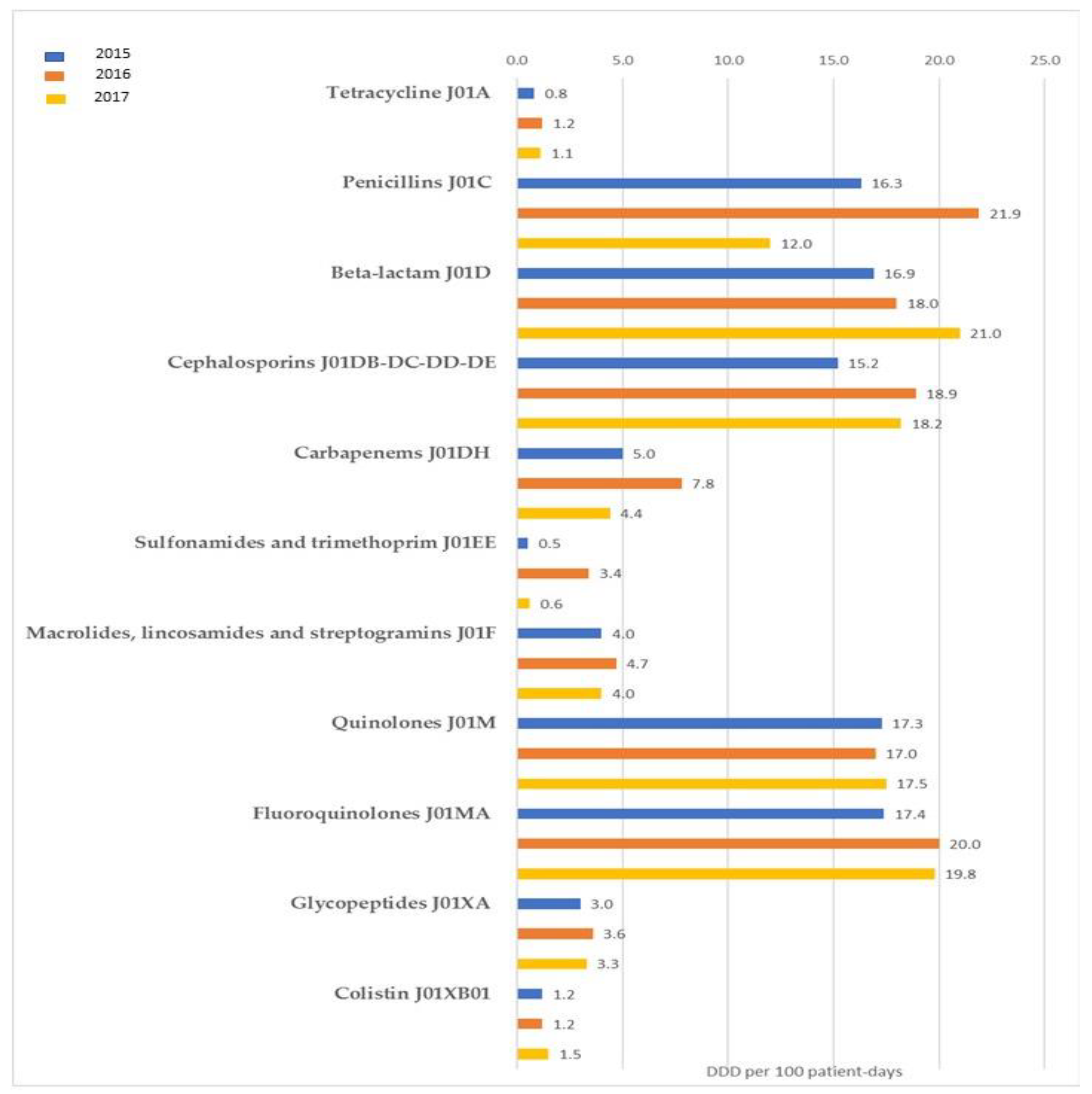
3.1. Antibiotic Consumption in Healthcare Facilities
3.2. Antibiotic Consumption in the Community
3.3. Resistance Rates
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ten Threats to Global Health in 2019. WHO, 2019. Available online: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed on 1 May 2019).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Tacconelli, E.; Pezzani, M.D. Public health burden of antimicrobial resistance in Europe. Lancet Infect. Dis. 2019, 19, 4–6. [Google Scholar] [CrossRef]
- Mathers, A.J.; Peirano, G.; Pitout, J.D. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef]
- Van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef]
- A European One Health Action Plan against Antimicrobial Resistance (AMR). Available online: https://ec.europa.eu/health/amr/sites/amr/files/amr_action_plan_2017_en.pdf (accessed on 1 May 2019).
- Nordmann, P.; Poirel, L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin. Microbiol. Infect. 2014, 20, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Aldisio, E.; Marchese, A.E.; Mattaliano, A.R.; Tsakris, A. Antibiotic trends of Klebsiella pneumoniae and Acinetobacter baumannii resistance indicators in an intensive care unit of Southern Italy, 2008–2013. Antimicrob. Resist. Infect. Control. 2015, 4, 43. [Google Scholar] [CrossRef]
- Agodi, A.; Auxilia, F.; Barchitta, M.; Brusaferro, S.; D’Errico, M.M.; Montagna, M.T.; Pasquarella, C.; Tardivo, S.; Mura, I. SPIN-UTI network of the GISIOWorking Group of the Italian Society of Hygiene, Preventive Medicine and Public Health (SItI). Antibiotic consumption and resistance: Results of the SPIN-UTI project of the GISIO-SItI. Epidemiol. Prev. 2015, 39, 94–98. [Google Scholar]
- Barchitta, M.; Cipresso, R.; Giaquinta, L.; Romeo, M.A.; Denaro, C.; Pennisi, C.; Agodi, A. Acquisition and spread of Acinetobacter baumannii and Stenotrophomonas maltophilia in intensive care patients. Int. J. Hyg. Environ. Health 2009, 212, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Voulgari, E.; Barchitta, M.; Quattrocchi, A.; Bellocchi, P.; Poulou, A.; Santangelo, C.; Castiglione, G.; Giaquinta, L.; Romeo, M.A.; et al. Spread of a carbapenem- and colistin-resistant Acinetobacter baumannii ST2 clonal strain causing outbreaks in two Sicilian hospitals. J. Hosp. Infect. 2014, 86, 260–266. [Google Scholar] [CrossRef] [PubMed]
- García-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Castro-Sánchez, E.; Moore, L.S.; Husson, F.; Holmes, A.H. What are the factors driving antimicrobial resistance? Perspectives from a public event in London, England. BMC Infect. Dis. 2016, 16, 465. [Google Scholar] [CrossRef]
- Tangcharoensathien, V.; Chanvatik, S.; Sommanustweechai, A. Complex determinants of inappropriate use of antibiotics. Bull. World Health Organ. 2018, 96, 141–144. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2012. In Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2013. [Google Scholar]
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; Rex, J.; et al. Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52 (Suppl. 5), S397–S428. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef] [PubMed]
- Squeri, R.; Genovese, C.; Palamara, M.A.; Trimarchi, G.; La Fauci, V. Clean care is safer care: Correct handwashing in the prevention of healthcare associated infections. Ann. Ig. 2016, 28, 409–415. [Google Scholar] [CrossRef] [PubMed]
- La Fauci, V.; Riso, R.; Facciolà, A.; Merlina, V.; Squeri, R. Surveillance of microbiological contamination and correct use of protective lead garments. Ann. Ig. 2016, 28, 360–366. [Google Scholar] [CrossRef]
- Barchitta, M.; Matranga, D.; Quattrocchi, A.; Bellocchi, P.; Ruffino, M.; Basile, G.; Agodi, A. Prevalence of surgical site infections before and after the implementation of a multimodal infection control programme. J. Antimicrob. Chemother. 2012, 67, 749–755. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Proposals for EU Guidelines on the Prudent Use of Antimicrobials in Humans; ECDC: Stockholm, Sweden, 2017. [Google Scholar]
- Piano Nazionale di Contrasto dell’Antimicrobico-Resistenza 2017–2020. PNCAR, 2017. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2660_allegato.pdf (accessed on 1 May 2019).
- Regione Siciliana. Assessorato della Salute. DASOE. Programma Regionale di Sorveglianza e Controllo Delle ICA. Available online: https://www.qualitasiciliassr.it/?q=infezioni-correlate-assistenza (accessed on 1 May 2019).
- European Centre for Disease Prevention and Control. ECDC Country Visit to Italy to Discuss Antimicrobial Resistance Issues; ECDC: Stockholm, Sweden, 2017. [Google Scholar]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Murolo, G. Sistema di Sorveglianza Delle Resistenze Antibiotiche e dei Consumi di Antibiotici Nella Regione Sicilia-Anni 2015–2016–2017. Available online: https://www.qualitasiciliassr.it/sites/default/files/field/download/sorveglianza/antibiotici/Report_2015-2017.pdf (accessed on 1 May 2019).
- World Health Organization Collaborating Centre for Drug Statistics Methodology ATC/DDD Methodology. WHO, 2018. Available online: https://www.whocc.no/atc_ddd_methodology/history (accessed on 1 May 2019).
- Bebell, L.M.; Muiru, A.N. Antibiotic use and emerging resistance: How can resource-limited countries turn the tide? Glob. Heart 2014, 9, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ivanovska, V.; Schweickert, B.; Muller, A. Proxy indicators for antibiotic consumption; surveillance needed to control antimicrobial resistance. Bull. World Health Organ. 2019, 97, 3. [Google Scholar] [CrossRef] [PubMed]
- Carlet, J.; Jarlier, V.; Harbarth, S.; Voss, A.; Goossens, H.; Pittet, D. The Participants of the 3rd World Healthcare-Associated Infections Forum. Ready for a world without antibiotics? The Pensières Antibiotic Resistance Call to Action. Antimicrob. Resist. Infect. Control 2012, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2017. [Google Scholar]
- Agodi, A.; Barchitta, M.; Gianninò, V.; Collura, A.; Pensabene, T.; Garlaschi, M.L.; Pasquarella, C.; Luzzaro, F.; Sinatra, F.; Mahenthiralingam, E.; et al. Burkholderia cepacia complex in cysticfibrosis and non-cystic fibrosis patients: Identification of a cluster of epidemic lineages. J. Hosp. Infect. 2002, 50, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Journal of Antimicrobial Chemotherapy, 5th ed.; Oxford University Press: Oxford, UK, 1 November 2017; Issue 11; pp. 3199–3204.
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption—Annual Epidemiological Report for 2017; ECDC: Stockholm, Sweden, 2018. [Google Scholar]
- Iacchini, S.; Sabbatucci, M.; Gagliotti, C.; Rossolini, G.M.; Moro, M.L.; Iannazzo, S.; D’Ancona, F.; Pezzotti, P.; Pantosti, A. Bloodstream infections due to carbapenemase-producing Enterobacteriaceae in Italy: Results from nationwide surveillance, 2014 to 2017. Eurosurveillace 2019, 24. [Google Scholar] [CrossRef]
- Mor, A.; Frøslev, T.; Thomsen, R.W.; Oteri, A.; Rijnbeek, P.; Schink, T.; Garbe, E.; Pecchioli, S.; Innocenti, F.; Bezemer, I.; et al. Antibiotic use varies substantially among adults: A cross-national study from five European Countries in the ARITMO project. Infection 2015, 43, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Holstiege, J.; Enders, D.; Schink, T.; Innocenti, F.; Oteri, A.; Bezemer, I.; Kaguelidou, F.; Molokhia, M.; Poluzzi, E.; Puccini, A.; et al. Trends in paediatric macrolide use in five European countries-a population-based study. Eur. J. Clin. Pharmacol. 2015, 71, 991–999. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. WHO, 2015. Available online: http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf (accessed on 1 May 2019).
- Regione Siciliana, Assessorato della Salute, DASOE. Programma Regionale per l’azzeramento delle Infezioni CVC Correlate—Targeting Zero. Available online: https://www.qualitasiciliassr.it/sites/doc/ica/D.A._n._1004_del_01.06.2016.pdf (accessed on 1 May 2019).
| Consumption of Antibiotics for Systemic Use (J01) per 1000 Inhabitants Per Day | 2015 | 2016 | 2017 | p-Trend |
|---|---|---|---|---|
| Antibacterials for systemic use (J01) | 23.4 | 22.5 | 22.5 | 0.667 |
| Penicillin (J01C) | 11.6 | 11.3 | 11.5 | 0.879 |
| Other beta-lactams (J01D) | 2.6 | 2.5 | 2.3 | 0.212 |
| Cephalosporins (J01DB-DC-DD-DE) | 2.6 | 2.5 | 2.3 | 0.212 |
| Macrolides, lincosamides, and streptogramins (J01F) | 4.6 | 4.3 | 4.3 | 0.407 |
| Quinolones (J01M) | 3.8 | 3.5 | 3.5 | 0.667 |
| Fluoroquinolones (J01MA) | 3.8 | 3.5 | 3.5 | 0.667 |
| Microorganism | Antibiotic | Resistance Rates | p-Trend * | ||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||
| K. pneumoniae | Third-generation cephalosporins | 70.3 | 73.0 | 81.3 | <0.001 |
| Carbapenems | 44.3 | 48.9 | 45.0 | 0.838 | |
| Fluoroquinolones | 71.5 | 73.0 | 83.8 | <0.001 | |
| Colistin | 13.6 | 15.2 | 20.9 | <0.001 | |
| E. coli | Third-generation cephalosporins | 46.5 | 42.4 | 38.7 | <0.001 |
| Carbapenems | 4.8 | 1.9 | 1.6 | <0.001 | |
| Fluoroquinolones | 58.9 | 56.4 | 61.0 | 0.510 | |
| P. aeruginosa | Carbapenems | 33.1 | 25.9 | 43.3 | 0.031 |
| Ceftazidime, Fluoroquinolones, Piperacillin + Tazobactam | 39.3 | 35.7 | 27.5 | 0.004 | |
| A. baumannii | Carbapenems | 86.8 | 85.9 | 86.9 | 0.982 |
| S. aureus | Methicillin | 55.8 | 55.0 | 54.2 | 0.626 |
| S. pneumoniae | Erythromycin | 38.5 | 35.3 | 50.0 | 0.461 |
| Penicillin | 27.3 | 28.6 | 33.3 | 0.715 | |
| E. faecium | Vancomycin | 8.3 | 9.1 | 7.5 | 0.849 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barchitta, M.; Quattrocchi, A.; Maugeri, A.; La Rosa, M.C.; La Mastra, C.; Sessa, L.; Cananzi, P.; Murolo, G.; Oteri, A.; Basile, G.; et al. Antibiotic Consumption and Resistance during a 3-Year Period in Sicily, Southern Italy. Int. J. Environ. Res. Public Health 2019, 16, 2253. https://doi.org/10.3390/ijerph16132253
Barchitta M, Quattrocchi A, Maugeri A, La Rosa MC, La Mastra C, Sessa L, Cananzi P, Murolo G, Oteri A, Basile G, et al. Antibiotic Consumption and Resistance during a 3-Year Period in Sicily, Southern Italy. International Journal of Environmental Research and Public Health. 2019; 16(13):2253. https://doi.org/10.3390/ijerph16132253
Chicago/Turabian StyleBarchitta, Martina, Annalisa Quattrocchi, Andrea Maugeri, Maria Clara La Rosa, Claudia La Mastra, Laura Sessa, Pasquale Cananzi, Giuseppe Murolo, Alessandro Oteri, Guido Basile, and et al. 2019. "Antibiotic Consumption and Resistance during a 3-Year Period in Sicily, Southern Italy" International Journal of Environmental Research and Public Health 16, no. 13: 2253. https://doi.org/10.3390/ijerph16132253
APA StyleBarchitta, M., Quattrocchi, A., Maugeri, A., La Rosa, M. C., La Mastra, C., Sessa, L., Cananzi, P., Murolo, G., Oteri, A., Basile, G., & Agodi, A. (2019). Antibiotic Consumption and Resistance during a 3-Year Period in Sicily, Southern Italy. International Journal of Environmental Research and Public Health, 16(13), 2253. https://doi.org/10.3390/ijerph16132253







