Study on the In Vitro Activity of Five Disinfectants against Nosocomial Bacteria
Abstract
1. Introduction
2. Materials and Methods
- 1)
- o-phenylphenol (11.4%), p-ter-amylphenol (2.3%), o-benzyl-p-chloro-phenol (+8.2%);
- 2)
- didecyldimethylammonium chloride (8.5%) (DDAC);
- 3)
- sodium hypochlorite (1.1% active chlorine);
- 4)
- isopropanol (17.2%) + ammonium compounds (0.28%) (IACs);
- 5)
- hydrogen peroxide (1.5%).
- Klebsiella pneumoniae: 58 strains, of which 30 were susceptible to carbapenems (KP) and 28 were resistant to carbapenems (KPC);
- Pseudomonas aeruginosa: 59 strains, of which 30 were isolated from hospital water samples;
- Staphylococcus aureus: 40 strains, of which 20 were susceptible to methicillin (MSSA) and 20 were resistant to methicillin (MRSA);
- Enterococcus faecalis: 30 strains.
Statistical Analysis
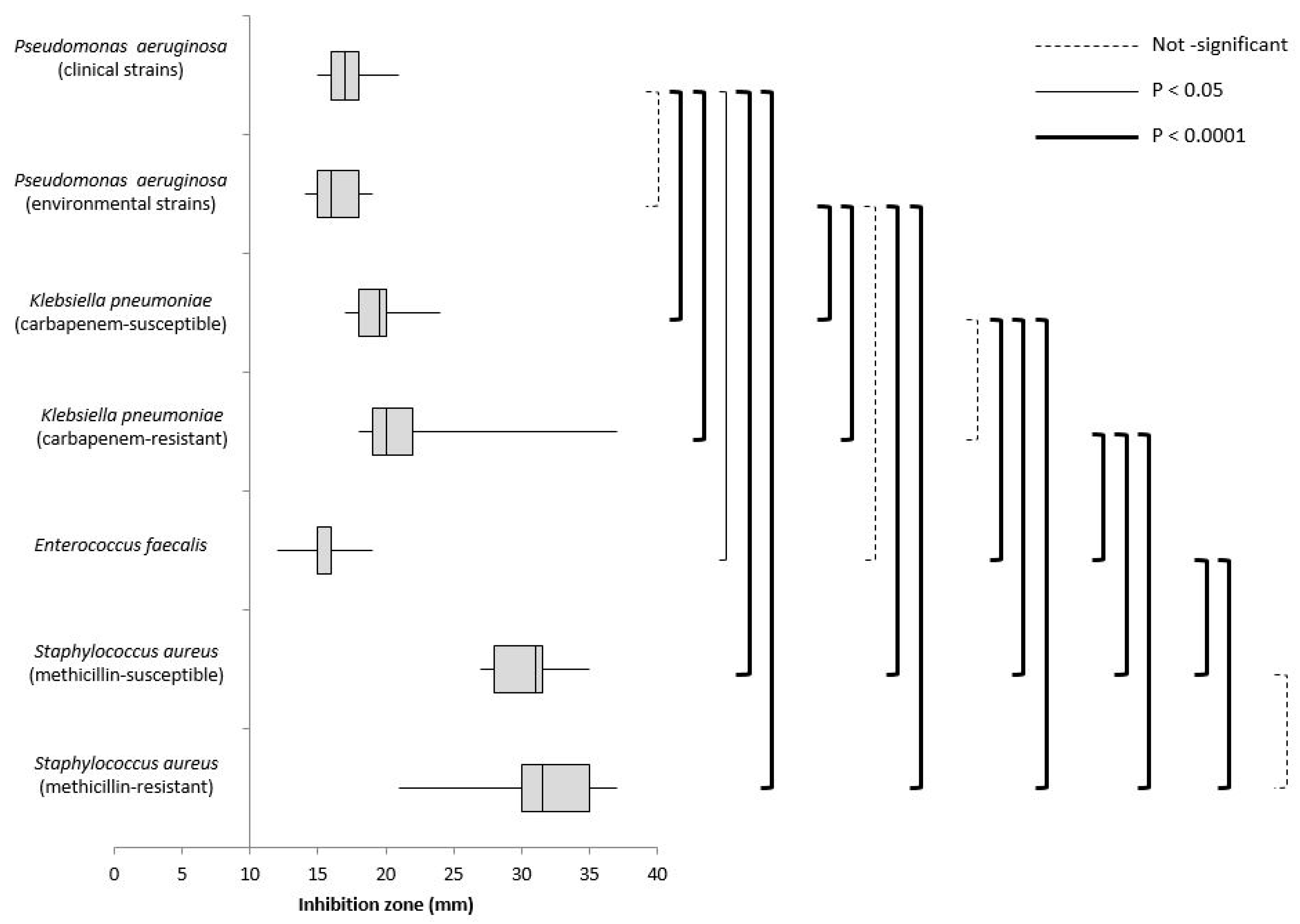
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 2018, 7, 154. [Google Scholar]
- Quinn, M.M.; Henneberger, P.K.; Braun, B.; Delclos, G.L.; Fagan, K.; Huang, V.; Knaack, J.L.; Kusek, L.; National Institute for Occupational Safety and Health (NIOSH); National occupational research agenda (NORA) cleaning and disinfecting in healthcare working group; et al. Cleaning and disinfecting environmental surfaces in health care: Toward an integrated framework for infection and occupational illness prevention. Am. J. Infect. Control 2015, 43, 424–434. [Google Scholar]
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infections Worldwide; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Block, S.S. Peroxygen compounds. In Disinfection, Sterilization, and Preservation, 5th ed.; Block, S.S., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 185–204. [Google Scholar]
- Ferreira, A.M.; De Andrade, D.; Rigotti, M.A.; De Almeida, M.T.G.; Guerra, O.G.; Dos Santos Junior, A.G. Assessment of disinfection of hospital surfaces using different monitoring methods. Rev. Lat. Am. Enferm. 2015, 23, 466–474. [Google Scholar] [CrossRef]
- Vickery, K.; Deva, A.; Jacombs, A.; Allan, J.; Valente, P.; Gosbell, I.B. Presence of biofilm containing viable multiresistant organisms despite terminal cleaning on clinical surfaces in an intensive care unit. J. Hosp. Infect. 2012, 80, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Anderson, D.; Rutala, W.A. The role of the surface environment in healthcare-associated infections. Curr. Opin. Infect. Dis. 2013, 26, 338–344. [Google Scholar]
- Liu, J.; Luo, Z.; Liu, K.; Zhang, Y.; Peng, H.; Hu, B.; Ren, H.; Zhou, X.; Qiu, S.; He, X.; et al. Flushing on the detachment of biofilms attached to the walls of metal pipes in water distribution systems. J. Zhejiang Univ.-Sci. A 2017, 18, 313–328. [Google Scholar]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacteria biofilms and associated infections. J. Chin. Med. Assoc. 2017, 81, 7–11. [Google Scholar]
- Paul, M.; Shani, V.; Mucihtar, E.; Kariv, G.; Robenshtok, E.; Leibovici, L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob. Agents Chemother. 2010, 54, 4851–4863. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- West, A.M.; Teska, P.J.; Lineback, C.B.; Oliver, H.F. Strain, disinfectant, concentration, and contact time quantitatively impact disinfectant efficacy. Antimicrob. Resist. Infect. Control 2018, 7, 49. [Google Scholar]
- Al-Tawfiq, J.A.; Tambyah, P.A. Healthcare associated infections (HAI) perspectives. J. Infect. Public Health 2014, 7, 339–344. [Google Scholar] [CrossRef]
- ECDC 2018. Available online: https: //www.thelancet.com/journals/laninf/article/PIIS1473-3099(18)30605-4/fulltext#sec1 (accessed on 23 January 2019).
- Italian Report PPS2 2016/2017 (page 2)—Italian Prevalence Study on Infections Related to the Assistance and Use of Antibiotics in Acute Care Hospitals—ECDC Protocol. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2791_allegato.pdf (accessed on 23 January 2019).
- D’Amico, A.; Montagna, M.T.; Caggiano, G.; De Giglio, O.; Rutigliano, S.; Lopuzzo, M.; Mascipinto, S.; Napoli, C.; Currà, E.; D’Alessandro, D. Observational study on hospital building heritage and microbiological air quality in the orthopedic operating theater: The IM.PA.C.T. Project. 2018. submitted. [Google Scholar]
- Boyce, J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 10. [Google Scholar] [CrossRef]
- Han, J.H.; Sullivan, N.; Leas, B.F.; Pegues, D.A.; Kaczmarek, J.L.; Umscheid, C.A. Cleaning hospital room surfaces to prevent health care-associated infections. Ann. Intern. Med. 2015, 163, 598–607. [Google Scholar] [CrossRef]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef]
- Donskey, C.J. Does improving surface cleaning and disinfection reduce health care-associated infections? Am. J. Infect. Control 2013, 41, S12–S19. [Google Scholar] [CrossRef]
- Rutala, W.A.; Weber, D.J. Disinfectants used for environmental disinfection and new room decontamination technology. Am. J. Infect. Control 2013, 41, 36–41. [Google Scholar] [CrossRef]
- Doerrbecker, J.; Friesland, M.; Ciesek, S. Inactivation and survival of hepatitis C virus on inanimate surfaces. J. Infect. Dis. 2011, 204, 1830–1838. [Google Scholar] [CrossRef]
- Falagas, M.E.; Thomaidis, P.C.; Kotsantis, I.K. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: A systematic review. J. Hosp. Infect. 2011, 78, 171–177. [Google Scholar] [CrossRef]
- Simões, M.; Simões, L.C.; Cleto, S.; Machado, I.; Pereira, M.O.; Vieira, M.J. Antimicrobial mechanisms of ortho-phthalaldehyde action. J. Basic Microbiol. 2007, 47, 230–242. [Google Scholar] [CrossRef]
- Russell, A.D. Bacterial resistance to disinfectants: Present knowledge and future problems. J. Hosp. Infect. 1999, 43, 57–68. [Google Scholar] [CrossRef]
- Campos, G.B.; Souza, S.G.; Lob, O.T.N.; Da Silva, D.C.; Sousa, D.S.; Oliveira, P.S.; Santos, V.M.; Amorim, A.T.; Farias, S.V.; Cruz, M.P.; et al. Isolation, molecular characteristics and disinfection of methicillin-resistant Staphylococcus aureus from ICU units in Brazil. New Microbiol. 2012, 35, 183–190. [Google Scholar]
- Chapman, J.S. Disinfectants resistance mechanisms, cross-resistance, and co-resistance. Int. Biodeterior. Biodegrad. 2003, 51, 271–276. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Reitzel, R.A.; Dvorak, T.L.; Hachem, R.Y.; Fang, X.; Jiang, Y.; Raad, I. Efficacy of novel antimicrobial gloves impregnated with antiseptic dyes in preventing the adherence of multidrug resistant nosocomial pathogens. Am. J. Infect. Control 2009, 37, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Eggimann, P.; Luyt, C.E.; Wolff, M.; Tamm, M.; François, B.; Mercier, E.; Garbino, J.; Laterre, P.F.; Koch, H.; et al. Pseudomonas aeruginosa serotypes in nosocomial pneumonia: Prevalence and clinical outcomes. Crit. Care 2014, 18, R17. [Google Scholar] [CrossRef]
- Le Berre, R.; Nguyen, S.; Nowak, E.; Kipnis, E.; Pierre, M.; Quenee, L.; Ader, F.; Lancel, S.; Courcol, R.; Guery, B.P.; et al. Relative contribution of three main virulence factors in Pseudomonas aeruginosa pneumonia. Crit. Care Med. 2011, 39, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, N.S.; Kocic, B.; Tiodorovic, B.; Brankovic, S.; Antic, S.M. Serotyping and analysis of produced pigments kinds by Pseudomonas aeruginosa clinical isolates. Vojnosanit. Pregl. 2011, 68, 923–929. [Google Scholar] [CrossRef]
- Nedeljković, N.S.; Tiodorović, B.; Kocić, B.; Cirić, B.; Milojković, M.; Waisi, H. Pseudomonas aeruginosa serotypes and resistance to antibiotics from wound swabs. Vojnosanit. Pregl. 2015, 72, 996–1003. [Google Scholar] [CrossRef]
- Fonseca, A.P.; Correia, P.; Sousa, J.C.; Tenreiro, R. Association patterns of Pseudomonas aeruginosa clinical isolates as revealed by virulence traits, antibiotic resistance, serotype and genotype. FEMS Immunol. Med. Microbiol. 2007, 51, 505–516. [Google Scholar] [CrossRef]
- Antisepsi, M.E.M.O. Disinfezione in Ambito Sanitario e Socio-Sanitario; Centro stampa regionale, Regione Emilia-Romagna: Bologna, Italy, 2011. [Google Scholar]
- Boyce, M.J.; Guercia, K.A.; Sullivan, L.; Havill, N.L.; Fekieta, R.; Kozakiewicz, J.; Goffman, D. Prospective cluster controlled crossover trial to compare the impact of an improved hydrogen peroxide disinfectant and a quaternary ammonium-based disinfectant on surface contamination and health care outcomes. Am. J. Infect. Control 2017, 45, 1006–1010. [Google Scholar] [CrossRef]
- De Giglio, O.; Coretti, C.; Lovero, G.; Barbuti, G.; Caggiano, G. Pilot study on the antibacterial activity of hydrogen peroxide and silver ions in the hospital environment. Annali di Igiene Medicina Preventiva e di Comunità 2014, 26, 181–185. [Google Scholar]
- French, G.L.; Otter, J.A.; Shannon, K.P.; Adams, N.M.T.; Watling, D.; Parks, M.J. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): A comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2004, 57, 31–37. [Google Scholar] [CrossRef]
- Estrela, C.; Estrela, C.R.A.; Barbin, E.L.; Spanó, J.C.; Marchesan, M.A.; Pécora, J.D. Mechanism of action of sodium hypoclorite. Braz. Dent. J. 2002, 2, 113–117. [Google Scholar] [CrossRef]
- Merritt, K.; Hitchins, V.M.; Brown, S.A. Safety and cleaning of medical materials and devices. J. Biomed. Mater. Res. 2000, 53, 131–136. [Google Scholar] [CrossRef]
- Goddard, P.A.; McCue, K.A. Phenolic compounds. In Disinfection, Sterilization, and Preservation, 5th ed.; Block, S.S., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 255–281. [Google Scholar]
- Omidbakhsh, N. Theoretical and experimental aspects of microbicidal activities of hard surface disinfectants: Are their label claims based on testing under field conditions? J. AOAC Int. 2010, 93, 1944–1951. [Google Scholar]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Bore, E.; Hébraud, M.; Chafsey, I.; Chambon, C.; Skjaeret, C.; Moen, B.; Møretrø, T.; Langsrud, Ø.; Rudi, K.; Langsrud, S. Adapted tolerance to benzalkonium chloride in Escherichia coli K-12 studied by transcriptome and proteome analyses. Microbiology 2007, 153, 935–946. [Google Scholar] [CrossRef]
- Wu, G.; Yang, Q.; Long, M.; Guo, L.; Li, B.; Meng, Y.; Zhang, A.; Wang, H.; Liu, S.; Zou, L. Evaluation of agar dilution and broth microdilution methods to determine the disinfectant susceptibility. J. Antibiot. 2015, 68, 661–665. [Google Scholar] [CrossRef]
- Russell, A.; Chopra, I. Understanding Antibacterial Action and Resistance; Ellis Horwood: London, UK, 1996. [Google Scholar]
- Gilbert, P.; McBain, A.J. Potential impact of increased use of biocides in consumer products on prevalence of antibiotic resistance. Clin. Microbiol. Rev. 2003, 16, 189–208. [Google Scholar] [CrossRef]
| Serotype | P. aeruginosa Strains | ||
|---|---|---|---|
| Clinical | Environmental | Total (%) | |
| O1 | 4 | 0 | 4 (6.8) |
| O3 | 6 | 6 | 12 (20.3) |
| O4 | 3 | 4 | 7 (11.9) |
| O6 | 6 | 9 | 15 (25.4) |
| O7 | 1 | 1 | 2 (3.4) |
| O9 | 2 | 2 | 4 (6.8) |
| O10 | 1 | 1 | 2 (3.4) |
| O11 | 7 | 6 | 13 (22.0) |
| Total | 30 (50.8%) | 29 (49.2%) | 59 (100%) |
| Microorganisms | INHIBITION ZONE (mm) | |||||
|---|---|---|---|---|---|---|
| Products Tested | ||||||
| Phenol Compounds | Sodium Hypochlorite | Hydrogen Peroxide | IACs | DDAC | ||
| Pseudomonas aeruginosa | Clinical (n = 29) | 0 | 0 | 17 | 0 | 0 |
| IQR 16–18 | ||||||
| Environmental (n = 30) | 0 | 0 | 16 | 0 | 0 | |
| IQR 15–18 | ||||||
| ATCC 27853 | 0 | 0 | 16 | 0 | 0 | |
| Klebsiella pneumoniae | Carbapenem-susceptible (n = 30) | 0 | 0 | 19.5 | 7 | 0 |
| IQR 18–20 | IQR 7–7 | |||||
| KPC (n = 30) | 0 | 0 | 20 | 7 | 0 | |
| IQR 19–22 | IQR 7–7 | |||||
| ATCC 43816 | 0 | 0 | 19 | 7 | 0 | |
| Enterococcus faecalis | (n = 30) | 0 | 0 | 16 | 10 | 13 |
| IQR 15–16 | IQR 10–11 | IQR 12–13 | ||||
| NCTC 775 | 0 | 0 | 20 | 11 | 13 | |
| Staphylococcus aureus | MSSA (n = 20) | 0 | 0 | 31 | 16 | 13 |
| IQR 28–31.5 | IQR 15.5–16.5 | IQR 13–14 | ||||
| MRSA (n = 20) | 0 | 0 | 31.5 | 16 | 14 | |
| IQR 30–35 | IQR 15–17 | IQR 14–14.5 | ||||
| NCTC 6571 | 0 | 0 | 30 | 15 | 13 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montagna, M.T.; Triggiano, F.; Barbuti, G.; Bartolomeo, N.; De Giglio, O.; Diella, G.; Lopuzzo, M.; Rutigliano, S.; Serio, G.; Caggiano, G. Study on the In Vitro Activity of Five Disinfectants against Nosocomial Bacteria. Int. J. Environ. Res. Public Health 2019, 16, 1895. https://doi.org/10.3390/ijerph16111895
Montagna MT, Triggiano F, Barbuti G, Bartolomeo N, De Giglio O, Diella G, Lopuzzo M, Rutigliano S, Serio G, Caggiano G. Study on the In Vitro Activity of Five Disinfectants against Nosocomial Bacteria. International Journal of Environmental Research and Public Health. 2019; 16(11):1895. https://doi.org/10.3390/ijerph16111895
Chicago/Turabian StyleMontagna, Maria Teresa, Francesco Triggiano, Giovanna Barbuti, Nicola Bartolomeo, Osvalda De Giglio, Giusy Diella, Marco Lopuzzo, Serafina Rutigliano, Gabriella Serio, and Giuseppina Caggiano. 2019. "Study on the In Vitro Activity of Five Disinfectants against Nosocomial Bacteria" International Journal of Environmental Research and Public Health 16, no. 11: 1895. https://doi.org/10.3390/ijerph16111895
APA StyleMontagna, M. T., Triggiano, F., Barbuti, G., Bartolomeo, N., De Giglio, O., Diella, G., Lopuzzo, M., Rutigliano, S., Serio, G., & Caggiano, G. (2019). Study on the In Vitro Activity of Five Disinfectants against Nosocomial Bacteria. International Journal of Environmental Research and Public Health, 16(11), 1895. https://doi.org/10.3390/ijerph16111895








