Dementia-Related Functional Disability in Moderate to Advanced Parkinson’s Disease: Assessment Using the World Health Organization Disability Assessment Schedule 2.0
Abstract
1. Introduction
2. Materials and Methods
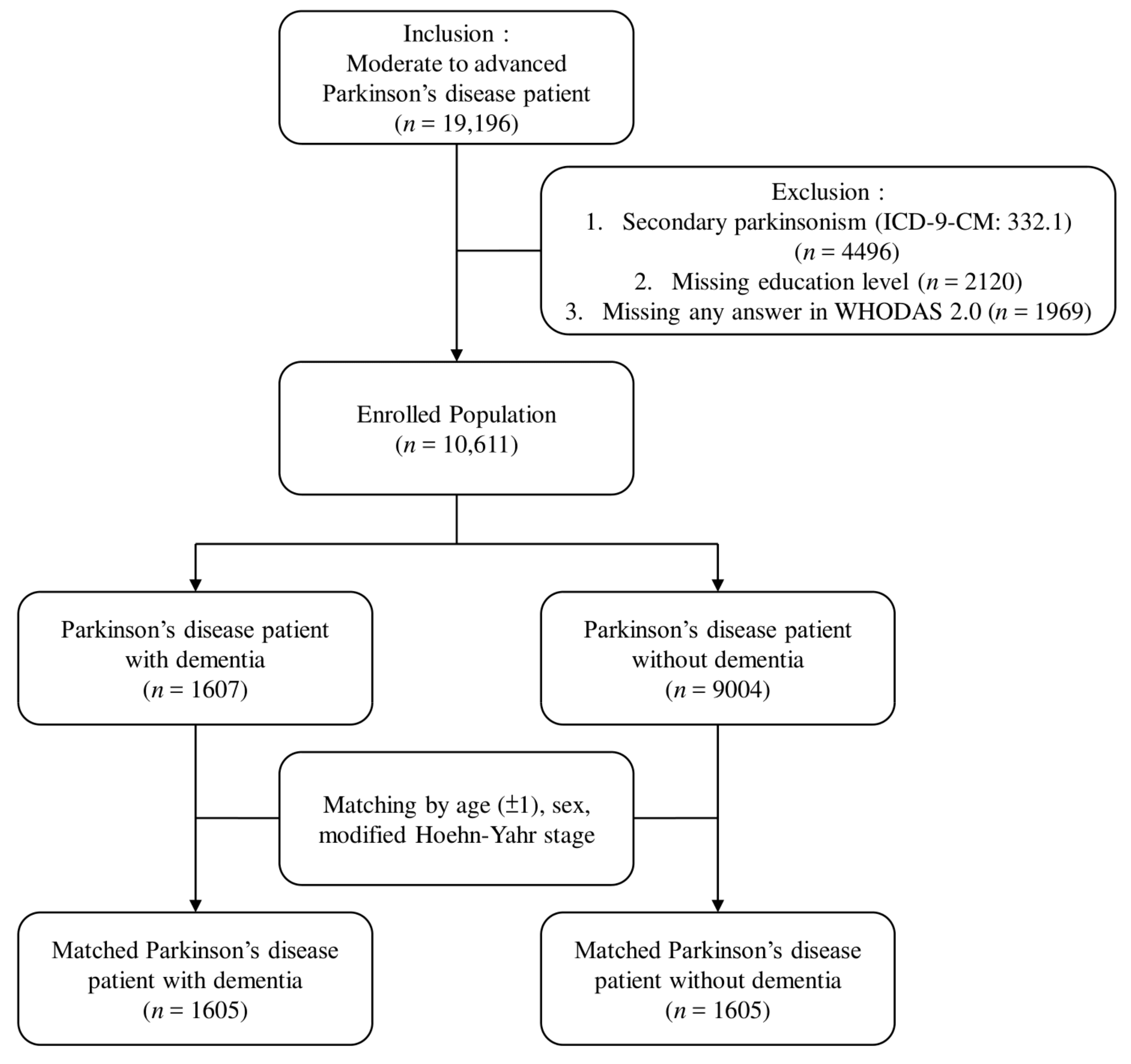
2.1. Study Design and Participants
2.2. Instruments and Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schapira, A.H.V. Etiology of Parkinson’s disease. Neurology 2006, 66, S10–S23. [Google Scholar] [CrossRef] [PubMed]
- Von Campenhausen, S.; Bornschein, B.; Wick, R.; Bötzel, K.; Sampaio, C.; Poewe, W.; Oertel, W.; Siebert, U.; Berger, K.; Dodel, R. Prevalence and incidence of Parkinson’s disease in Europe. Eur. Neuropsychopharmacol. 2005, 15, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Strickland, D.; Bertoni, J.M. Parkinson’s prevalence estimated by a state registry. Mov. Disord. 2004, 19, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Bauso, D.J.; Tartari, J.P.; Stefani, C.V.; Rojas, J.I.; Giunta, D.H.; Cristiano, E. Incidence and prevalence of Parkinson’s disease in Buenos Aires City, Argentina: Incidence of Parkinson′s disease. Eur. J. Neurol. 2012, 19, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Okubadejo, N.U.; Bower, J.H.; Rocca, W.A.; Maraganore, D.M. Parkinson’s disease in Africa: A systematic review of epidemiologic and genetic studies. Mov. Disord. 2006, 21, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Muangpaisan, W.; Hori, H.; Brayne, C. Systematic review of the prevalence and incidence of Parkinson’s disease in Asia. J. Epidemiol. 2009, 19, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Benamer, H.T.S.; de Silva, R.; Siddiqui, K.A.; Grosset, D.G. Parkinson’s disease in Arabs: A systematic review. Mov. Disord. 2008, 23, 1205–1210. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Lerman, S.F.; Bronner, G.; Cohen, O.S.; Elincx-Benizri, S.; Strauss, H.; Yahalom, G.; Hassin-Baer, S. Catastrophizing mediates the relationship between non-motor symptoms and quality of life in Parkinson’s disease. Disabil. Health J. 2019. [Google Scholar] [CrossRef]
- Garcia-Ptacek, S.; Kramberger, M.G. Parkinson Disease and Dementia. J. Geriatr. Psychiatry Neurol. 2016, 29, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Gauthier, S.; Aarsland, D.; Leverenz, J.B.; Barone, P.; Weintraub, D.; Tolosa, E.; Dubois, B. Diagnosis and management of Parkinson’s disease dementia: Diagnosis and management of PDD. Int. J. Clin. Pract. 2008, 62, 1581–1587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aarsland, D.; Kurz, M.W. The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol. 2010, 20, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Zaccai, J.; Brayne, C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov. Disord. 2005, 20, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Stella, F.; Banzato, C.E.M.; Quagliato, E.M.A.B.; Viana, M.A.; Christofoletti, G. Dementia and functional decline in patients with Parkinson’s disease. Dement. Neuropsychol. 2008, 2, 96–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiseman, J.P.; Fackrell, R. Caregiver burden and the nonmotor symptoms of Parkinson’s disease. Int. Rev. Neurobiol. 2017, 133, 479–497. [Google Scholar]
- Kowal, S.L.; Dall, T.M.; Chakrabarti, R.; Storm, M.V.; Jain, A. The current and projected economic burden of Parkinson’s disease in the United States. Mov. Disord. 2013, 28, 311–318. [Google Scholar] [CrossRef]
- Fredericks, D.; Norton, J.C.; Atchison, C.; Schoenhaus, R.; Pill, M.W. Parkinson’s disease and Parkinson’s disease psychosis: A perspective on the challenges, treatments, and economic burden. Am. J. Manage. Care 2017, 23, S83–S92. [Google Scholar]
- Yamabe, K.; Liebert, R.; Flores, N.; Pashos, C. Health-related quality-of-life, work productivity, and economic burden among patients with Parkinson’s disease in Japan. J. Med. Econ. 2018, 21, 1206–1212. [Google Scholar] [CrossRef]
- Women With Disabilities Australia Schedule. WHODAS 2.0; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Liu, W.-M.; Wu, R.-M.; Lin, J.-W.; Liu, Y.-C.; Chang, C.-H.; Lin, C.-H. Time trends in the prevalence and incidence of Parkinson’s disease in Taiwan: A nationwide, population-based study. J. Formos. Med. Assoc. 2016, 115, 531–538. [Google Scholar] [CrossRef]
- Wang, S.J.; Fuh, J.L.; Teng, E.L.; Liu, C.Y.; Lin, K.P.; Chen, H.M.; Lin, C.H.; Wang, P.N.; Ting, Y.C.; Wang, H.C.; et al. A door-to-door survey of Parkinson’s disease in a Chinese population in Kinmen. Arch. Neurol. 1996, 53, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Lo Buono, V.; Corallo, F.; Foti, M.; Di Lorenzo, G.; Bramanti, P.; Marino, S. Nonmotor symptoms in Parkinson disease: A descriptive review on social cognition ability. J. Geriatr. Psychiatry Neurol. 2017, 30, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Murata, C.; Saito, M.; Takeda, T.; Kondo, K. Influence of social relationship domains and their combinations on incident dementia: A prospective cohort study. J. Epidemiol. Community Health 2018, 72, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.S.; Zuidersma, M.; Oude Voshaar, R.C.; Zuidema, S.U.; van den Heuvel, E.R.; Stolk, R.P.; Smidt, N. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res. Rev. 2015, 22, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Bronner, G.; Aharon-Peretz, J.; Hassin-Baer, S. Sexuality in patients with Parkinson’s disease, Alzheimer’s disease, and other dementias. Handb. Clin. Neurol. 2015, 130, 297–323. [Google Scholar]
- Fratiglioni, L.; Wang, H.X.; Ericsson, K.; Maytan, M.; Winblad, B. Influence of social network on occurrence of dementia: A community-based longitudinal study. Lancet 2000, 355, 1315–1319. [Google Scholar] [CrossRef]
- Berkman, L.F.; Glass, T.; Brissette, I.; Seeman, T.E. From social integration to health: Durkheim in the new millennium. Soc. Sci. Med. 2000, 51, 843–857. [Google Scholar] [CrossRef]
- Eisele, M.; Zimmermann, T.; Köhler, M.; Wiese, B.; Heser, K.; Tebarth, F.; Weeg, D.; Olbrich, J.; Pentzek, M.; Fuchs, A.; et al. Influence of social support on cognitive change and mortality in old age: Results from the prospective multicentre cohort study AgeCoDe. BMC Geriatr. 2012, 12, 9. [Google Scholar] [CrossRef]
- Rasovska, H.; Rektorova, I. Instrumental activities of daily living in Parkinson’s disease dementia as compared with Alzheimer’s disease: Relationship to motor disability and cognitive deficits: A pilot study. J. Neurol. Sci. 2011, 310, 279–282. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Silverberg, N.; Bircea, S.; Majeed, B.; Samant, S.; Caviness, J.N.; Reisberg, B.; Adler, C.H. Is the functional decline of Parkinson’s disease similar to the functional decline of Alzheimer’s disease? Parkinsonism Relat. Disord. 2005, 11, 311–315. [Google Scholar] [CrossRef]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar]
- Augustine, E.F.; Pérez, A.; Dhall, R.; Umeh, C.C.; Videnovic, A.; Cambi, F.; Wills, A.-M.A.; Elm, J.J.; Zweig, R.M.; Shulman, L.M.; et al. Sex differences in clinical features of early, treated Parkinson’s disease. PLoS ONE 2015, 10, e0133002. [Google Scholar] [CrossRef]
- Szeto, J.Y.Y.; Lewis, S.J.G. Current treatment options for Alzheimer’s disease and Parkinson’s disease dementia. Curr. Neuropharmacol. 2016, 14, 326–338. [Google Scholar] [CrossRef]
- Li, S.; Dong, J.; Cheng, C.; Le, W. Therapies for Parkinson’s diseases: Alternatives to current pharmacological interventions. J. Neural Transm. 2016, 123, 1279–1299. [Google Scholar] [CrossRef]
- Hindle, J.V.; Watermeyer, T.J.; Roberts, J.; Martyr, A.; Lloyd-Williams, H.; Brand, A.; Gutting, P.; Hoare, Z.; Edwards, R.T.; Clare, L. Cognitive rehabilitation for Parkinson’s disease dementia: A study protocol for a pilot randomised controlled trial. Trials 2016, 17, 152. [Google Scholar] [CrossRef][Green Version]
- McCormick, S.A.; McDonald, K.R.; Vatter, S.; Orgeta, V.; Poliakoff, E.; Smith, S.; Silverdale, M.A.; Fu, B.; Leroi, I. Psychosocial therapy for Parkinson’s-related dementia: Study protocol for the INVEST randomised controlled trial. BMJ Open 2017, 7, e016801. [Google Scholar] [CrossRef]
| Variables | PwPD (n = 1607) (n/%) | PwPND (n = 9004) (n/%) | p-Value |
|---|---|---|---|
| Sex | <0.001 | ||
| Male | 732/45.55% | 4551/50.545% | |
| Female | 875/54.45% | 4453/49.46% | |
| Age | |||
| 18–44 | 5/0.3% | 89/1.0% | |
| 45–64 | 82/5.1% | 1926/21.4% | |
| 65 or older | 1520/94.6% | 6989/77.6% | |
| Total mean ± SD | 77.5 ± 7.2 | 72.4 ± 9.8 | <0.001 |
| Education | <0.001 | ||
| College or higher | 32/2.0% | 191/2.1% | |
| Senior high school | 97/6.0% | 762/8.5% | |
| Junior high school | 126/7.8% | 1380/15.4% | |
| Primary school | 1011/62.9% | 5583/62.0% | |
| No education | 341/21.2% | 1088/12.1% | |
| Residence | <0.001 | ||
| Community dwelling | 1315/81.8% | 8086/89.8% | |
| Care facility | 292/18.2% | 918/10.2% | |
| Urbanization level | <0.001 | ||
| Rural | 260/16.2% | 1037/11.5% | |
| Suburban | 590/36.7% | 3141/34.9% | |
| Urban | 757/47.1% | 4826/53.6% | |
| Hoehn-Yahr Stage | <0.001 | ||
| Stage 3 | 481/29.9% | 3452/38.3% | |
| Stage 4 | 693/43.1% | 3711/41.2% | |
| Stage 5 | 433/26.9% | 1841/20.5% |
| Variables | Male | Female | ||||
|---|---|---|---|---|---|---|
| PwPD (n = 731) (n/%) | PwPND (n = 731) (n/%) | p-Value | PwPD (n = 874) (n/%) | PwPND (n = 874) (n/%) | p-Value | |
| Age | 0.97 | 1 | ||||
| 18–44 | 3/0.4% | 3/0.4% | 1/0.1% | 1/0.1% | ||
| 45–64 | 52/7.1% | 52/7.1% | 30/3.4% | 31/3.6% | ||
| 65 or older | 676/92.5% | 676/92.5% | 843/96.5% | 842/96.3% | ||
| Total (mean ± SD) | 76.8 ± 7.8 | 76.8 ± 7.8 | 0.92 | 78.2 ± 6.4 | 78.1 ± 6.4 | 0.93 |
| Education | 0.06 | 0.17 | ||||
| College or higher | 25/3.4% | 24/3.3% | 7/0.8% | 5/0.6% | ||
| Senior high school | 70/9.6% | 66/9.0% | 27/3.1% | 34/3.9% | ||
| Junior high school | 80/10.9% | 70/9.6% | 45/5.2% | 49/5.6% | ||
| Primary school | 482/65.9% | 525/71.8% | 528/60.4% | 563/64.4% | ||
| No education | 74/10.1% | 46/6.3% | 267/30.6% | 233/25.5% | ||
| Residence | <0.01 | <0.001 | ||||
| Community dwelling | 603/82.5% | 645/88.2% | 711/81.4% | 768/87.9% | ||
| Care facility | 128/17.5% | 86/11.8% | 163/18.7% | 106/12.1% | ||
| Urbanization level | <0.01 | 0.01 | ||||
| Rural | 104/14.2% | 80/10.9% | 155/17.7% | 123/14.1% | ||
| Suburban | 260/35.6% | 224/30.6% | 330/37.8% | 305/34.9% | ||
| Urban | 367/50.2% | 427/58.4% | 389/44.5% | 446/51.0% | ||
| Hoehn–Yahr stage | 1 | 1 | ||||
| Stage 3 | 267/36.5% | 267/36.5% | 213/24.4% | 213/24.4% | ||
| Stage 4 | 304/41.6% | 304/41.6% | 389/44.5% | 389/44.5% | ||
| Stage 5 | 160/21.9% | 160/21.9% | 272/31.1% | 272/31.1% | ||
| WHODAS 2.0 score (mean ± SD) | ||||||
| Cognition (domain 1) | 62.7 ± 27.7 | 50.0 ± 29.1 | <0.001 | 64.5 ± 27.7 | 54.5 ± 30.1 | <0.001 |
| Mobility (domain 2) | 59.2 ± 28.3 | 59.6 ± 27.5 | 0.88 | 62.9 ± 28.5 | 65.2 ± 26.5 | 0.15 |
| Self-care (domain 3) | 40.2 ± 31.6 | 40.0 ± 30.9 | 0.90 | 42.8 ± 33.8 | 42.1 ± 33.4 | 0.73 |
| Getting along with people (domain 4) | 69.3 ± 28.4 | 60.9 ± 30.4 | <0.001 | 69.7 ± 28.3 | 62.6 ± 31.8 | <0.001 |
| Life activities (domain 5-1) | 76.8 ± 35.6 | 70.2 ± 38.7 | <0.001 | 76.9 ± 35.0 | 74.1 ± 37.8 | 0.43 |
| Participation in society (domain 6) | 47.1 ± 24.1 | 48.7 ± 24.7 | 0.25 | 49.0 ± 24.8 | 50.5 ± 25.2 | 0.17 |
| Average score | 58.0 ± 21.5 | 53.8 ± 22.4 | <0.001 | 59.9 ± 22.1 | 57.2 ±22.5 | <0.01 |
| Questions | Male | Female | |||||
|---|---|---|---|---|---|---|---|
| PwPD (n = 731) | PwPND (n = 731) | p-Value | PwPD (n = 874) | PwPND (n = 874) | p-Value | ||
| Getting along (domain 4) (mean ± SD) | |||||||
| D4.1 | Dealing with people you do not know | 2.4 ± 1.3 | 2.0 ± 1.5 | <0.001 | 2.4 ± 1.3 | 2.1 ± 1.5 | <0.001 |
| D4.2 | Maintaining friendships | 2.6 ± 1.3 | 2.2 ± 1.4 | <0.001 | 2.6 ± 1.3 | 2.3 ± 1.5 | <0.001 |
| D4.3 | Getting along with people who are close to you | 1.8 ± 1.3 | 1.4 ± 1.3 | <0.001 | 1.8 ± 1.4 | 1.5 ± 1.4 | <0.001 |
| D4.4 | Making new friends | 3.0 ± 1.2 | 2.7 ± 1.4 | <0.001 | 3.0 ± 1.2 | 2.9 ± 1.3 | 0.06 |
| D4.5 | Sexual activity | 3.1 ± 1.3 | 3.0 ± 1.4 | 0.34 | 3.2 ± 1.2 | 3.1 ± 1.3 | 0.56 |
| Life activities (domain 5-1) (mean ± SD) | |||||||
| D5.1 | Taking care of your household responsibilities | 2.9 ± 1.5 | 2.7 ± 1.6 | <0.01 | 2.9 ± 1.4 | 2.8 ± 1.5 | 0.16 |
| D5.2 | Doing most important household tasks well | 2.9 ± 1.5 | 2.6 ± 1.6 | <0.001 | 3.0 ± 1.4 | 2.8 ± 1.5 | 0.11 |
| D5.3 | Getting all the household work done that you needed to do | 2.9 ± 1.5 | 2.6 ± 1.6 | <0.001 | 2.9 ± 1.5 | 2.8 ± 1.5 | 0.34 |
| D5.4 | Getting your household work done as quickly as needed | 3.0 ± 1.4 | 2.7 ± 1.6 | <0.001 | 3.0 ± 1.4 | 2.9 ± 1.5 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-H.; Hong, C.-T.; Wu, D.; Chi, W.-C.; Yen, C.-F.; Liao, H.-F.; Chan, L.; Liou, T.-H. Dementia-Related Functional Disability in Moderate to Advanced Parkinson’s Disease: Assessment Using the World Health Organization Disability Assessment Schedule 2.0. Int. J. Environ. Res. Public Health 2019, 16, 2230. https://doi.org/10.3390/ijerph16122230
Chen J-H, Hong C-T, Wu D, Chi W-C, Yen C-F, Liao H-F, Chan L, Liou T-H. Dementia-Related Functional Disability in Moderate to Advanced Parkinson’s Disease: Assessment Using the World Health Organization Disability Assessment Schedule 2.0. International Journal of Environmental Research and Public Health. 2019; 16(12):2230. https://doi.org/10.3390/ijerph16122230
Chicago/Turabian StyleChen, Jia-Hung, Chien-Tai Hong, Dean Wu, Wen-Chou Chi, Chia-Feng Yen, Hua-Fang Liao, Lung Chan, and Tsan-Hon Liou. 2019. "Dementia-Related Functional Disability in Moderate to Advanced Parkinson’s Disease: Assessment Using the World Health Organization Disability Assessment Schedule 2.0" International Journal of Environmental Research and Public Health 16, no. 12: 2230. https://doi.org/10.3390/ijerph16122230
APA StyleChen, J.-H., Hong, C.-T., Wu, D., Chi, W.-C., Yen, C.-F., Liao, H.-F., Chan, L., & Liou, T.-H. (2019). Dementia-Related Functional Disability in Moderate to Advanced Parkinson’s Disease: Assessment Using the World Health Organization Disability Assessment Schedule 2.0. International Journal of Environmental Research and Public Health, 16(12), 2230. https://doi.org/10.3390/ijerph16122230







