Personalized Moderate-Intensity Exercise Training Combined with High-Intensity Interval Training Enhances Training Responsiveness
Abstract
1. Introduction
2. Materials and Methods
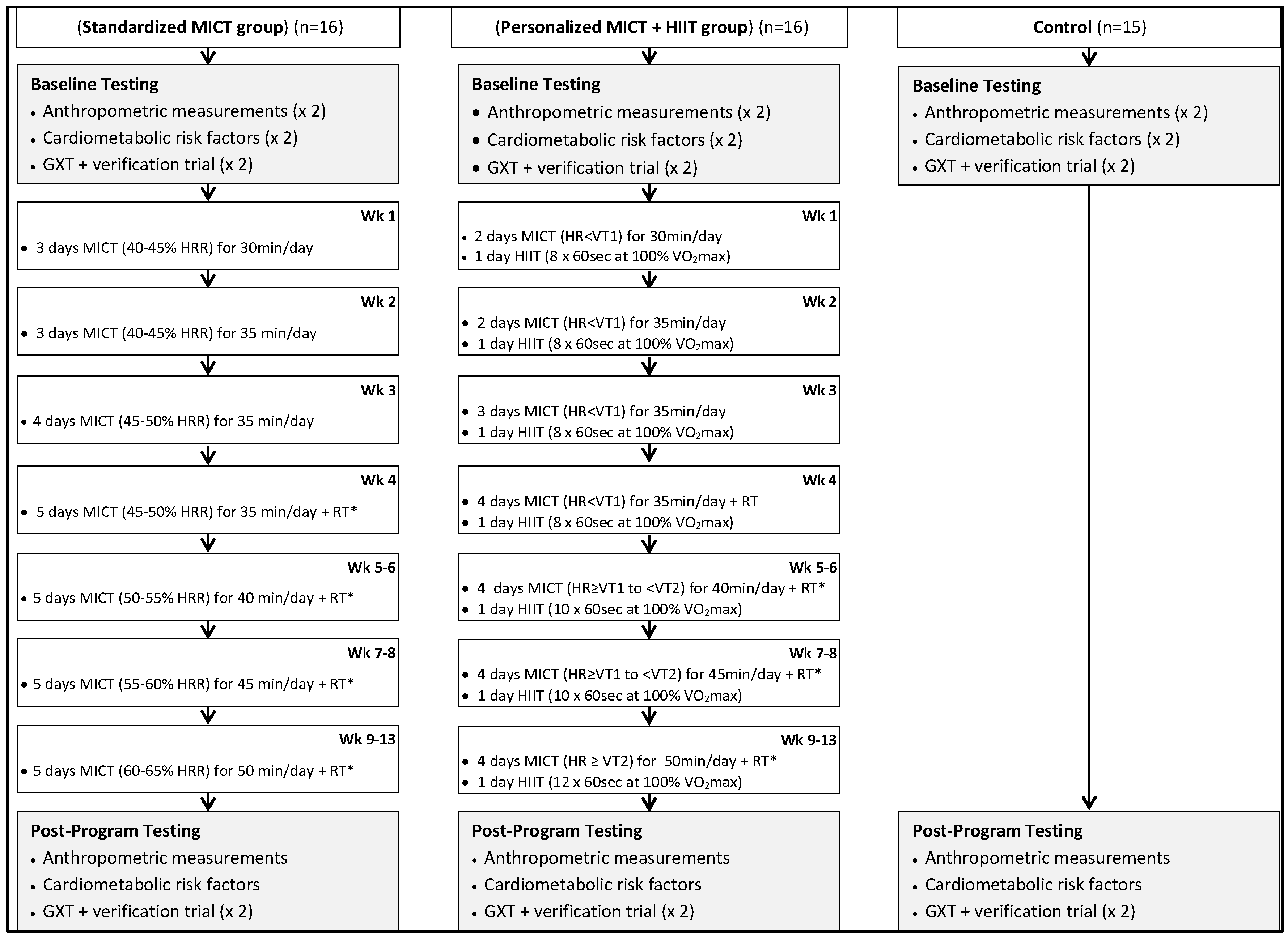
2.1. Experimental Design
2.2. Moderate-Intensity Continuous Training (MICT)
- Wk 1–4 (HR < VT1): target HR = HR range of 10–15 bpm just below VT1
- Wk 5–8 (HR ≥ VT1 to < VT2): target HR = HR range of 10–20 bpm (above VT1 and below VT2)
- Wk 9–13 (HR ≥ VT2): target HR = HR range of 10–15 bpm at or just above VT2
2.3. High-Intensity Interval Training (HIIT)
2.4. Resistance Exercise Prescription
2.5. Resistance Training for the Standardized Group
2.6. Resistance Training for the Personalized Group
2.7. Anthropometric, Cardiovascular, and Cardiometabolic Measurements
2.8. Metabolic Syndrome z-Score
2.9. Muscular Fitness Assessments
- 10 repetitions of a weight the participant felt comfortable lifting (40–60% of estimated 5-RM) were performed to warm up muscles followed by 1 min rest period
- 5 repetitions at a weight of 60–80% estimated 5-RM was performed as a further warm up and followed by a 2 min rest period
- First 5-RM attempt at a weight of 2.5–20 kg greater than warm up
- If the first 5-RM lift was deemed successful by the researcher (appropriate lifting form) weight was increased until maximum weight participant can lift was established with 3 min between each attempt.
- If the first 5-RM lift deemed unsuccessful by the researcher, weight was decreased until the participant successfully lifted the heaviest weight possible.
2.10. Maximal Exercise Testing and Verification Bout Trial Procedures
2.11. Quantification of Ventilatory Thresholds
2.12. Statistical Analyses
3. Results
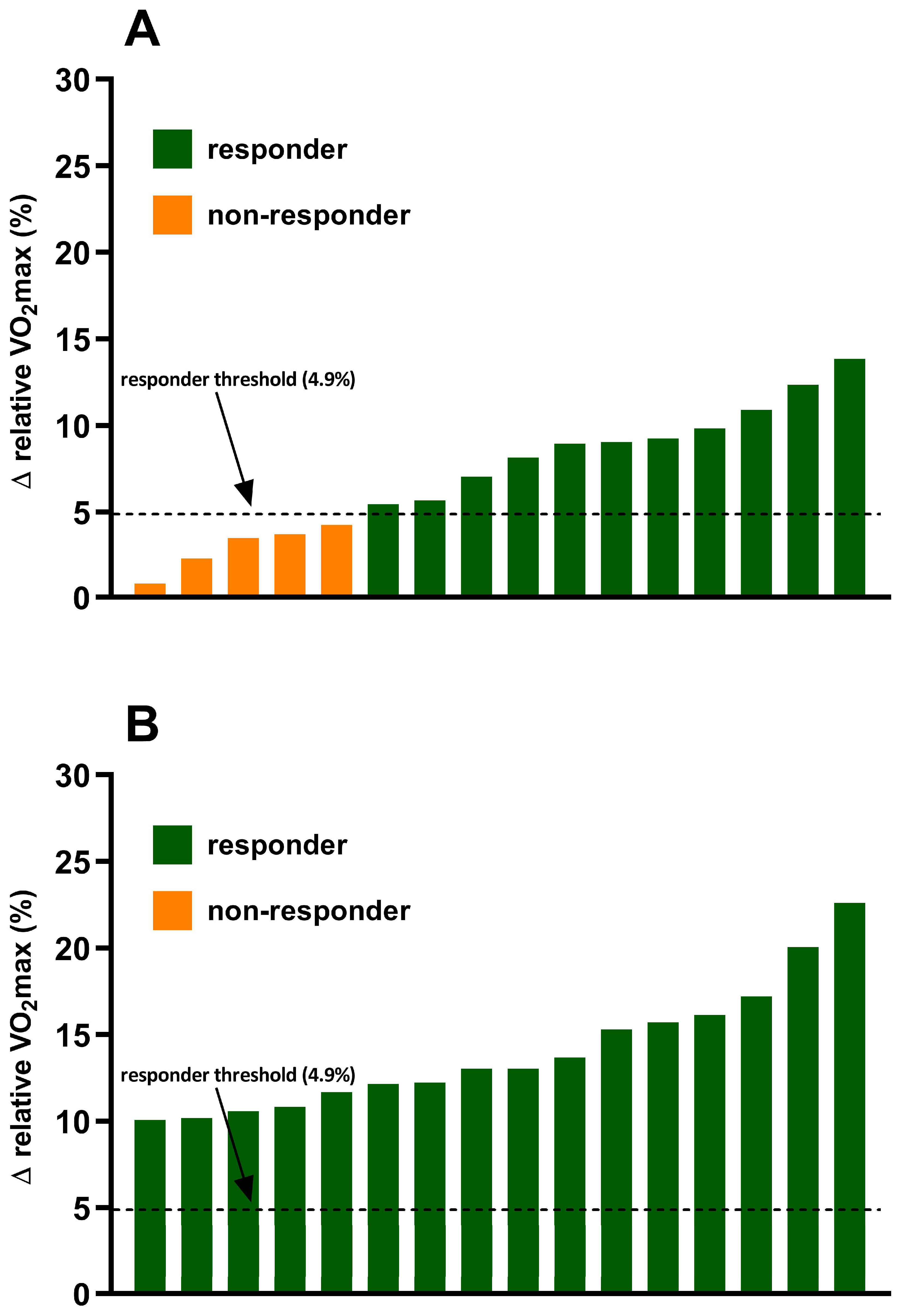
3.1. Incidence of VO2max Responders and Non-Responders
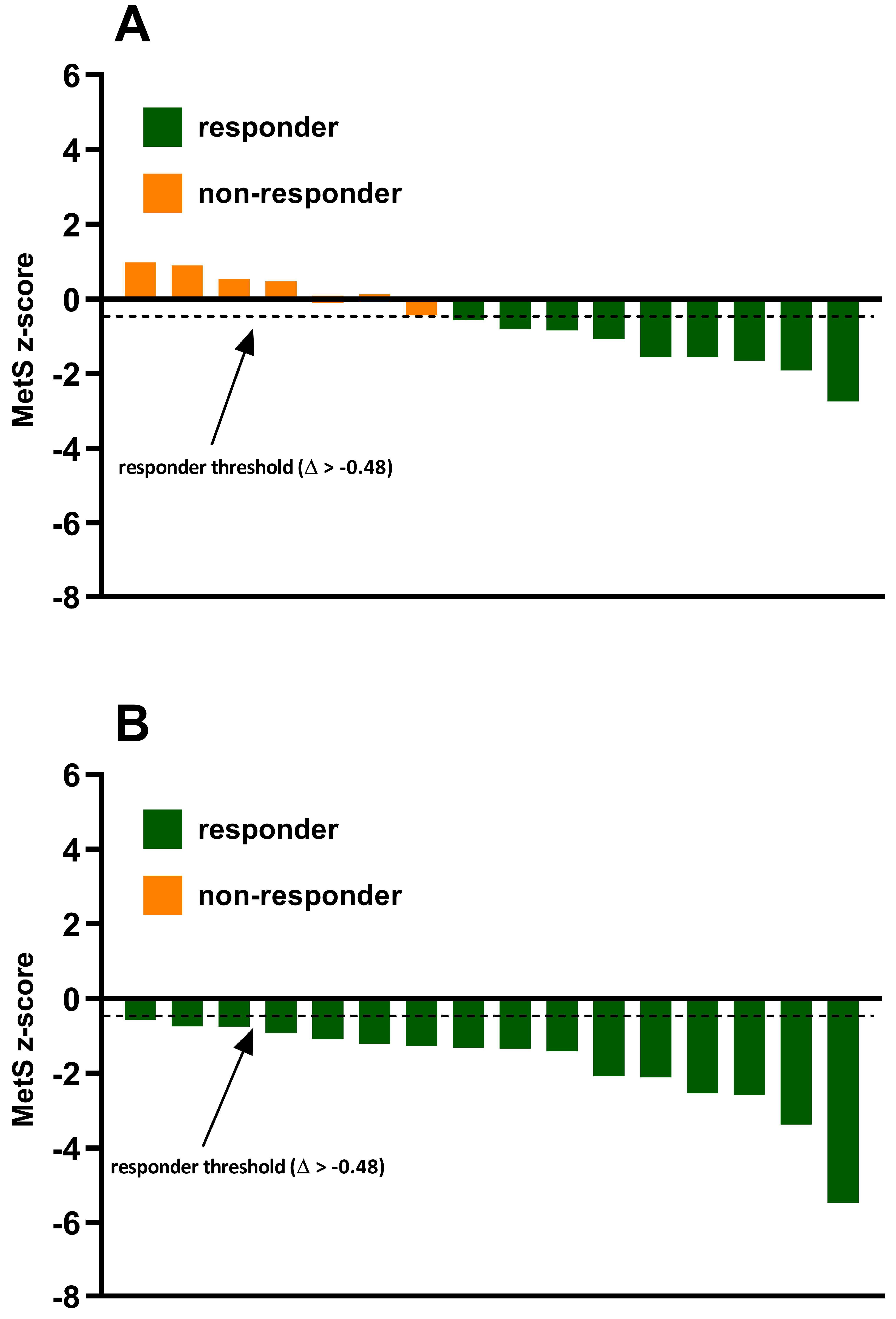
3.2. Incidence of MetS z-Score Responders and Non-Responders
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swift, D.L.; Lavie, C.J.; Johannsen, N.M.; Arena, R.; Earnest, C.P.; O’Keefe, J.H.; Milani, R.V.; Blair, S.N.; Church, T.S. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ. J. 2013, 77, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; An, P.; Rice, T.; Skinner, J.S.; Wilmore, J.H.; Gagnon, J.; Pérusse, L.; Leon, A.S.; Rao, D.C. Familial aggregation of VO2max response to exercise training: Results from the HERITAGE Family Study. J. Appl. Physiol. 1999, 87, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Couchard, C.; Blair, S.N.; Church, T.S.; Earnest, C.P.; Hagberg, J.M.; Häkkinen, K.; Jenkins, N.T.; Karavirta, L.; Kraus, W.E.; Leon, A.S.; et al. Adverse metabolic response to regular exercise: Is it a rare or common occurrence? PLoS ONE 2012, 7, e37887. [Google Scholar] [CrossRef]
- Weatherwax, R.M.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. The incidence of training responsiveness to cardiorespiratory fitness and cardiometabolic measurements following personalized and standardized exercise prescription: Study protocol for a randomized controlled trial. Trials 2016, 17, 601. [Google Scholar] [CrossRef]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wolpern, A.E.; Burgos, D.J.; Janot, J.M.; Dalleck, L.C. Is a threshold-based model a superior method to the relative percent concept for establishing individual exercise intensity? A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2015, 7, 16. [Google Scholar] [CrossRef]
- Weatherwax, R.M.; Ramos, J.S.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. Changes in Metabolic Syndrome Severity Following Individualized Versus Standardized Exercise Prescription: A Feasibility Study. Int. J. Environ. Res. Public Health 2018, 15, 2594. [Google Scholar] [CrossRef]
- Katch, V.; Weltman, A.; Sady, S.; Freedson, P. Validity of the relative percent concept for equating training intensity. Eur. J. Appl. Physiol. Occup. Physiol. 1978, 39, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Tjønna, A.E.; Lee, S.J.; Rognmo, Ø.; Stølen, T.O.; Bye, A.; Haram, P.M.; Loennechen, J.P.; Al-Share, Q.Y.; Skogvoll, E.; Slørdahl, S.A.; et al. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: A pilot study. Circulation 2008, 118, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.S.; Dalleck, L.C.; Borrani, F.; Beetham, K.S.; Wallen, M.P.; Mallard, A.R.; Clark, B.; Gomersall, S.; Keating, S.E.; Fassett, R.G.; et al. Low-volume high-intensity interval training is sufficient to ameliorate the severity of metabolic syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 319–328. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2018. [Google Scholar]
- Roxburgh, B.H.; Nolan, P.B.; Weatherwax, R.M.; Dalleck, L.C. Is moderate intensity exercise training combined with high intensity interval training more effective at improving cardiorespiratory fitness than moderate intensity exercise training alone? J. Sports Sci. Med. 2014, 13, 702–707. [Google Scholar] [PubMed]
- Bryant, C.X.; Green, D.J. ACE Personal Trainer Manual; American Council on Exercise: San Diego, CA, USA, 2010. [Google Scholar]
- Borg, G. Psychophysical bases of perceived exertion. Med. Sci. Sports. Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Sweet, T.; Foster, C.; McGuigan, M.; Brice, G. Quantitation of resistance training using the session rating of perceived exertion method. J. Strength Cond. Res. 2004, 18, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.K.; Nightingale, J.; Choi, S.E.; Chipkin, S.R.; Braun, B. Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adults. Obesity 2013, 21, 93–100. [Google Scholar] [CrossRef]
- Dalleck, L.C.; Astorino, T.A.; Erickson, R.M.; McCarthy, C.M.; Beadell, A.A.; Botten, B.H. Suitability of verification testing to confirm attainment of VO2max in middle-aged and older adults. Res. Sports. Med. 2012, 20, 118–128. [Google Scholar] [CrossRef]
- Wasserman, K.; McIlroy, M.B. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. Am. J. Cardiol. 1964, 14, 844–852. [Google Scholar] [CrossRef]
- Skinner, J.S.; Jaskólski, A.; Jaskólska, A.; Krasnoff, J.; Gagnon, J.; Leon, A.S.; Rao, D.C.; Wilmore, J.H.; Bouchard, C.; HERITAGE Family Study. Age, sex, race, initial fitness, and response to training: The HERITAGE Family Study. J. Appl. Physiol. 2001, 90, 1770–1776. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Dalleck, L.C.; Allen, B.A.; Hanson, B.A.; Borresen, E.C.; Erickson, M.E.; De Lap, S.L. Dose-response relationship between moderate-intensity exercise duration and coronary heart disease risk factors in postmenopausal women. J. Womens Health (Larchmt.) 2009, 18, 105–113. [Google Scholar] [CrossRef]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Weatherwax, R.M.; Harris, N.K.; Kilding, A.E.; Dalleck, L.C. Using a site-specific technical error to establish training responsiveness: A preliminary explorative study. Open Access J. Sports Med. 2018, 9, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.N.; Lamberts, R.P.; Lambert, M.I. High responders and low responders: Factors associated with individual variation in response to standardized training. Sports Med. 2014, 44, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Rankinen, T. Individual differences in response to regular physical activity. Med. Sci. Sports Exerc. 2001, 33, S446–S451. [Google Scholar] [CrossRef] [PubMed]
- Scharhag-Rosenberger, F.; Meyer, T.; Gässler, N.; Faude, O.; Kindermann, W. Exercise at given percentages of VO2max: Heterogeneous metabolic responses between individuals. J. Sci. Med. Sport. 2010, 13, 74–79. [Google Scholar] [CrossRef]
- Williams, C.J.; Gurd, B.J.; Bonafiglia, J.T.; Voisin, S.; Li, Z.; Harvey, N.; Croci, I.; Taylor, J.L.; Gajanand, T.; Ramos, J.S.; et al. A Multi-Center Comparison of VO2peak Trainability between Interval Training and Moderate Intensity Continuous Training. Front. Physiol. 2019, 10, 19. [Google Scholar] [CrossRef]
- Montero, D.; Lundby, C. Refuting the myth of non-response to exercise training: ‘Non-responders’ do respond to higher dose of training. J. Physiol. 2017, 595, 3377–3387. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Batterham, A.M. True and false interindividual differences in the physiological response to an intervention. Exp. Physiol. 2015, 100, 577–588. [Google Scholar] [CrossRef]
- Hecksteden, A.; Pitsch, W.; Rosenberger, F.; Meyer, T. Repeated testing for the assessment of individual response to exercise training. J. Appl. Physiol. 2018, 124, 1567–1579. [Google Scholar] [CrossRef]
- Katch, V.L.; Sady, S.S.; Freedson, P. Biological variability in maximum aerobic power. Med. Sci. Sports Exerc. 1982, 14, 21–25. [Google Scholar] [CrossRef]
- Bateman, L.A.; Slentz, C.A.; Willis, L.H.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the Studies of a Targeted Risk Reduction Intervention through Defined Exercise-STRRIDE-AT/RT). Am. J. Cardiol. 2011, 108, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.; Thoma, C.; Houghton, D.; Trenell, M.I. High-intensity interval training: A review of its impact on glucose control and cardiometabolic health. Diabetologia 2017, 60, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Schaun, G.Z. The maximal oxygen uptake verification phase: A light at the end of the tunnel? Sports Med. Open 2017, 3, 44. [Google Scholar] [CrossRef] [PubMed]
- Astorino, T.A.; Robergs, R.A.; Ghiasvand, F.; Marks, D.; Burns, S. Incidence of the oxygen plateau at VO2max during exercise testing to volitional fatigue. J. Exerc. Physiol. Online 2000, 3, 1–12. [Google Scholar]
- Chmelo, E.A.; Crotts, C.I.; Newman, J.C.; Brinkley, T.E.; Lyles, M.F.; Leng, X.; Marsh, A.P.; Nicklas, B.J. Heterogeneity of physical function responses to exercise training in older adults. J. Am. Geriatr. Soc. 2015, 63, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; de Lannoy, L.; Stotz, P.J. Separate effects of intensity and amount of exercise on interindividual cardiorespiratory fitness response. Mayo Clin. Proc. 2015, 90, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Scharhag, J.; Kindermann, W. Peak oxygen uptake: Myth and truth about an internationally accepted reference value. Z. Kardiol. 2005, 94, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kampert, J.B.; Kohl, H.W., 3rd; Barlow, C.E.; Macera, C.A.; Paffenbarger, R.S., Jr.; Gibbons, L.W. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996, 276, 205–210. [Google Scholar] [CrossRef]
- Gulati, M.; Pandey, D.K.; Arnsdorf, M.F.; Lauderdale, D.S.; Thisted, R.A.; Wicklund, R.H.; Al-Hani, A.J.; Black, H.R. Exercise capacity and the risk of death in women: The St James Women Take Heart Project. Circulation 2003, 108, 1554–1559. [Google Scholar] [CrossRef]
| Parameter | Control Group (n = 15; Women = 8, Men = 7) | Standardized MICT Group (n = 16; Women = 9, Men = 7) | Personalized MICT + HIIT Group (n = 16; Women = 8, Men = 8) | |||
|---|---|---|---|---|---|---|
| Baseline | 13 Week | Baseline | 13 Week | Baseline | 13 Week | |
| Age (year) | 33.9 ± 6.9 | ____ | 34.2 ± 9.8 | ____ | 32.1 ± 6.9 | ____ |
| Height (cm) | 168.2 ± 5.9 | ____ | 167.4 ± 10.0 | ____ | 170.5 ± 8.2 | ____ |
| Weight (kg) | 77.3 ± 9.0 | 77.5 ± 8.6 | 80.5 ± 16.5 | 79.6 ± 16.0 * | 79.8 ± 19.7 | 79.4 ± 19.6 |
| Waist circumference (cm) | 81.5 ± 7.5 | 81.8 ± 7.3 | 84.0 ± 9.3 | 82.8 ± 8.7 *,† | 80.5 ± 11.9 | 78.8 ± 11.5 *,† |
| Body fat (%) | 23.2 ± 4.9 | 23.8 ± 4.5 * | 26.1 ± 6.2 | 24.6 ± 5.5 *,† | 24.5 ± 8.5 | 22.1 ± 7.3 *,† |
| Resting heart rate (b∙min−1) | 62.3 ± 6.8 | 63.7 ± 5.7 | 61.4 ± 8.9 | 60.3 ± 6.9 | 64.3 ± 5.9 | 62.8 ± 6.6 |
| Bench press 5-RM (kg) | 43.4 ± 13.1 | 43.7 ± 12.7 | 42.0 ± 5.7 | 48.1 ± 5.2 *,† | 44.8 ± 11.8 | 54.3 ± 14.1 *,‡ |
| Leg press 5-RM (kg) | 125.2 ± 37.3 | 126.6 ± 34.0 | 124.9 ± 16.6 | 145.3 ± 10.4 *,† | 121.1 ± 47.6 | 151.0 ± 43.8 *,† |
| VO2max (mL⋅kg−1⋅min−1) | 31.5 ± 6.7 | 30.9 ± 6.4 | 28.6 ± 6.0 | 30.8 ± 7.1 *,† | 31.8 ± 4.8 | 36.3 ± 5.5 *,‡ |
| Mean arterial pressure (mmHg) | 91.8 ± 7.8 | 93.3 ± 5.4 | 94.1 ± 9.9 | 92.7 ± 6.4 | 92.7 ± 10.7 | 88.6 ± 8.3 *,† |
| HDL cholesterol (mg∙dL−1) | 53.9 ± 21.0 | 52.5 ± 19.0 | 52.8 ± 12.4 | 55.6 ± 12.2 | 52.7 ± 13.4 | 57.2 ± 12.2 *,† |
| Triglycerides (mg∙dL−1) | 109.7 ± 39.6 | 117.7 ± 38.5 | 145.1 ± 80.8 | 137.3 ± 71.7 | 138.4 ± 62.5 | 108.2 ± 33.2 *,† |
| Blood Glucose (mg∙dL−1) | 88.7 ± 5.4 | 90.1 ± 6.7 | 96.1 ± 11.5 | 95.1 ± 9.0 | 97.4 ± 7.5 | 90.6 ± 6.1 *,‡ |
| MetS z-score | −4.52 ± 3.45 | −4.01 ± 3.35 | −2.92 ± 2.98 | −3.57 ± 2.61 *,† | −3.35 ± 2.87 | −5.15 ± 2.34 *,‡ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byrd, B.R.; Keith, J.; Keeling, S.M.; Weatherwax, R.M.; Nolan, P.B.; Ramos, J.S.; Dalleck, L.C. Personalized Moderate-Intensity Exercise Training Combined with High-Intensity Interval Training Enhances Training Responsiveness. Int. J. Environ. Res. Public Health 2019, 16, 2088. https://doi.org/10.3390/ijerph16122088
Byrd BR, Keith J, Keeling SM, Weatherwax RM, Nolan PB, Ramos JS, Dalleck LC. Personalized Moderate-Intensity Exercise Training Combined with High-Intensity Interval Training Enhances Training Responsiveness. International Journal of Environmental Research and Public Health. 2019; 16(12):2088. https://doi.org/10.3390/ijerph16122088
Chicago/Turabian StyleByrd, Bryant R., Jamie Keith, Shawn M. Keeling, Ryan M. Weatherwax, Paul B. Nolan, Joyce S. Ramos, and Lance C. Dalleck. 2019. "Personalized Moderate-Intensity Exercise Training Combined with High-Intensity Interval Training Enhances Training Responsiveness" International Journal of Environmental Research and Public Health 16, no. 12: 2088. https://doi.org/10.3390/ijerph16122088
APA StyleByrd, B. R., Keith, J., Keeling, S. M., Weatherwax, R. M., Nolan, P. B., Ramos, J. S., & Dalleck, L. C. (2019). Personalized Moderate-Intensity Exercise Training Combined with High-Intensity Interval Training Enhances Training Responsiveness. International Journal of Environmental Research and Public Health, 16(12), 2088. https://doi.org/10.3390/ijerph16122088






