Application of Graphene Oxide for Adsorption Removal of Geosmin and 2-Methylisoborneol in the Presence of Natural Organic Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Water Samples and Testing
2.3. Batch Adsorption Experiments
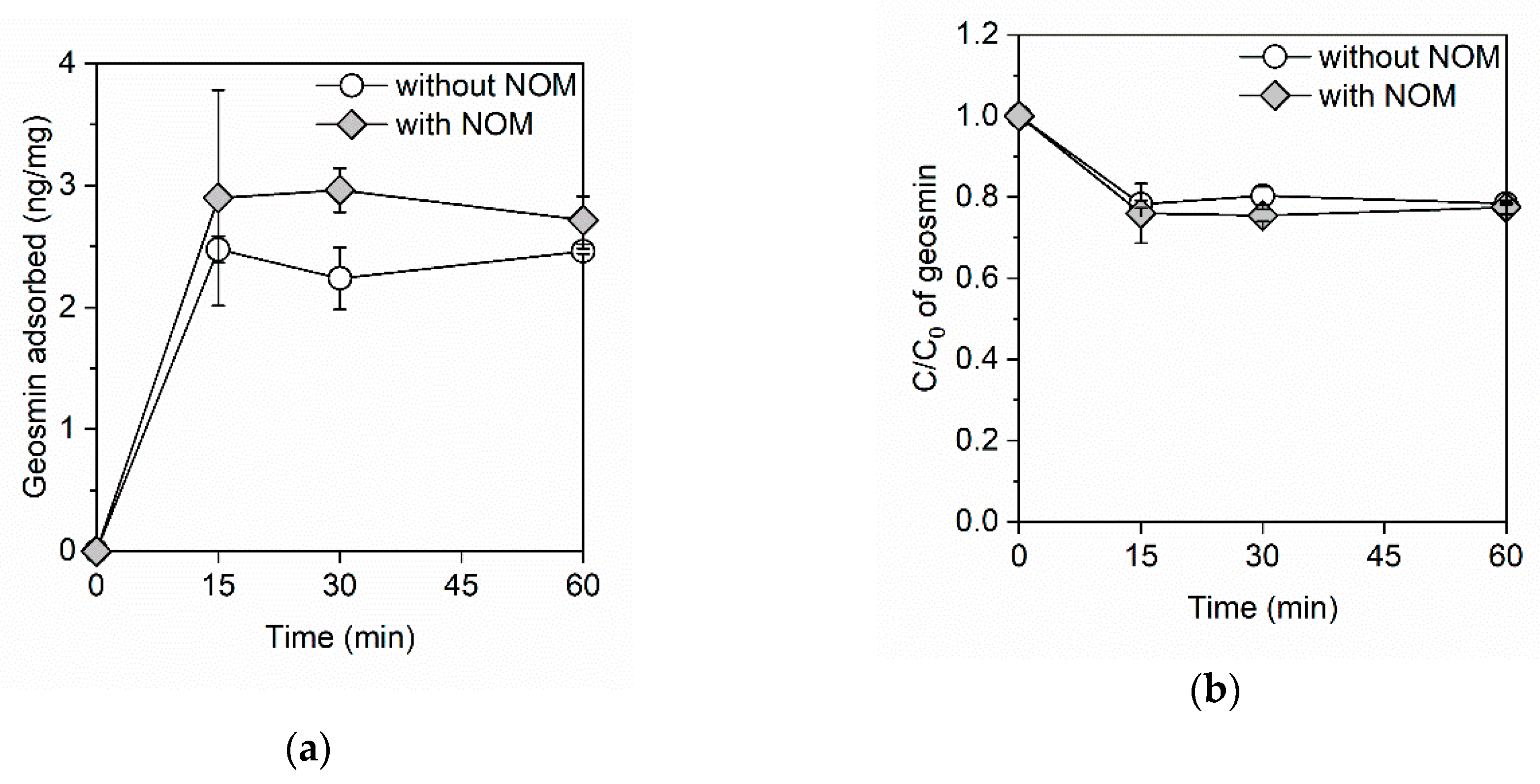
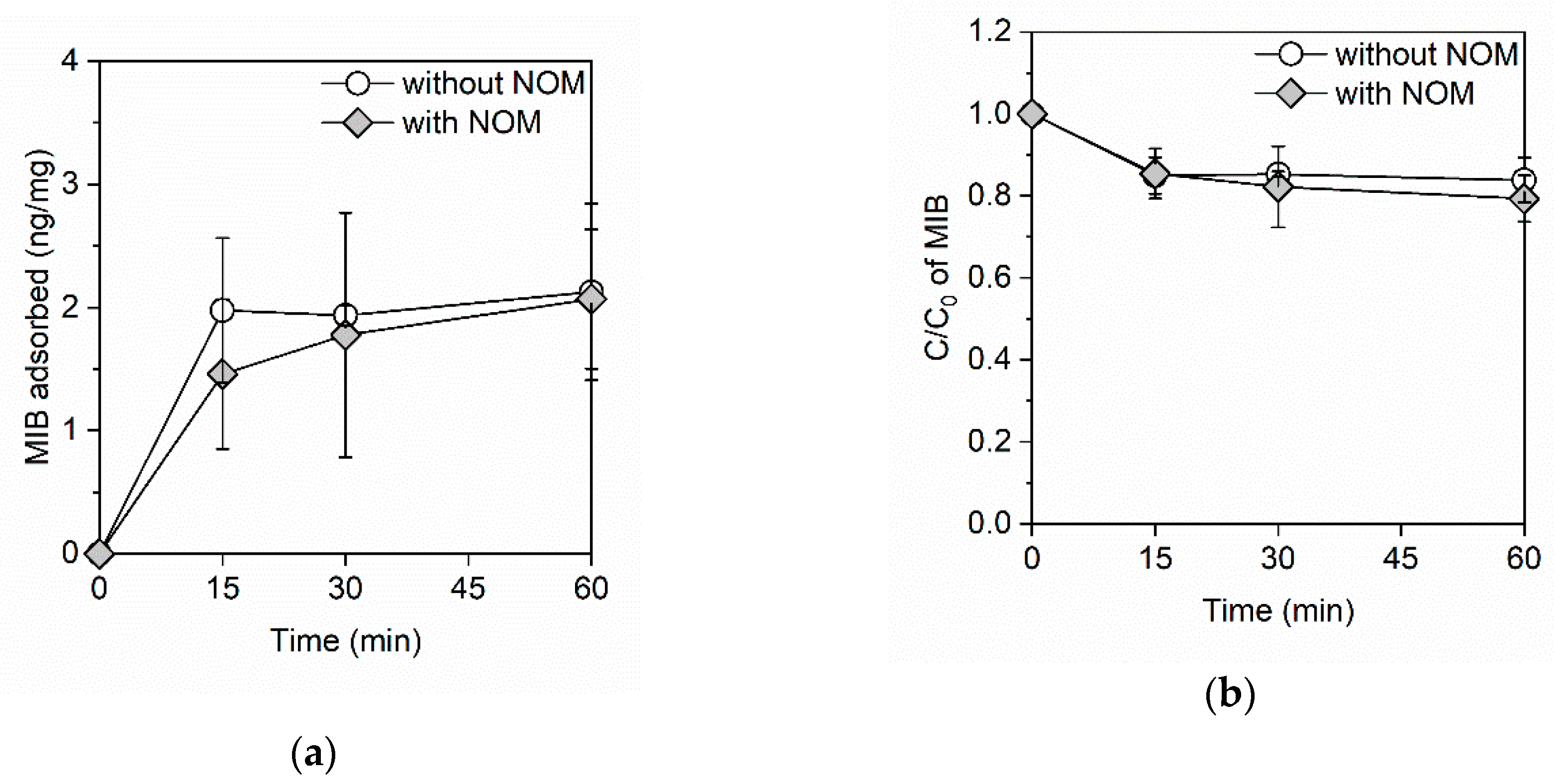
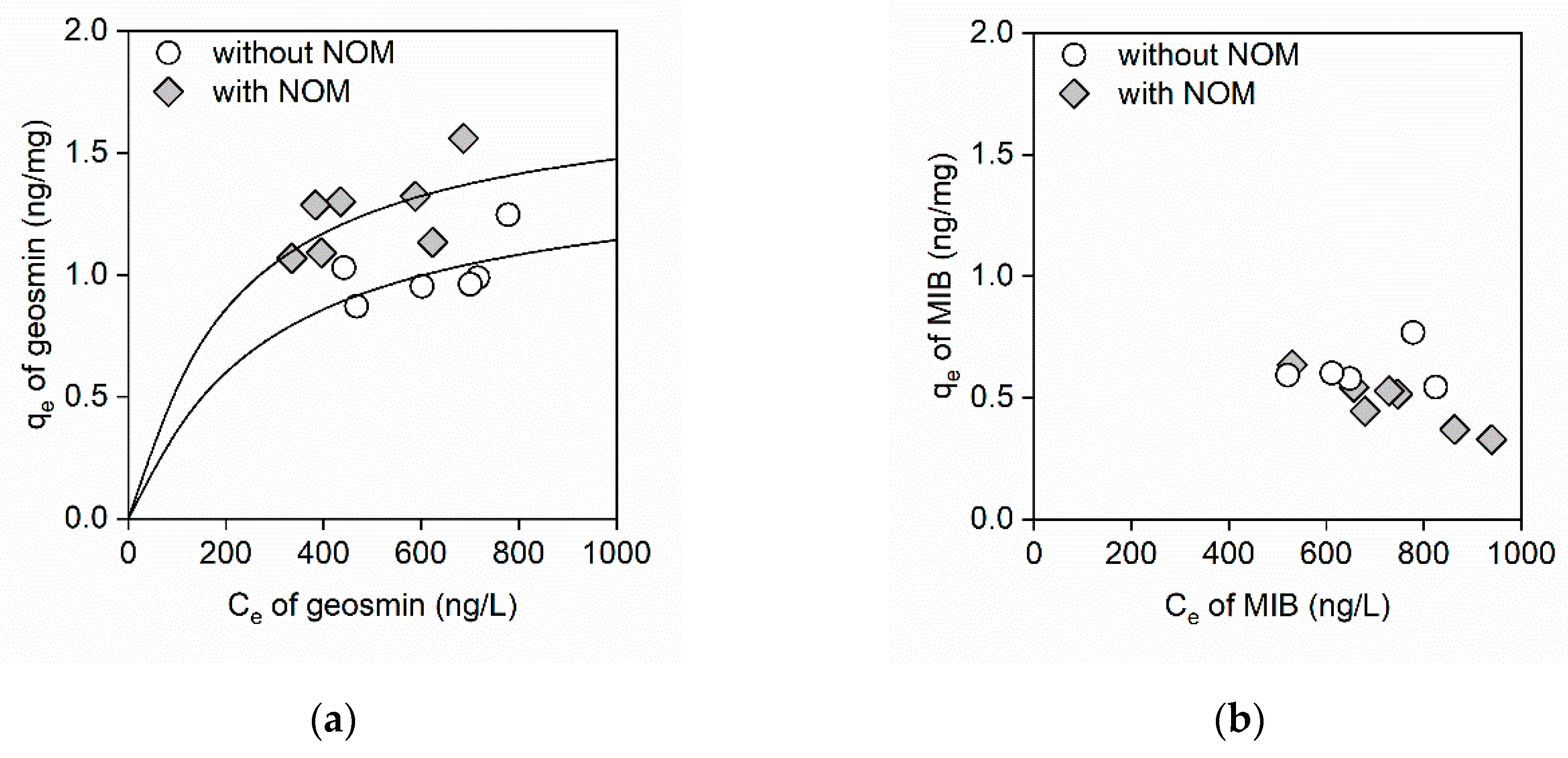
3. Results
3.1. Adsorption Kinetics
3.2. Adsorption Isotherms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suurnäkki, S.; Gomez-Saez, G.V.; Rantala-Ylinen, A.; Jokela, J.; Fewer, D.P.; Sivonen, K. Identification of geosmin and 2-methylisoborneol in cyanobacteria and molecular detection methods for the producers of these compounds. Water Res. 2015, 68, 56–66. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sorial, G.A. Treatment of taste and odor causing compounds 2-methyl isoborneol and geosmin in drinking water: A critical review. J. Environ. Sci. 2011, 23, 1–13. [Google Scholar] [CrossRef]
- Cook, D.; Newcombe, G.; Sztajnbok, P. The application of powdered activated carbon for MIB and geosmin removal: Predicting PAC doses in four raw waters. Water Res. 2001, 35, 1325–1333. [Google Scholar] [CrossRef]
- Matsui, Y.; Murase, R.; Sanogawa, T.; Aoki, H.; Mima, S.; Inoue, T.; Matsushita, T. Rapid adsorption pretreatment with submicrometre powdered activated carbon particles before microfiltration. Water Sci. Technol. 2005, 51, 249–256. [Google Scholar] [CrossRef]
- Matsui, Y.; Nakao, S.; Taniguchi, T.; Matsushita, T. Geosmin and 2-methylisoborneol removal using superfine powdered activated carbon: Shell adsorption and branched-pore kinetic model analysis and optimal particle size. Water Res. 2013, 47, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Yoshida, T.; Nakao, S.; Knappe, D.R.U.; Matsushita, T. Characteristics of competitive adsorption between 2-methylisoborneol and natural organic matter on superfine and conventionally sized powdered activated carbons. Water Res. 2012, 46, 4741–4749. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Chhowalla, M. Chemically Derived Graphene Oxide: Towards Large-Area Thin-Film Electronics and Optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Wu, Z.S.; Zhou, G.M.; Yin, L.C.; Ren, W.; Li, F.; Cheng, H.M. Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 2012, 1, 107–131. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Ersan, G.; Apul, O.G.; Perreault, F.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets: A review. Water Res. 2017, 126, 385–398. [Google Scholar] [CrossRef]
- Catherine, H.N.; Ou, M.H.; Manu, B.; Shih, Y.H. Adsorption mechanism of emerging and conventional phenolic compounds on graphene oxide nanoflakes in water. Sci. Total Environ. 2018, 635, 629–638. [Google Scholar] [CrossRef]
- Zhang, X.R.; Qin, C.B.; Gong, Y.N.; Song, Y.R.; Zhang, G.F.; Chen, R.Y.; Gao, Y.; Xiao, L.T.; Jia, S.T. Co-adsorption of an anionic dye in the presence of a cationic dye and a heavy metal ion by graphene oxide and photoreduced graphene oxide. RSC Adv. 2019, 9, 5313–5324. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Liu, Y.G.; Liu, S.B.; Zeng, G.M.; Jiang, L.H.; Tan, X.F.; Zhou, L.; Zeng, W.; Li, T.T.; Yang, C.P. Adsorption of emerging contaminant metformin using graphene oxide. Chemosphere 2017, 179, 20–28. [Google Scholar] [CrossRef]
- Pirbazari, M.; Borow, H.S.; Craig, S.; Ravindran, V.; McGuire, M.J. Physical-chemical characterization of 5 earthy-musty-smelling compounds. Water Sci. Technol. 1992, 25, 81–88. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Fabbricino, M.; Pontoni, L. Use of non-treated shrimp-shells for textile dye removal from wastewater. J. Environ. Chem. Eng. 2016, 4, 4100–4106. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaria, R.; Menendez, R.; Corma, A.; Garcia, H. Surface Area Measurement of Graphene Oxide in Aqueous Solutions. Langmuir 2013, 29, 13443–13448. [Google Scholar] [CrossRef]
- Apul, O.G.; Wang, Q.L.; Zhou, Y.; Karanfil, T. Adsorption of aromatic organic contaminants by graphene nanosheets: Comparison with carbon nanotubes and activated carbon. Water Res. 2013, 47, 1648–1654. [Google Scholar] [CrossRef]
- Ersan, G.; Kaya, Y.; Apul, O.G.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets, carbon nanotubes and granular activated carbons under natural organic matter preloading conditions. Sci. Total Environ. 2016, 565, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Matsui, Y.; Matsushita, T.; Shirasaki, N. Superiority of wet-milled over dry-milled superfine powdered activated carbon for adsorptive 2-methylisoborneol removal. Water Res. 2016, 102, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Takaesu, H.; Matsui, Y.; Nishimura, Y.; Matsushita, T.; Shirasaki, N. Micro-milling super-fine powdered activated carbon decreases adsorption capacity by introducing oxygen/hydrogen-containing functional groups on carbon surface from water. Water Res. 2019, 155, 66–75. [Google Scholar] [CrossRef] [PubMed]
| Compound Name | Chemical Structure | Molecular Weight (g/mol) | log Kow 1 | Cs 1 (mg/L) |
|---|---|---|---|---|
| Geosmin | 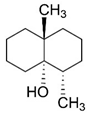 | 182.3 | 3.70 | 150.2 |
| MIB |  | 168.3 | 3.13 | 194.5 |
| Model | Adsorbate | qm (ng/mg) | kb | Qm (ng/mg) | b | r2 |
|---|---|---|---|---|---|---|
| BET | Geosmin | 1.46 | 5.4 × 105 | - | - | 0.69 |
| Geosmin–NOM | 1.78 | 7.4 × 105 | - | - | 0.73 | |
| MIB | 0.67 | 3.0 × 105 | - | - | 0.58 | |
| MIB–NOM | - | - | - | - | - | |
| Langmuir | Geosmin | - | - | 1.46 | 3.6 × 10−3 | 0.69 |
| Geosmin–NOM | - | - | 1.78 | 4.9 × 10−3 | 0.73 | |
| MIB | - | - | 0.67 | 1.5 × 10−2 | 0.58 | |
| MIB–NOM | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafuka, A.; Nagasato, T.; Yamamura, H. Application of Graphene Oxide for Adsorption Removal of Geosmin and 2-Methylisoborneol in the Presence of Natural Organic Matter. Int. J. Environ. Res. Public Health 2019, 16, 1907. https://doi.org/10.3390/ijerph16111907
Hafuka A, Nagasato T, Yamamura H. Application of Graphene Oxide for Adsorption Removal of Geosmin and 2-Methylisoborneol in the Presence of Natural Organic Matter. International Journal of Environmental Research and Public Health. 2019; 16(11):1907. https://doi.org/10.3390/ijerph16111907
Chicago/Turabian StyleHafuka, Akira, Takahiro Nagasato, and Hiroshi Yamamura. 2019. "Application of Graphene Oxide for Adsorption Removal of Geosmin and 2-Methylisoborneol in the Presence of Natural Organic Matter" International Journal of Environmental Research and Public Health 16, no. 11: 1907. https://doi.org/10.3390/ijerph16111907
APA StyleHafuka, A., Nagasato, T., & Yamamura, H. (2019). Application of Graphene Oxide for Adsorption Removal of Geosmin and 2-Methylisoborneol in the Presence of Natural Organic Matter. International Journal of Environmental Research and Public Health, 16(11), 1907. https://doi.org/10.3390/ijerph16111907




