Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in their Mixed Solution by UV/H2O2 Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Procedures
2.3. Analytical Methods
3. Results and Discussion
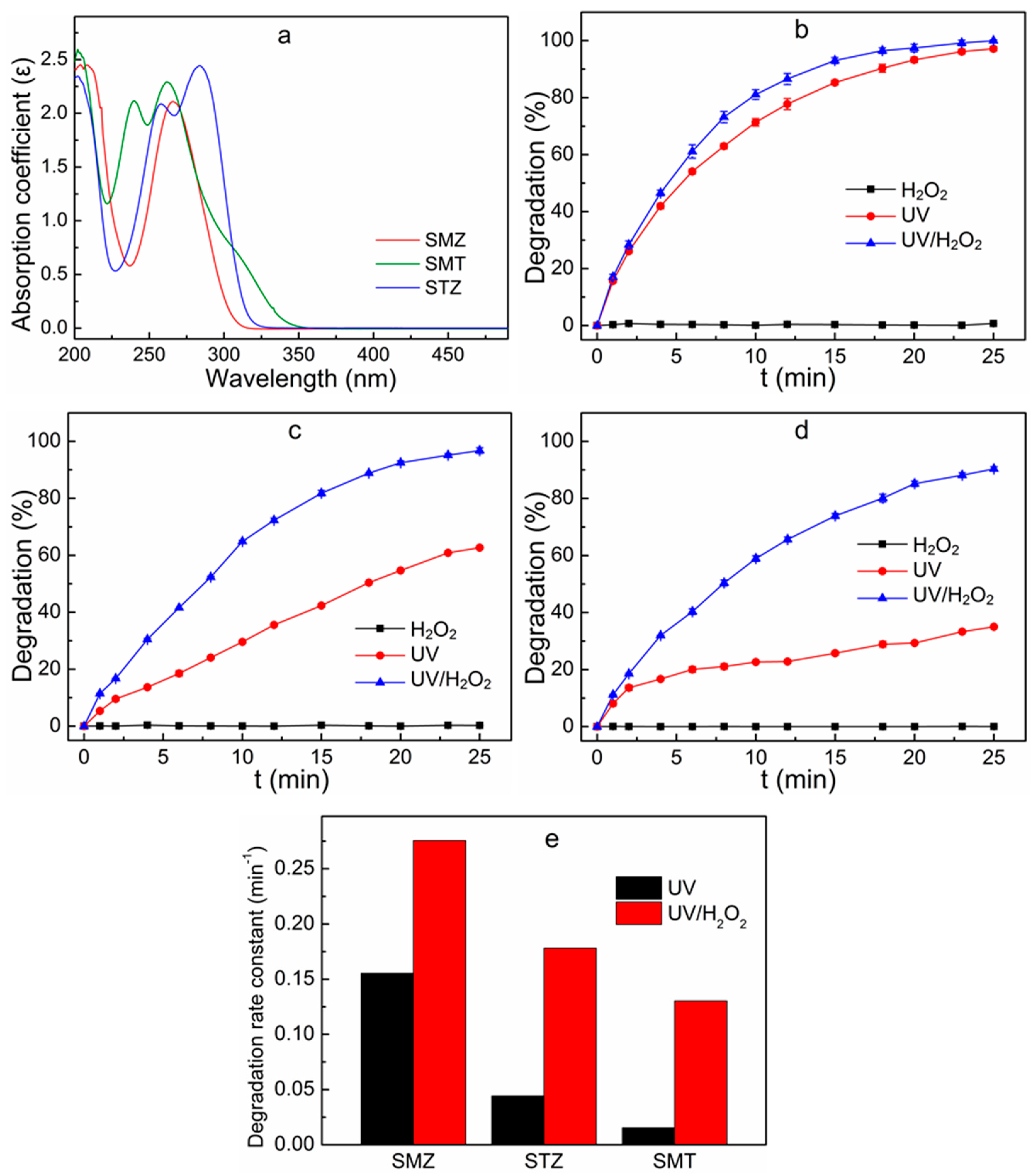
3.1. Comparison of Absorption Spectra of Target Compounds
3.2. Decomposition of Sulfonamides in the Mixed System
3.3. Effect of Initial Concentration of the Mixed Solution on Degradation Efficiency
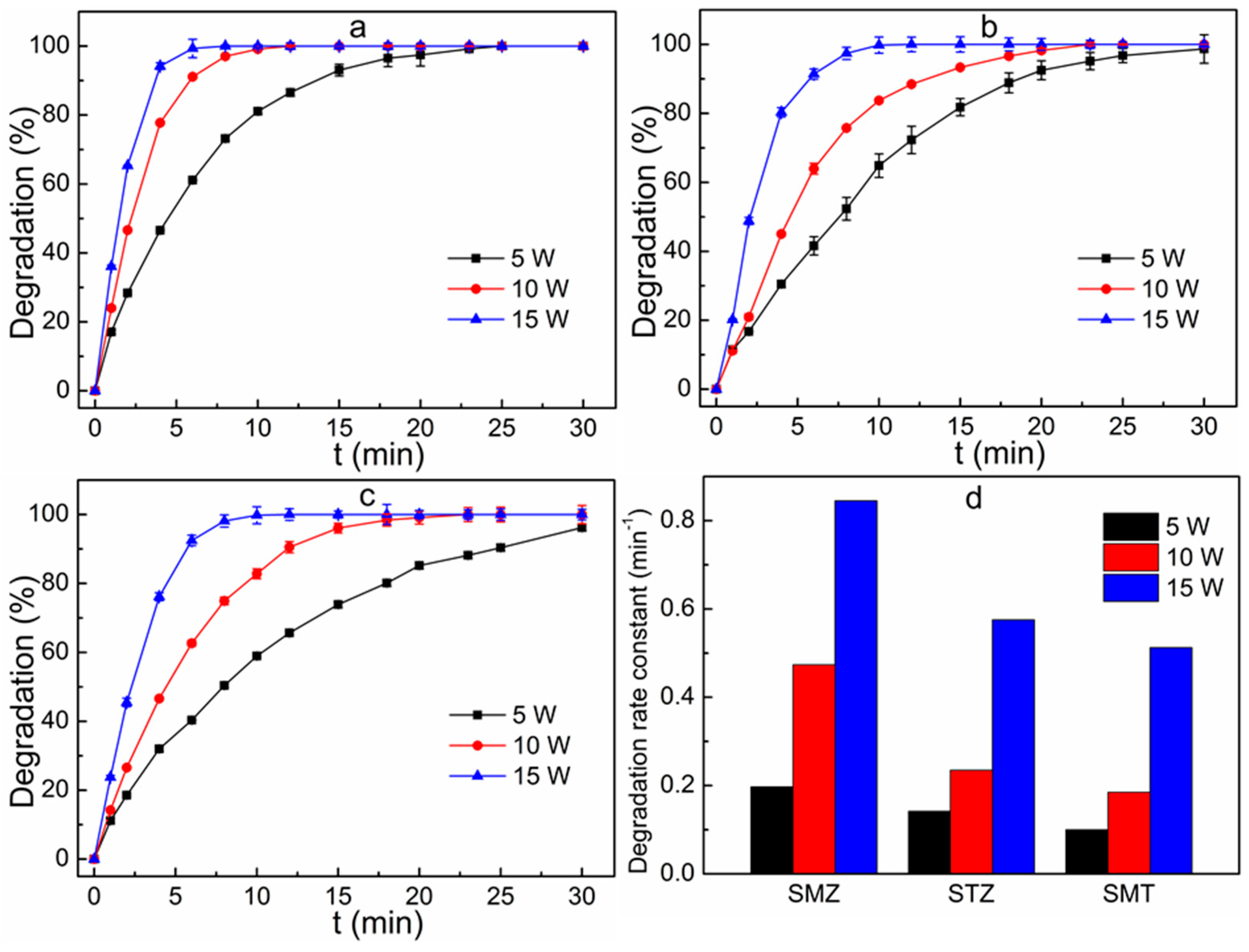
3.4. Effect of UV Light Intensity on Degradation Efficiency
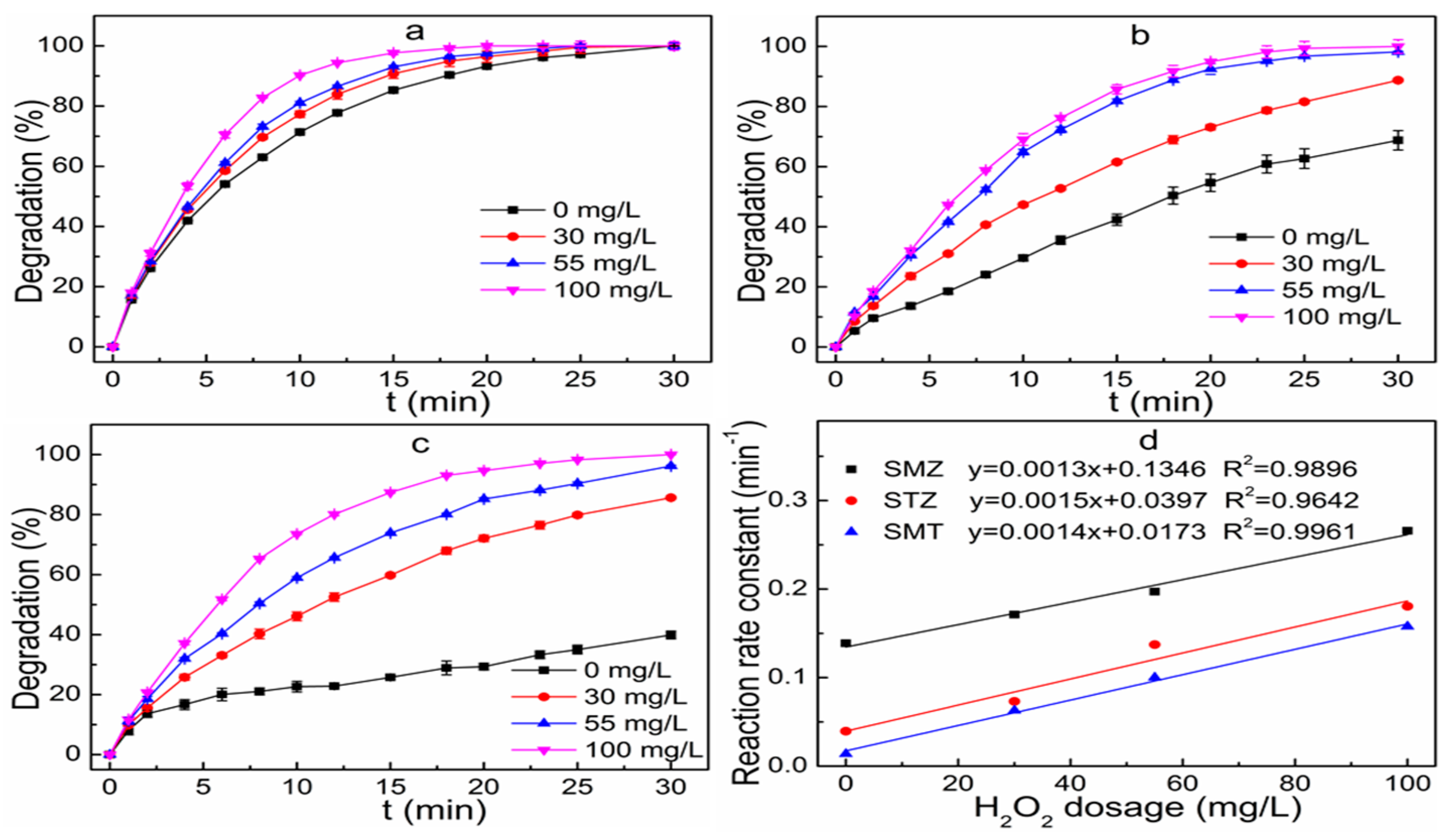
3.5. Effect of H2O2 Dosages on Degradation Efficiency
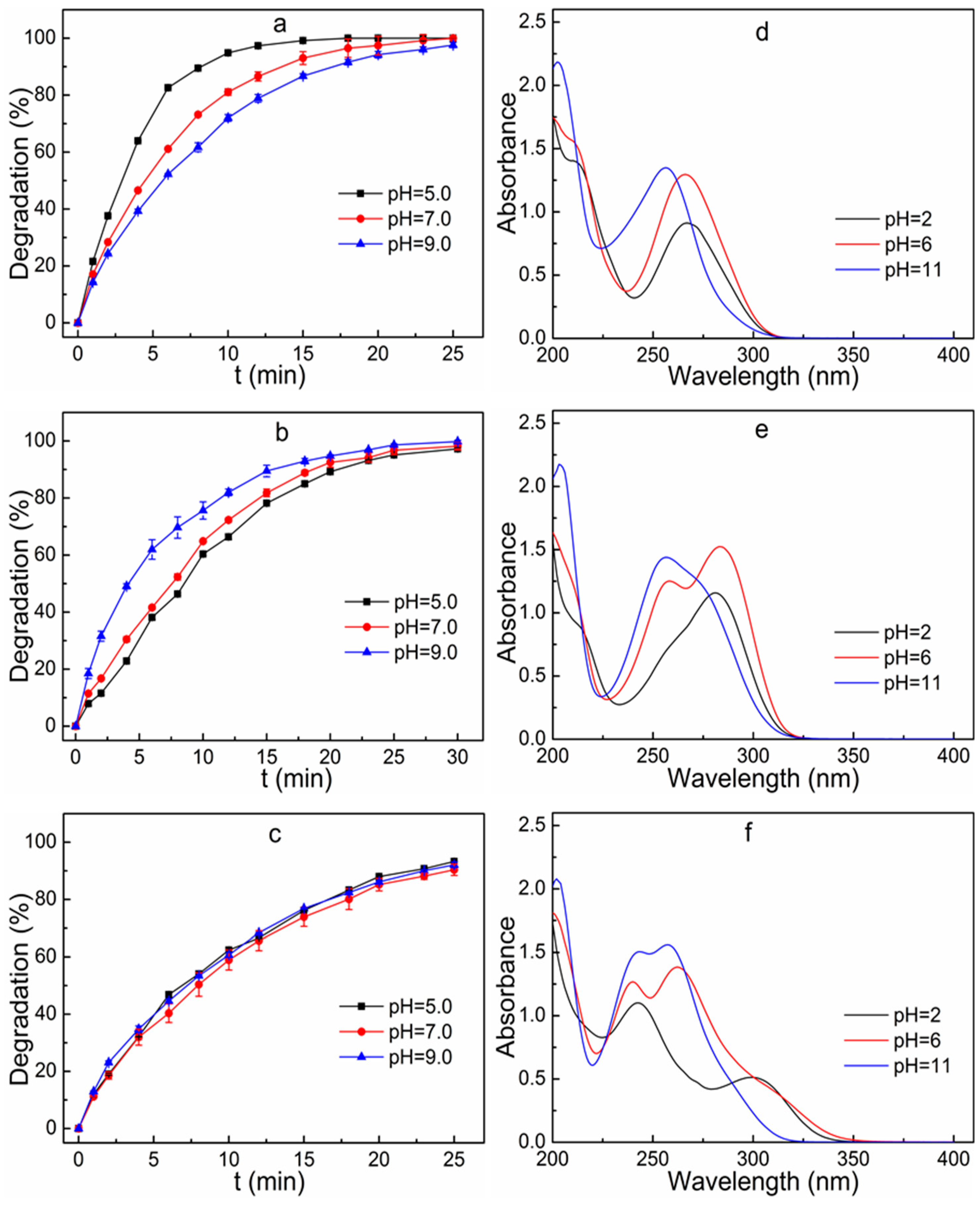
3.6. Effect of Initial Solution pH on Degradation Efficiency
3.7. Identification of Sulfonamides Degradation Intermediates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Długosz, M.; Żmudzki, P.; Kwiecień, A.; Szczubiałka, K.; Krzek, J.; Nowakowska, M. Photocatalytic degradation of sulfamethoxazole in aqueous solution using a floating TiO2-expanded perlite photocatalyst. J. Hazard. Mater. 2015, 298, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Khataee, A.; Kıranşan, M.; Karaca, S.; Sheydaei, M. Photocatalytic ozonation of metronidazole by synthesized zinc oxide nanoparticles immobilized on montmorillonite. J. Taiwan Inst. Chem. E 2017, 74, 196–204. [Google Scholar] [CrossRef]
- Li, G.; Ben, W.; Ye, H.; Zhang, D.; Qiang, Z. Performance of ozonation and biological activated carbon in eliminating sulfonamides and sulfonamide-resistant bacteria: A pilot-scale study. Chem. Eng. J. 2018, 341, 327–334. [Google Scholar] [CrossRef]
- Niu, X.Z.; Glady-Croué, J.; Croué, J.P. Photodegradation of sulfathiazole under simulated sunlight: Kinetics, photo-induced structural rearrangement, and antimicrobial activities of photoproducts. Water Res. 2017, 124, 576–583. [Google Scholar] [CrossRef]
- Zhao, W.; Sui, Q.; Mei, X.; Cheng, X. Efficient elimination of sulfonamides by an anaerobic/anoxic/oxic-membrane bioreactor process: Performance and influence of redox condition. Sci. Total Environ. 2018, 633, 668–676. [Google Scholar] [CrossRef]
- Garoma, T.; Umamaheshwar, S.K.; Mumper, A. Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere 2010, 79, 814–820. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Ziemiańska, J.; Sobczak, A. Effects of the presence of sulfonamides in the environment and their influence on human health. J. Hazard. Mater. 2011, 196, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Garoma, T.; Chen, Z.; Wang, L.; Wu, Y. SMX degradation by ozonation and UV radiation: A kinetic study. Chemosphere 2012, 87, 1134–1140. [Google Scholar] [CrossRef]
- Nasuhoglu, D.; Yargeau, V.; Berk, D. Photo-removal of sulfamethoxazole (SMX) by photolytic and photocatalytic processes in a batch reactor under UV-C radiation (λmax = 254 nm). J. Hazard. Mater. 2011, 186, 67–75. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Y.; Lu, S.; Yan, P.; Sui, Q. Recent advances in pharmaceuticals and personal care products in the surface water and sediments in China. Front. Environ. Sci. Eng. 2016, 10, 2. [Google Scholar] [CrossRef]
- Yao, H.; Sun, P.; Minakata, D.; Crittenden, J.C.; Huang, C.H. Kinetics and modeling of degradation of ionophore antibiotics by UV and UV/H2O2. Environ. Sci. Technol. 2013, 47, 4581–4589. [Google Scholar] [CrossRef]
- Benito, A.; Penadés, A.; Lliberia, J.L.; Gonzalez-Olmos, R. Degradation pathways of aniline in aqueous solutions during electro-oxidation with BDD electrodes and UV/H2O2 treatment. Chemosphere 2017, 166, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Trovó, A.G.; Nogueira, R.F.P.; Agüera, A.; Fernandez-Alba, A.R.; Sirtori, C.; Malato, S. Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res. 2009, 43, 3922–3931. [Google Scholar] [CrossRef]
- Liao, Q.N.; Ji, F.; Li, J.C.; Zhan, X.; Hu, Z.H. Decomposition and mineralization of sulfaquinoxaline sodium during UV/H2O2 oxidation processes. Chem. Eng. J. 2016, 284, 494–502. [Google Scholar] [CrossRef]
- Urbano, V.R.; Peres, M.S.; Maniero, M.G.; Guimarães, J.R. Abatement and toxicity reduction of antimicrobials by UV/H2O2 process. J. Environ. Manag. 2017, 193, 439–447. [Google Scholar] [CrossRef]
- Mouamfon, M.V.N.; Li, W.; Lu, S.; Chen, N.; Qiu, Z.; Lin, K. Photodegradation of sulfamethoxazole applying UV- and VUV-based processes. Water Air Soil Poll. 2011, 218, 265–274. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Kinetic studies and characterization of photolytic products of sulfamethazine, sulfapyridine and their acetylated metabolites in water under simulated solar irradiation. Water Res. 2012, 46, 711–722. [Google Scholar] [CrossRef]
- Mohajerani, M.; Mehrvar, M.; Ein-Mozaffari, F. CFD modeling of metronidazole degradation in water by the UV/H2O2 process in single and multilamp photoreactors. Ind. Eng. Chem. Res. 2010, 49, 5367–5382. [Google Scholar] [CrossRef]
- Pereira, V.J.; Weinberg, H.S.; Linden, K.G.; Singer, P.C. UV degradation kinetics and modeling of pharmaceutical compounds in laboratory grade and surface water via direct and indirect photolysis at 254 nm. Environ. Sci. Technol. 2007, 41, 1682–1688. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, C.; Wang, Z.; Chen, Y.; Mao, K.; Zhang, X.; Xiong, Y.; Zhu, M. Photodegradation of methyl tert-butyl ether (MTBE) by UV/H2O2 and UV/TiO2. J. Hazard. Mater. 2008, 154, 795–803. [Google Scholar] [CrossRef]
- Mouamfon, M.V.N.; Li, W.; Lu, S.; Qiu, Z.; Chen, N.; Lin, K. Photodegradation of sulphamethoxazole under UV-light irradiation at 254 nm. Environ. Technol. 2010, 31, 489–494. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photochemical oxidation of reactive azo dye with UV-H2O2 process. Dyes Pigments 2004, 62, 269–275. [Google Scholar] [CrossRef]
- Ghaly, M.Y.; Härtel, G.; Mayer, R.; Haseneder, R. Photochemical oxidation of p-chlorophenol by UV/H2O2 and photo-Fenton process. A comparative study. Waste Manag. 2001, 21, 41–47. [Google Scholar] [CrossRef]
- Xu, B.; Gao, N.Y.; Cheng, H.; Xia, S.J.; Rui, M.; Zhao, D.D. Oxidative degradation of dimethyl phthalate (DMP) by UV/H2O2 process. J. Hazard. Mater. 2009, 162, 954–959. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Zhou, S.; Xiao, Y.; Zhuang, Z. Kinetic study of acetaminophen degradation by UV-based advanced oxidation processes. Chem. Eng. J. 2014, 253, 229–236. [Google Scholar] [CrossRef]
- Aleboyeh, A.; Moussa, Y.; Aleboyeh, H. The effect of operational parameters on UV/H2O2 decolourisation of Acid Blue 74. Dyes Pigments 2005, 66, 129–134. [Google Scholar] [CrossRef]
- Fabiańska, A.; Białkbielińska, A.; Stepnowski, P.; Stolte, S.; Siedlecka, E.M. Electrochemical degradation of sulfonamides at BDD electrode: Kinetics, reaction pathway and eco-toxicity evaluation. J. Hazard. Mater. 2014, 280, 579–587. [Google Scholar] [CrossRef]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef]
- Justo, A.; González, O.; Aceña, J.; Pérez, S.; Barceló, D.; Sans, C.; Esplugas, S. Pharmaceuticals and organic pollution mitigation in reclamation osmosis brines by UV/H2O2 and ozone. J. Hazard. Mater. 2013, 263, 268–274. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, H.; Wang, L.; Chen, L.; Xiong, Y.; Xue, X. Removal of sulfamethoxazole from aqueous solution by sono-ozonation in the presence of a magnetic catalyst. Sep. Purif. Technol. 2013, 117, 46–52. [Google Scholar] [CrossRef]
- Guo, W.Q.; Yin, R.L.; Zhou, X.J.; Cao, H.O.; Chang, J.S.; Ren, N.Q. Ultrasonic-assisted ozone oxidation process for sulfamethoxazole removal: Impact factors and degradation process. Desalin. Water Treat. 2016, 57, 21015–21022. [Google Scholar] [CrossRef]


| Compound Name | Sulfamethoxazole | Sulfathiazole | Sulfamethazine |
|---|---|---|---|
| Acronym | SMZ | STZ | SMT |
| Molecular formula | C10H11N3O3S | C9H9N3O2S2 | C12H14N4O2S |
| Molecular weight (g/mol) | 253.3 | 255.3 | 278.3 |
| Pka1 | 1.6 ± 0.2 | 2.2 ± 0.1 | 2.6 ± 0.2 |
| Pka2 | 5.7 ± 0.2 | 7.2 ± 0.4 | 8 ± 1 |
| Molecular structure | 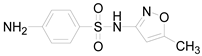 | 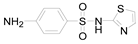 | 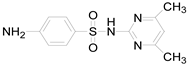 |
| Chemical speciation | 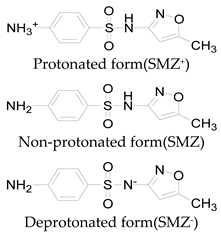 | 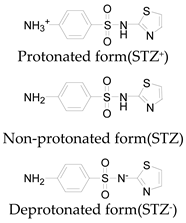 | 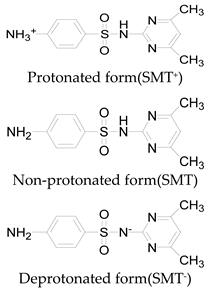 |
| Reference | [16,21] | [7] | [27] |
| Name | Retention Time (min) | Molecular Weight (MW) | Characteristic Ions | Molecular Structure |
|---|---|---|---|---|
| S1 | 1.666 | 189 | 190.0182,172.0045, 122.0243,109.0524 | 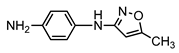 |
| S2 | 1.713 | 132 | 133.0609,72.0449 | 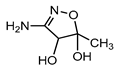 |
| S3 | 2.615 | 287 | 288.0661,156.0119, 108.0452,92.0504 | 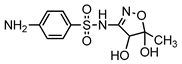 |
| S4 | 2.843 | 98 | 99.0562,72.0462 | 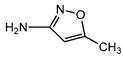 |
| S5 | 2.785 | 215 | 216.0437,156.0113, 108.0444,92.0507, 65.0395 |  |
| S6 | 4.049 | 269 | 270.0556,172.0065, 124.0398,109.0528 | 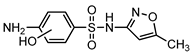 |
| S7 | 2.882 | 269 | 270.0552,156.0116, 108.0446,92.0504 |  |
| S8 | 4.184 | 253 | 254.0608,156.0120, 108.0451,92.0505 | 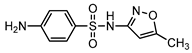 |
| Name | Retention Time (min) | Molecular Weight (MW) | Characteristic Ions | Molecular Structure |
|---|---|---|---|---|
| S1 | 2.033 | 139 | 140.0824,123.0558, 99.0563,82.0302 | 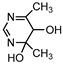 |
| S2 | 2.160 | 123 | 124.0878,107.0610,67.0314 | 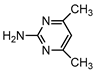 |
| S3 | 2.965 | 214 | 215.1301,108.0689,198.1035 | 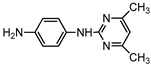 |
| S4 | 3.812 | 294 | 295.0875,124.0874, 186.0336,108.0450,172.0067 | 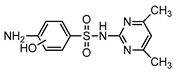 |
| S5 | 3.218 | 292 | 293.0718,212.0823,184.0876 | 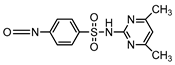 |
| S6 | 3.984 | 278 | 279.0934,186.0347,124.0882, 108.0458,92.0512,156.0126 | 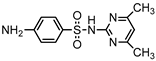 |
| S7 | 4.313 | 312 | 313.0537,186.0336, 142.0052,124.0864 | 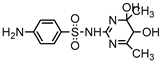 |
| Name | Retention Time (min) | Molecular Weight (MW) | Characteristic Ions | Molecular Structure |
|---|---|---|---|---|
| S1 | 1.692 | 100 | 101.0179,74.0078,58.9978 | 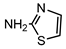 |
| S2 | 2.208 | 225 | 226.0662,124.0222, 151.0334 | 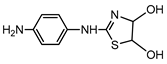 |
| S3 | 2.483 | 172 | 173.0318,93.0582 | 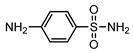 |
| S4 | 2.648 | 289 | 290.0274,156.0120, 108.0452,92.0507 | 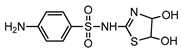 |
| S5 | 3.423 | 271 | 272.0176,172.0069, 124.0400,108.0453 | 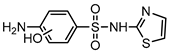 |
| S6 | 3.864 | 255 | 256.0217,156.0120, 108.0452,92.0506 | 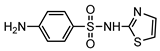 |
| S7 | 3.900 | 269 | 270.0017,189.0123, 161.0174 | 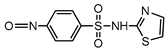 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Sun, Q.; Wang, C.; Yang, Z.; Xue, Q. Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in their Mixed Solution by UV/H2O2 Process. Int. J. Environ. Res. Public Health 2019, 16, 1797. https://doi.org/10.3390/ijerph16101797
Zhu G, Sun Q, Wang C, Yang Z, Xue Q. Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in their Mixed Solution by UV/H2O2 Process. International Journal of Environmental Research and Public Health. 2019; 16(10):1797. https://doi.org/10.3390/ijerph16101797
Chicago/Turabian StyleZhu, Guangcan, Qi Sun, Chuya Wang, Zhonglian Yang, and Qi Xue. 2019. "Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in their Mixed Solution by UV/H2O2 Process" International Journal of Environmental Research and Public Health 16, no. 10: 1797. https://doi.org/10.3390/ijerph16101797
APA StyleZhu, G., Sun, Q., Wang, C., Yang, Z., & Xue, Q. (2019). Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in their Mixed Solution by UV/H2O2 Process. International Journal of Environmental Research and Public Health, 16(10), 1797. https://doi.org/10.3390/ijerph16101797






