Combination of Coagulation and Ozone Catalytic Oxidation for Pretreating Coking Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Test Methods
3. Results and Discussions
3.1. O3 Catalytic Oxidation Treatment of Coking Wastewater
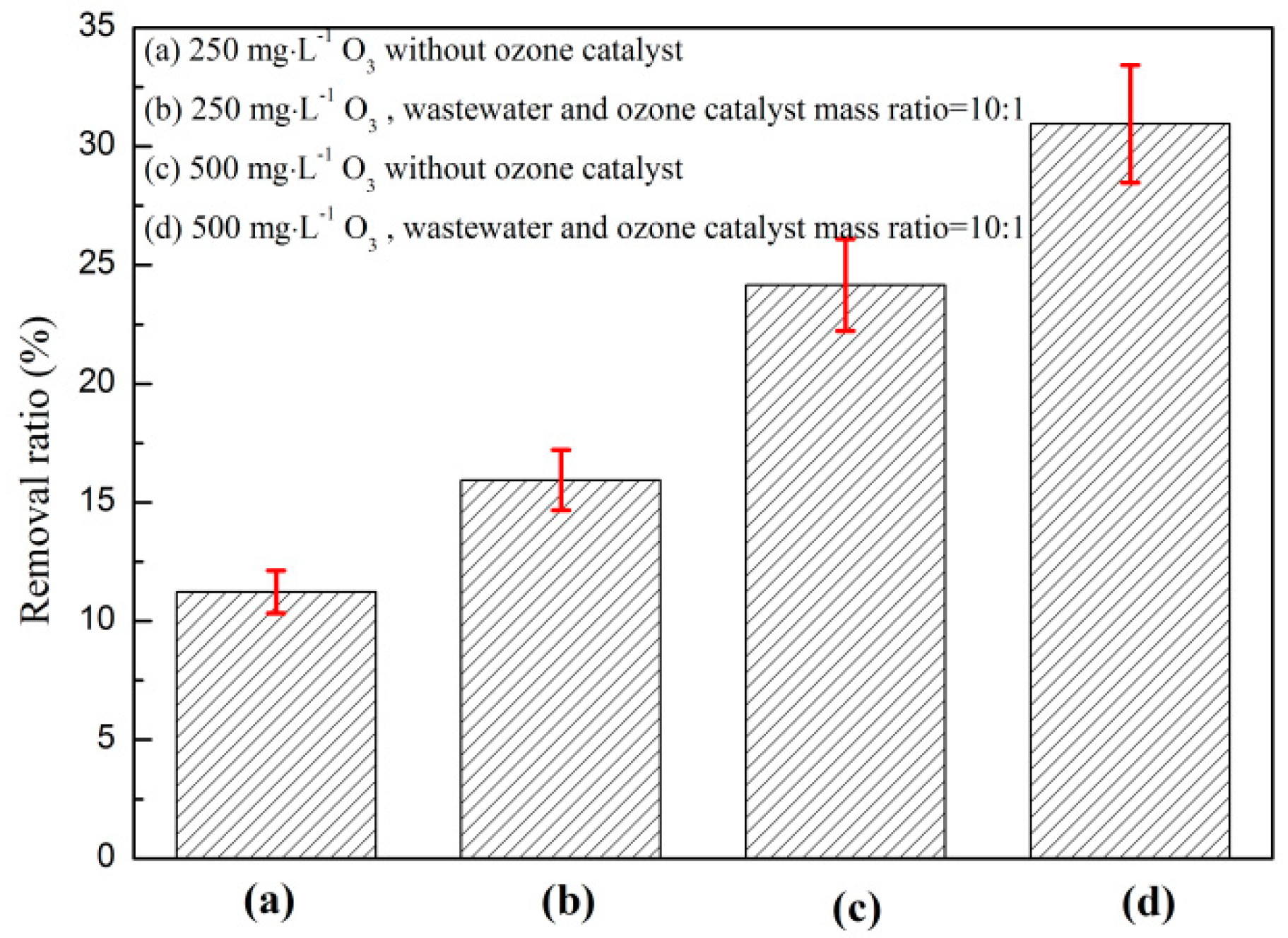
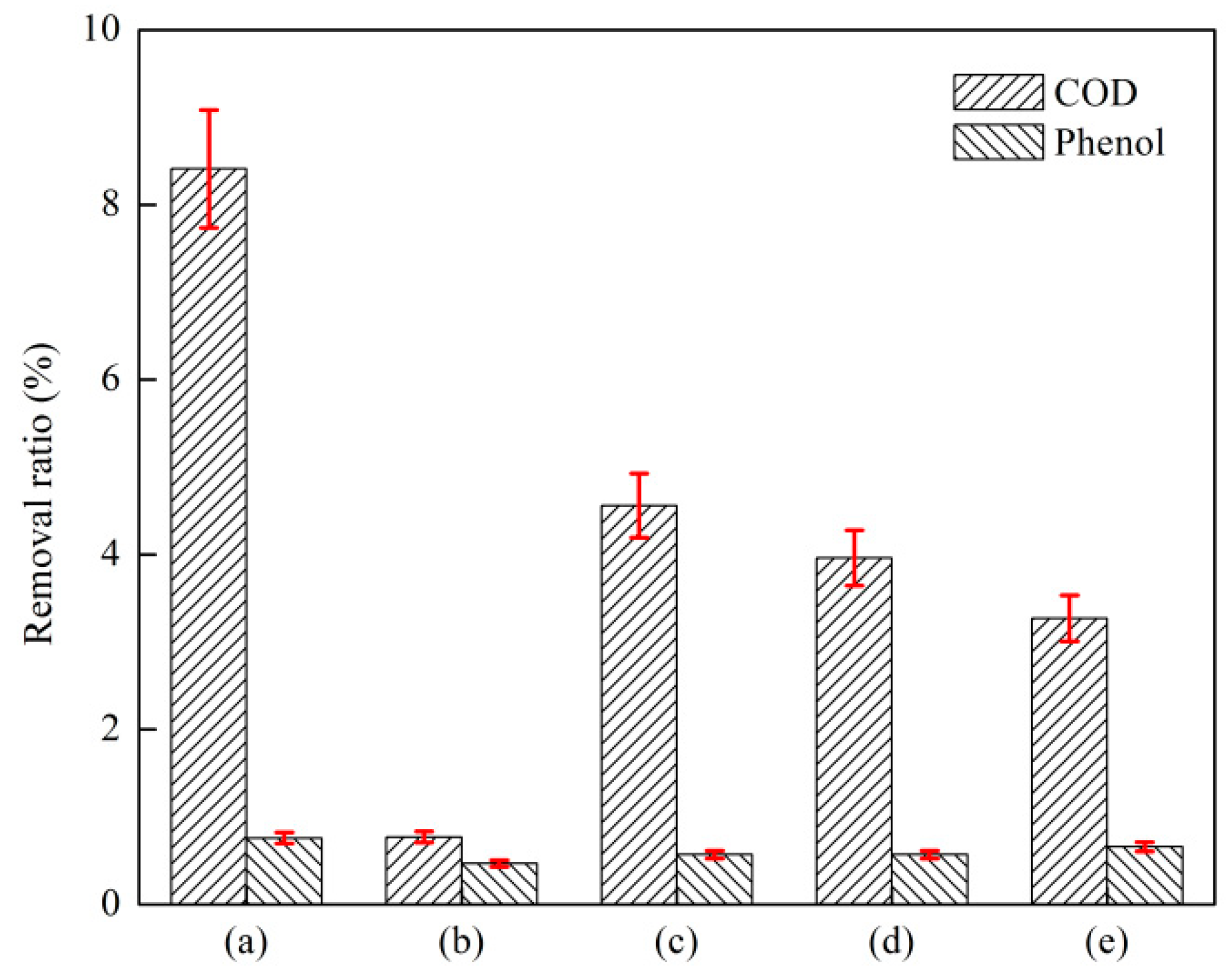
3.1.1. Effect of Catalyst and O3 on O3 Catalytic Oxidation and Phenol Removal
3.1.2. Effect of Catalyst Dosage Ratio on O3 Catalytic Oxidation Performance of Phenol Removal
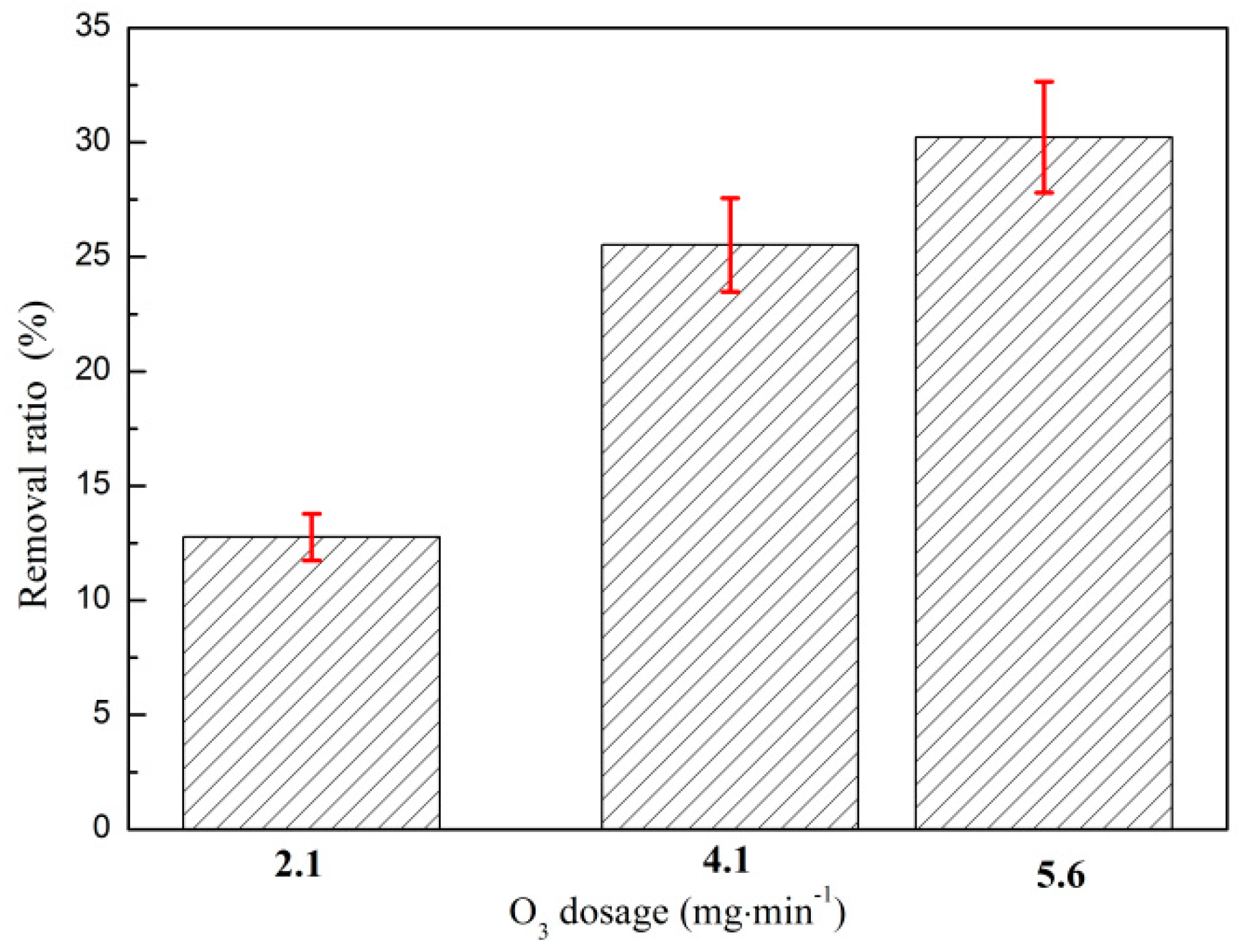
3.1.3. Effect of O3 Mass Flow on O3 Catalytic Oxidation Performance of Phenol Removal
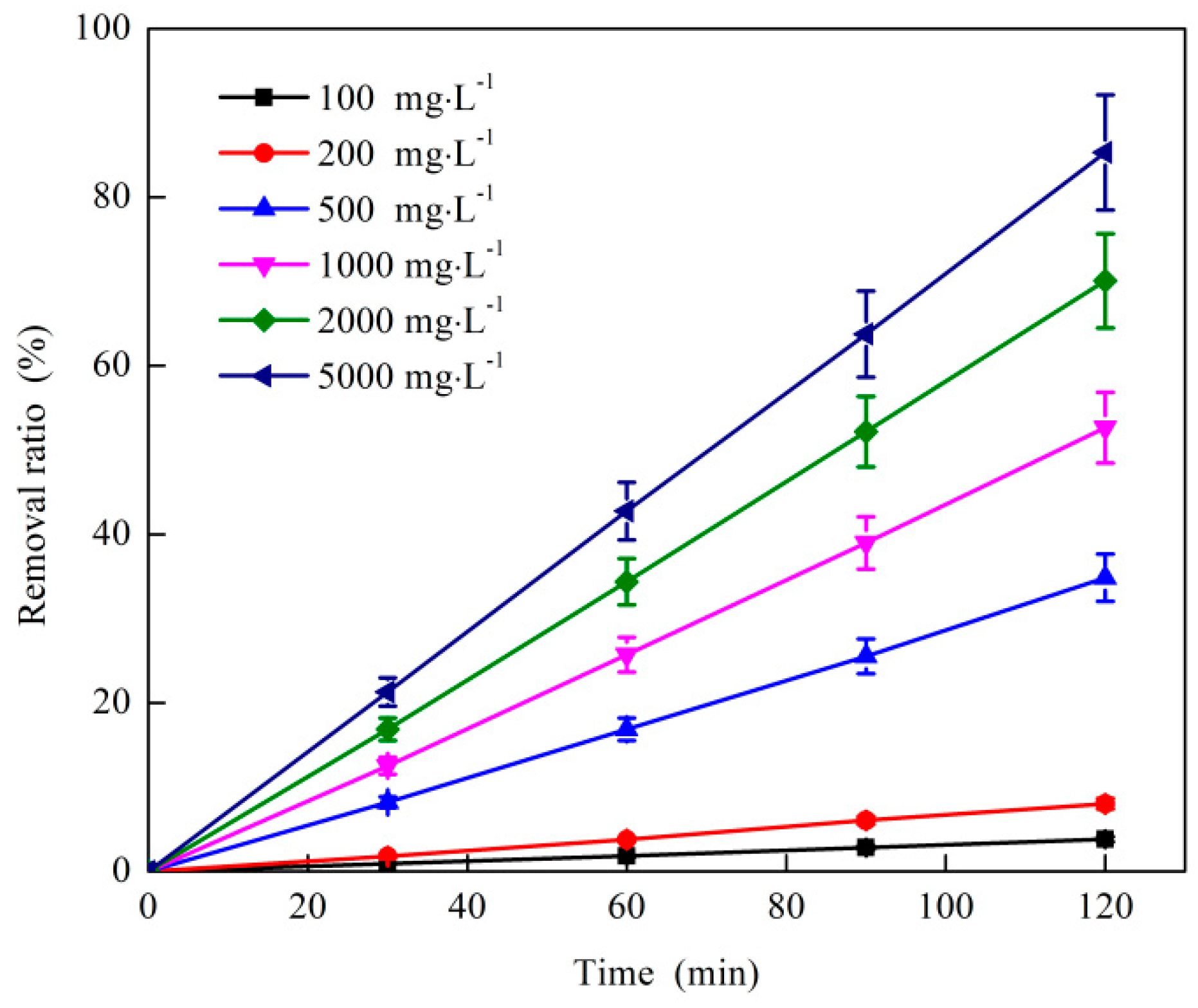
3.1.4. Effect of O3 Dosage on O3 Catalytic Oxidation Performance of Phenol Removal
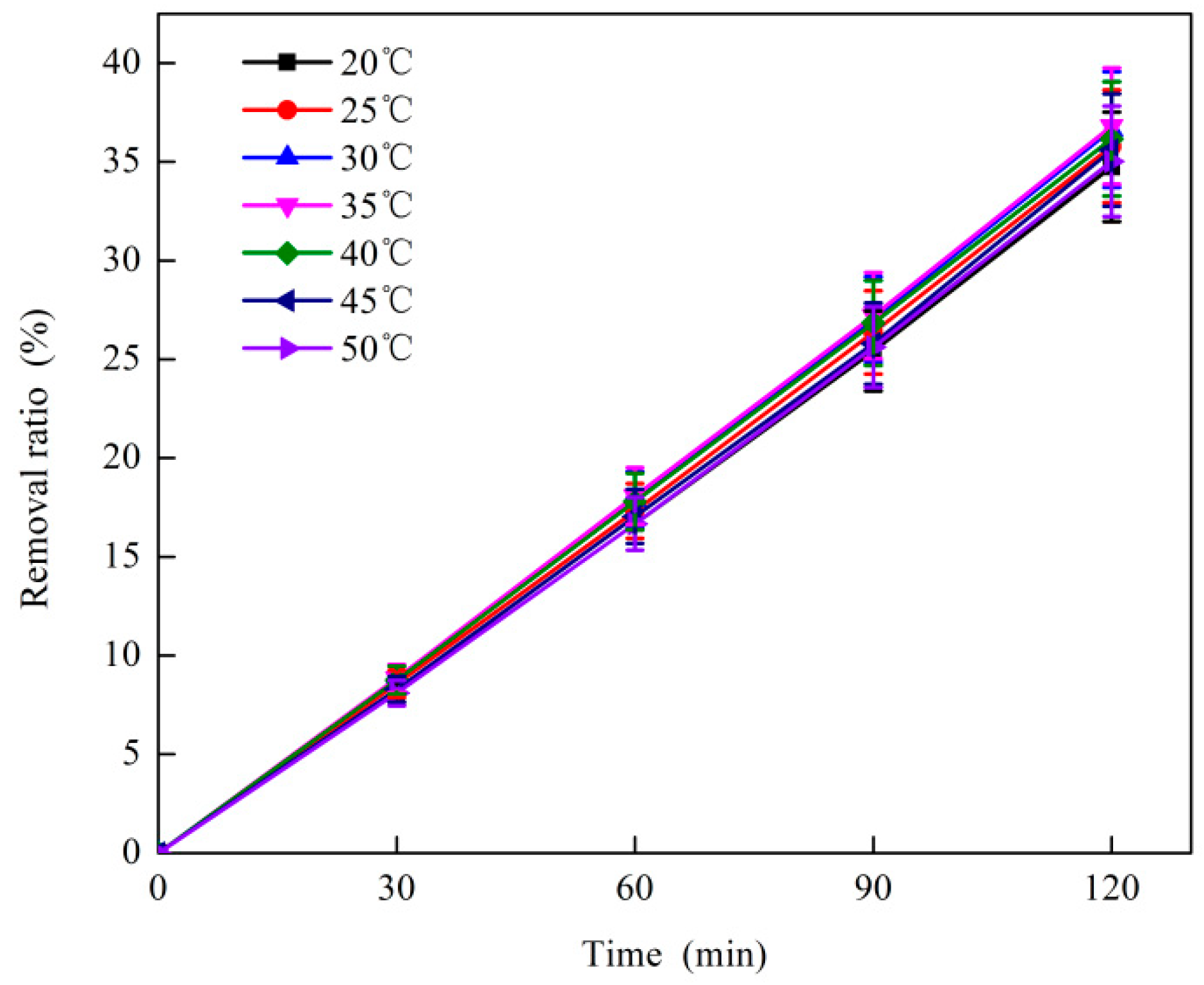
3.1.5. Effect of Reaction Temperature on O3 Catalytic Oxidation Performance of Phenol Removal
3.1.6. Effect of H2O2 Dosage on O3 Catalytic Oxidation Performance of Phenol Removal
3.2. Coagulation for Coking Wastewater Treatment
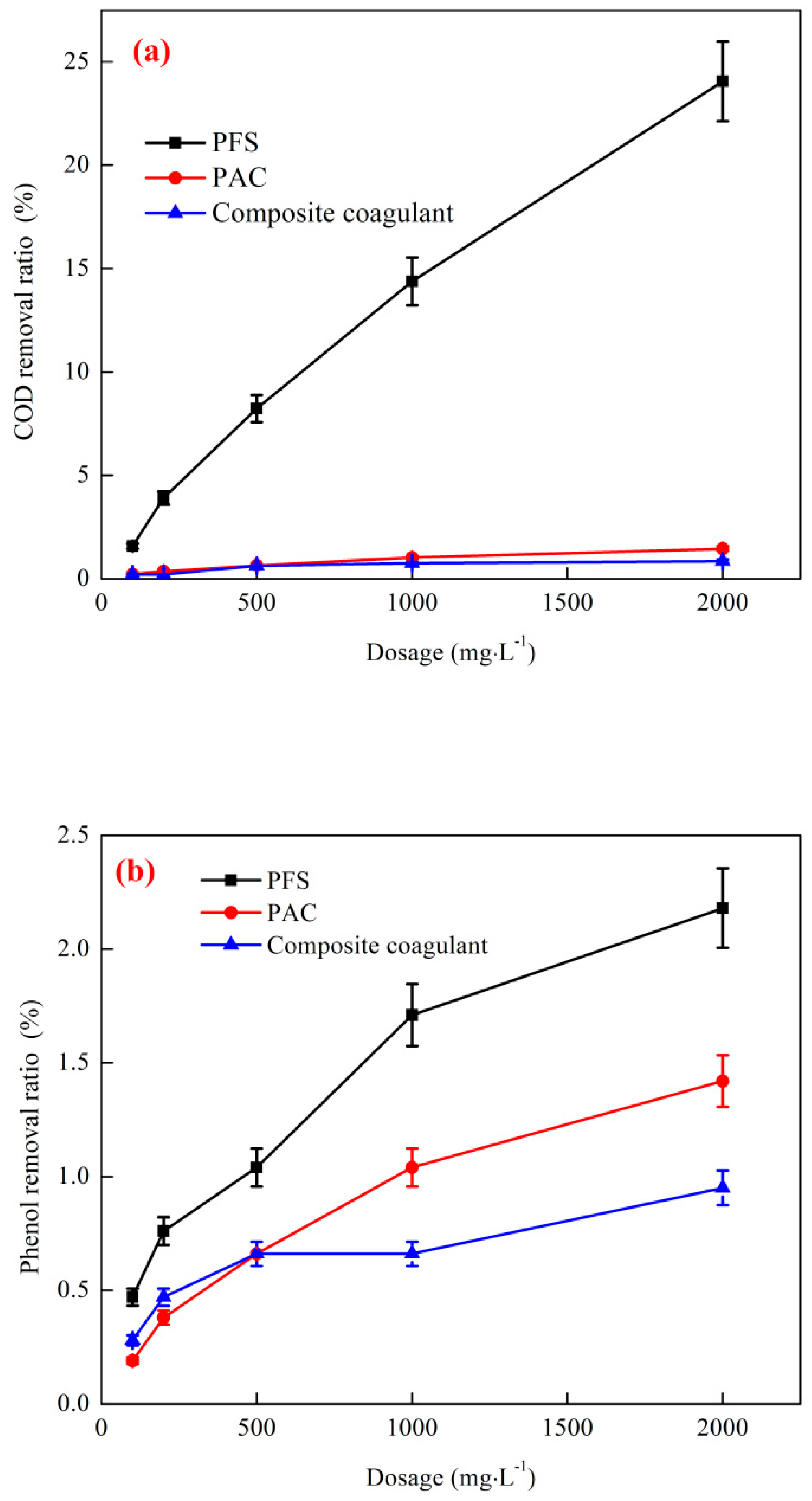
3.2.1. Effect of a Single Coagulant on the Removal of COD and Phenol by Coagulation
3.2.2. Effect of a Composite Coagulant on the Removal of COD and Phenol by Coagulation
3.3. Combined Process for the Treatment of Coking Wastewater
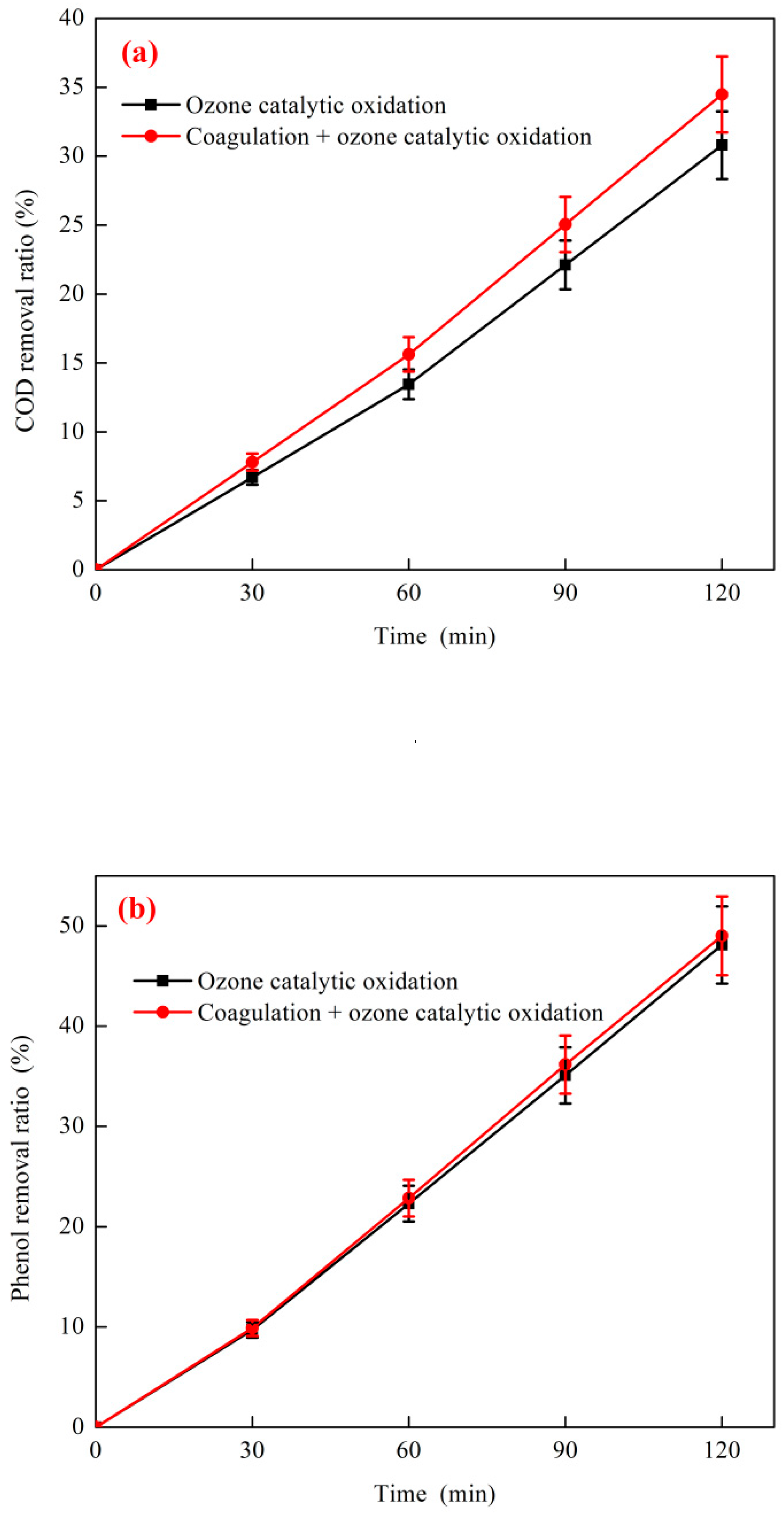
3.3.1. Coagulation + O3 Catalytic Oxidation Treatment of Coking Wastewater
3.3.2. Effect of H2O2 on the Combined Process of Coagulation + O3 Catalytic Oxidation
3.4. Mechanism Analysis
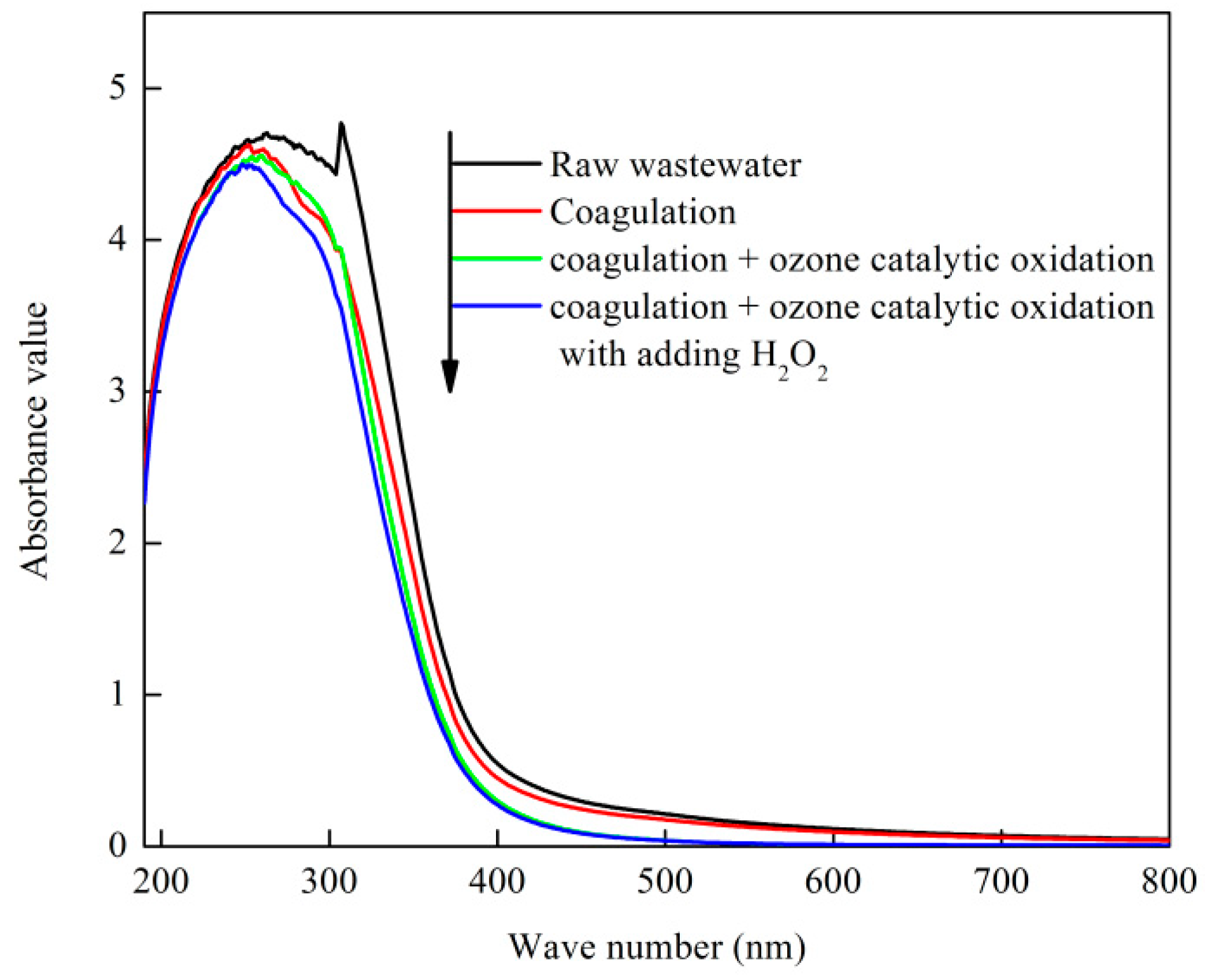
3.4.1. UV Analysis
3.4.2. GC–MS Analysis
3.5. Application of Coagulation and Ozone Catalytic Oxidation in Healthy Watershed Management
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.H.; Cheng, C.J.; Bai, S.; Jing, L.D.; Zhao, Z.G.; Liu, L.F. The performance of Pd-rGO electro-deposited PVDF/carbon fiber cloth composite membrane in MBR/MFC coupled system. Chem. Eng. J. 2019, 365, 317–324. [Google Scholar] [CrossRef]
- He, L.; Niu, Z.D.; Miao, R.R.; Chen, Q.L.; Guan, Q.Q.; Ning, P. Selective hydrogenation of phenol by the porous Carbon/ZrO2 supported Ni-Co nanoparticles in subcritical water medium. J. Clean. Prod. 2019, 215, 375–381. [Google Scholar] [CrossRef]
- Xu, H.X.; Qin, Q.Z.; Zhang, C.F.; Ning, K.J.; Zhao, R.; Wang, P.H.; Deng, J.S.; Huang, G. Adsorption of Organic Constituents from Reverse Osmosis Concentrate in Coal Chemical Industry by Coking Coal. Processes 2019, 7, 1. [Google Scholar] [CrossRef]
- Singh, H.; Mishra, B.K. Degradation of cyanide, aniline and phenol in pre-treated coke oven wastewater by peroxide assisted electro-oxidation process. Water Sci. Technol. 2018, 78, 2214–2227. [Google Scholar] [CrossRef]
- Li, S.X.; Feng, Z.T.; Hu, Y.; Wei, C.H.; Wu, H.Z.; Huang, J. In-Situ Synthesis and High-Efficiency Photocatalytic Performance of Cu(I)/Cu(II) Inorganic Coordination Polymer Quantum Sheets. Inorg. Chem. 2018, 57, 13289–13295. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.; Liu, L.F.; Nahyoon, N.A.; Zhang, Y.Z.; Idris, A.M. Enhanced Rhodamine B and coking wastewater degradation and simultaneous electricity generation via anodic g-C3N4/Fe-0(1%)/TiO2 and cathodic WO3 in photocatalytic fuel cell system under visible light irradiation. Electrochim. Acta 2019, 298, 430–439. [Google Scholar] [CrossRef]
- Oshiki, M.; Masuda, Y.; Yamaguchi, T.; Araki, N. Synergistic inhibition of anaerobic ammonium oxidation (anammox) activity by phenol and thiocyanate. Chemosphere 2018, 213, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.H.; Liang, C.Y.; Wang, C.Y.; Wang, Y.; Wang, H. Characterization of a phenanthrene-degrading methanogenic community. Front. Env. Sci. Eng. 2018, 12, 5. [Google Scholar] [CrossRef]
- Tu, Y.N.; Feng, P.; Ren, Y.G.; Cao, Z.H.; Wang, R.; Xu, Z.Q. Adsorption of ammonia nitrogen on lignite and its influence on coal water slurry preparation. Fuel 2019, 238, 34–43. [Google Scholar] [CrossRef]
- Tong, Y.J.; Zhang, Q.; Cai, J.J.; Gao, C.K.; Wang, L.Y.; Li, P. Water consumption and wastewater discharge in China’s steel industry. Ironmak. Steelmak. 2018, 45, 868–877. [Google Scholar] [CrossRef]
- Kudlek, E.; Dudziak, M. Toxicity and degradation pathways of selected micropollutants in water solutions during the O3 and O3/H2O2 process. Desalin. Water Treat 2018, 117, 88–100. [Google Scholar] [CrossRef]
- Kudlek, E. Decomposition of Contaminants of Emerging Concern in Advanced Oxidation Processes. Water 2018, 10, 9557. [Google Scholar] [CrossRef]
- Wang, N.N.; Zhao, Q.; Xu, H.; Niu, W.Y.; Ma, L.; Lan, D.C.; Hao, L.L. Adsorptive treatment of coking wastewater using raw coal fly ash: Adsorption kinetic, thermodynamics and regeneration by Fenton process. Chemosphere 2018, 210, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Kozak, J.; Wlodarczyk-Makula, M. Comparison of the PAHs degradation effectiveness using CaO2 or H2O2 under the photo-Fenton reaction. Desalin. Water Treat 2018, 134, 57–64. [Google Scholar] [CrossRef]
- Sun, Y.; Ren, M.; Zhu, C.; Xu, Y.; Zheng, H.; Xiao, X.; Wu, H.; Xia, T.; You, Z. UV-Initiated Graft Copolymerization of Cationic Chitosan-Based Flocculants for Treatment of Zinc Phosphate-Contaminated Wastewater. Ind. Eng. Chem. Res. 2016, 55, 10025–10035. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Zhu, C.; Guo, J.; Zhao, C.; Liao, Y.; Guan, Q. UV-initiated polymerization of hydrophobically associating cationic flocculants: Synthesis, characterization, and dewatering properties. Chem. Eng. J. 2013, 234, 318–326. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Guo, J.; Lo, F.; Fan, W.; Liao, Y.; Guan, Q. Characterization and Evaluation of Dewatering Properties of PADB, a Highly Efficient Cationic Flocculant. Ind. Eng. Chem. Res. 2014, 53, 2572–2582. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Wu, K.Y.; Zhou, H.T.; Hu, Y.; Sergei, P.; Wu, H.Z.; Wei, C.H. Ozonation of aqueous phenol catalyzed by biochar produced from sludge obtained in the treatment of coking wastewater. J. Environ. Manag. 2018, 224, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.M.; Ye, Y.L.; Lei, Z.C.; Feng, C.H.; Wei, C.H.; Chen, S.W. Phenol-degrading sludge as a promising precursor for a capacitive carbon material: Disclosing key factors for the nanostructure and high capacitance. Carbon 2018, 134, 53–61. [Google Scholar] [CrossRef]
- Li, J.F.; Li, J.G.; Liu, X.Y.; Du, Z.P.; Cheng, F.Q. Effect of silicon content on preparation and coagulation performance of poly-silicic-metal coagulants derived from coal gangue for coking wastewater treatment. Sep. Purif. Technol. 2018, 202, 149–156. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.F.; Chen, Z.L.; Cheng, F.Q. Fate and wetting potential of bio-refractory organics in membrane distillation for coke wastewater treatment. Chemosphere 2018, 208, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.T.; Sui, Q.; Huang, X. Removal and fate of polycyclic aromatic hydrocarbons in a hybrid anaerobic-anoxic-oxic process for highly toxic coke wastewater treatment. Sci. Total Environ. 2018, 635, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Zhang, Z.; Lin, A.G.; Song, Q.H.; Ji, G.D.; Wang, D.; Zhang, A.D. Treatment of super heavy oil wastewater by a combined process of lignite-activated coke adsorption and immobilized biological filter degradation: Performance and the relevant microbial community analysis. J. Chem. Technol. Biot. 2018, 93, 2942–2951. [Google Scholar] [CrossRef]
- Zhu, P.Y.; Zhu, K.J.; Puzey, R.; Ren, X.L. Degradation analysis of A(2)/O combined with AgNO3 + K2FeO4 on coking wastewater. Chin. J. Chem. Eng. 2018, 26, 1555–1560. [Google Scholar] [CrossRef]
- Zhang, X.W.; Qu, Y.Y.; You, S.N.; Ma, Q.; Zhou, H.; Zhang, L.Z.; Zhang, L.H.; Jing, J.W.; Liu, L.F. Bioremediation of nitrogen-containing organic pollutants using phenol-stimulated activated sludge: Performance and microbial community analysis. J. Chem. Technol. Biot. 2018, 93, 3199–3207. [Google Scholar] [CrossRef]
- Li, R.Q.; Wang, J.X.; Li, H.J. Isolation and characterization of organic matter-degrading bacteria from coking wastewater treatment plant. Water Sci. Technol. 2018, 78, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Li, E.C.; Jin, X.W.; Lu, S.G. Microbial communities in biological denitrification system using methanol as carbon source for treatment of reverse osmosis concentrate from coking wastewater. J. Water Reuse. Desalin. 2018, 8, 360–371. [Google Scholar] [CrossRef]
- Zhou, H.T.; Wei, C.H.; Zhang, F.Z.; Hu, Y.; Wu, H.Z.; Kraslawski, A. Energy Balance Evaluation in Coking Wastewater Treatment: Optimization and Modeling of Integrated Biological and Adsorption Treatment System. ACS Sustain. Chem. Eng. 2018, 6, 16448–16458. [Google Scholar] [CrossRef]
- Rychlewska, K.; Kwiecinska, A.; Kochel, M.; Figa, J. The use of polymeric and ceramic ultrafiltration in biologically treated coke oven wastewater polishing. Desalin. Water Treat 2018, 128, 207–213. [Google Scholar] [CrossRef]
- Cui, W.Q.; He, J.; Wang, H.; Hu, J.S.; Liu, L.; Liang, Y.H. Polyaniline hybridization promotes photo-electro-catalytic removal of organic contaminants over 3D network structure of rGH-PANI/TiO2 hydrogel. Appl. Catal. B Environ. 2018, 232, 232–245. [Google Scholar] [CrossRef]
- Zhu, X.B.; Tian, Y.; Li, F.F.; Liu, Y.P.; Wang, X.H.; Hu, X. Preparation and application of magnetic superhydrophobic polydivinylbenzene nanofibers for oil adsorption in wastewater. Environ. Sci. Pollut. R. 2018, 25, 22911–22919. [Google Scholar] [CrossRef]
- Song, X.L.; Liu, M.Q. Advanced treatment of biotreated coking wastewater with peroxymonosulfate oxidation catalyzed by granular activated carbon. J. Chem. Technol. Biot. 2018, 93, 2191–2198. [Google Scholar] [CrossRef]
- Zou, S.S.; Zhang, B.B.; Yan, N.; Zhang, C.Y.; Xu, H.; Zhang, Y.M.; Rittmann, B.E. Competition for molecular oxygen and electron donor between phenol and quinoline during their simultaneous biodegradation. Process Biochem. 2018, 70, 136–143. [Google Scholar] [CrossRef]
- Kong, Q.P.; Wu, H.Z.; Liu, L.; Zhang, F.Z.; Preis, S.; Zhu, S.; Wei, C.H. Solubilization of polycyclic aromatic hydrocarbons (PAHs) with phenol in coking wastewater treatment system: Interaction and engineering significance. Sci. Total Environ. 2018, 628–629, 467–473. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.; Sun, W.; Shah, K.J.; Xu, Y.; Zheng, H. Efficient cationic flocculant MHCS-g-P(AM-DAC) synthesized by UV-induced polymerization for algae removal. Sep. Purif. Technol. 2019, 210, 10–19. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, W.; Shah, K.J.; Chiang, P.; Zheng, H. Characterization and flocculation evaluation of a novel carboxylated chitosan modified flocculant by UV initiated polymerization. Carbohyd. Polym. 2019, 208, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, C.; Zheng, H.; Sun, W.; Xu, Y.; Xiao, X.; You, Z.; Liu, C. Characterization and coagulation behavior of polymeric aluminum ferric silicate for high-concentration oily wastewater treatment. Chem. Eng. Res. Des. 2017, 119, 23–32. [Google Scholar] [CrossRef]
- Sun, W.Q.; Zhu, H.; Sun, Y.J.; Chen, L.; Xu, Y.H.; Zheng, H.L. Enhancement of waste-activated sludge dewaterability using combined Fenton pre-oxidation and flocculation process. Desalin. Water Treat 2018, 126, 314–323. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, H.; Sun, Y.J.; Chiang, P.C.; Sun, W.Q.; Xu, Y.H.; Zheng, H.L.; Shah, K.J. Characterization and sludge dewatering performance evaluation of the photo-initiated cationic flocculant PDD. J. Taiwan Inst. Chem. E 2018, 93, 253–262. [Google Scholar] [CrossRef]
- Tang, M.; Sun, Y.; Zhu, C.; Xu, Y.; Zheng, H.; Xiao, X.; Sun, W.; Wu, H.; Liu, C. Preparation of polymeric aluminum ferric silicate for the pre-treatment of oily wastewater through response surface method. Desalin. Water Treat 2017, 65, 284–293. [Google Scholar] [CrossRef]
- Lu, X.; Xu, Y.; Sun, W.; Sun, Y.; Zheng, H. UV-initiated synthesis of a novel chitosan-based flocculant with high flocculation efficiency for algal removal. Sci. Total Environ. 2017, 609, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shah, K.J.; Sun, W.; Zheng, H. Performance evaluation of chitosan-based flocculants with good pH resistance and high heavy metals removal capacity. Sep. Purif. Technol. 2019, 215, 208–216. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, H.; Xiong, Z.; Wang, Y.; Tang, X.; Chen, W.; Ding, Y. Algae removal from raw water by flocculation and the fractal characteristics of flocs. Desalin. Water Treat 2015, 56, 894–904. [Google Scholar] [CrossRef]
- Egea-Corbacho, A.; Gutierrez, S.; Quiroga, J.M. Removal of emerging contaminants from wastewater through pilot plants using intermittent sand/coke filters for its subsequent reuse. Sci. Total Environ. 2019, 646, 1232–1240. [Google Scholar] [CrossRef]
- Zheng, M.Q.; Han, Y.X.; Xu, C.Y.; Zhang, Z.W.; Han, H.J. Selective adsorption and bioavailability relevance of the cyclic organics in anaerobic pretreated coal pyrolysis wastewater by lignite activated coke. Sci. Total Environ. 2019, 653, 64–73. [Google Scholar] [CrossRef]
- Malakootian, M.; Heidari, M.R. Removal of phenol from steel wastewater by combined electrocoagulation with photo-Fenton. Water Sci. Technol. 2018, 78, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Ledakowicz, S.; Stolarek, P.; Malinowski, A.; Lepez, O. Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification. Renew. Sust. Energ. Rev. 2019, 104, 319–327. [Google Scholar] [CrossRef]
- Cheng, Z.W.; Yang, B.W.; Chen, Q.C.; Gao, X.P.; Tan, Y.J.; Yuan, T.; Shen, Z.M. Quantitative-Structure-Activity-Relationship (QSAR) models for the reaction rate and temperature of nitrogenous organic compounds in supercritical water oxidation (SCWO). Chem. Eng. J. 2018, 354, 12–20. [Google Scholar] [CrossRef]
- Huang, J.J.; Hou, B.H.; Guo, N.N.; Xu, R.L.; Su, N.N.; Bairu, A.G.; Huang, X.; Xie, C.; Hao, H.X. Solid-liquid phase equilibria of ternary system Na2S2O3-Na2SO4-H2O in a wide range of temperatures: Measurement and application. J. Chem. Thermodyn. 2018, 125, 1–10. [Google Scholar] [CrossRef]



| Index | Characteristic | pH | COD (mg·L−1) | Total Nitrogen (mg·L−1) | Kjeldahl Nitrogen (mg·L−1) | NH3-N (mg·L−1) | Cyanide (mg·L−1) | Salinity (mg·L−1) | Total Phenols (mg·L−1) | Petroleum (mg·L−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Value | Yellowish brown, translucent, with a strong pungent odor | 9.6 | 4176 | 285 | 118 | 116 | 49.3 | 8500 | 716 | 184 |
| Process | Degradation Products List |
|---|---|
| Coking wastewater | Phenol, aniline, 2-methylphenol, 3-methylphenol, 2,4-dimethylphenol, 3,5-dimethylphenol, 3,4-dimethylphenol, 2,3-dihydrobenzene And furan, quinoline, anthracene, 1 (2H)-isoquinoline, |
| Coagulation | Phenol, aniline, 2-methylphenol, 3-methylphenol, 2,4-dimethylphenol, 3,5-dimethylphenol, 3,4-dimethylphenol, 2,3-dihydrobenzene Furan, quinoline, anthracene, 1(2H)-isoquinoline |
| Coagulation + O3 catalytic oxidation | Phenol, 5-methylfurfural, 2-methylphenylhydrazine, 4,5-dimethyl-2-hydroxypyrimidine, rosin acetate, 2,3,4,5-tetramethyl-2-cyclopentenone, 2-methylphenol, 4,5,6-trimethyl-2-pyrimidinone, benzofuran, quinoline, 4-bromo-3-methyl-phenol, 2,3-dihydroindole-4- Alcohol-2-ketone, N-phenylformamide |
| Coagulation + O3 catalytic oxidation with addition of H2O2 | Phenol, 5-methylfurfural, 4,5-dimethyl-2-hydroxypyrimidine, 2,3,4,5-tetramethyl-2-cyclopentenone, 4,5,6 trimethyl-2 -pyrimidinone, 2-hydroxy-4,4-dimethyl-3-indolyl-2-cyclopentanone, N-phenylformamide, quinoline, 2,3-dihydroindole-4-Alcohol-2-ketone |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Xu, Y.; Sun, Y. Combination of Coagulation and Ozone Catalytic Oxidation for Pretreating Coking Wastewater. Int. J. Environ. Res. Public Health 2019, 16, 1705. https://doi.org/10.3390/ijerph16101705
Chen L, Xu Y, Sun Y. Combination of Coagulation and Ozone Catalytic Oxidation for Pretreating Coking Wastewater. International Journal of Environmental Research and Public Health. 2019; 16(10):1705. https://doi.org/10.3390/ijerph16101705
Chicago/Turabian StyleChen, Lei, Yanhua Xu, and Yongjun Sun. 2019. "Combination of Coagulation and Ozone Catalytic Oxidation for Pretreating Coking Wastewater" International Journal of Environmental Research and Public Health 16, no. 10: 1705. https://doi.org/10.3390/ijerph16101705
APA StyleChen, L., Xu, Y., & Sun, Y. (2019). Combination of Coagulation and Ozone Catalytic Oxidation for Pretreating Coking Wastewater. International Journal of Environmental Research and Public Health, 16(10), 1705. https://doi.org/10.3390/ijerph16101705






