Occurrence, Removal and Bioaccumulation of Perfluoroalkyl Substances in Lake Chaohu, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
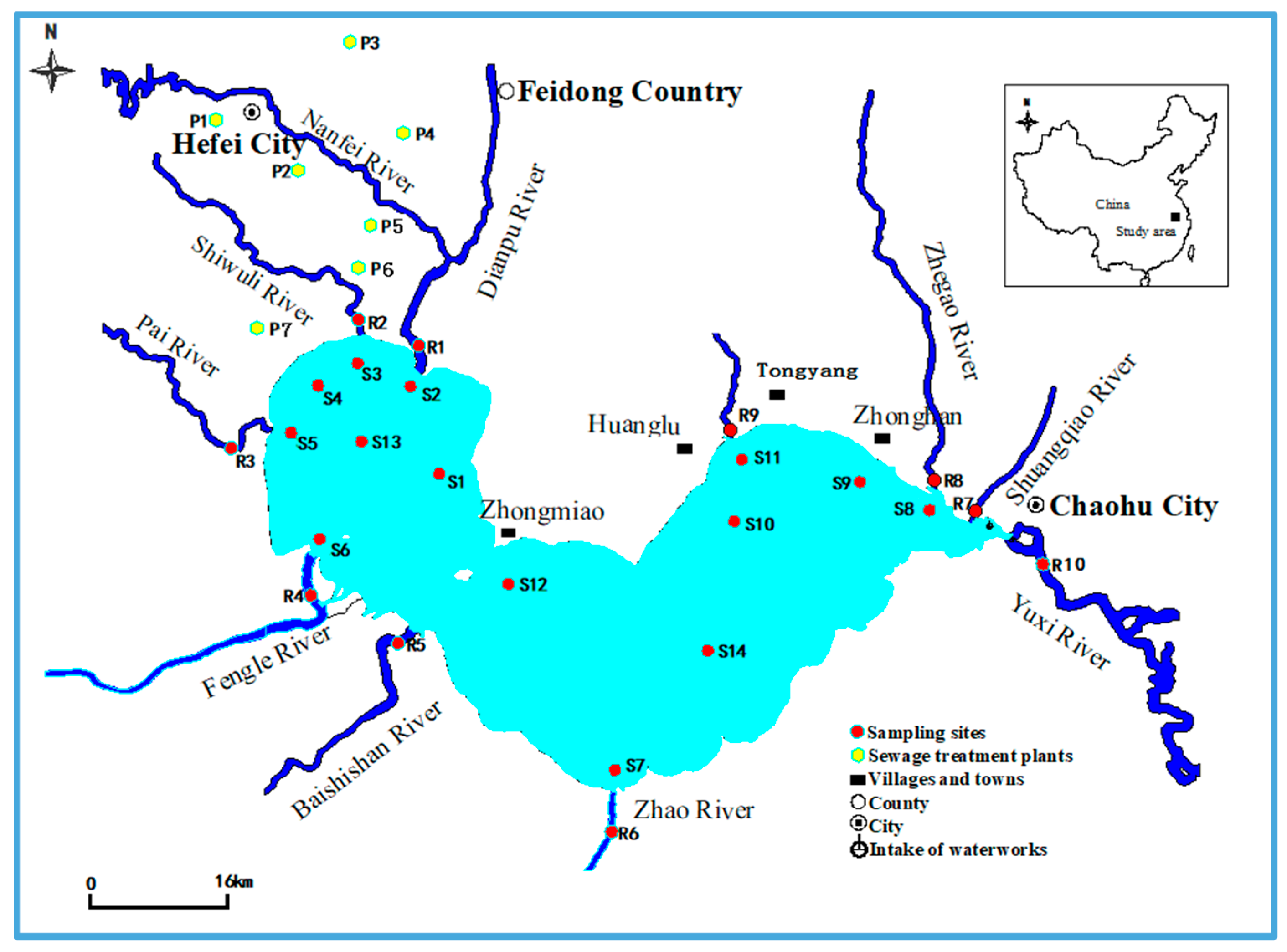
2.2. Sample Collection
2.3. Sample Preparation and Analysis
2.4. Quality Assurance/Quality Control (QA/QC)
3. Results and Discussion
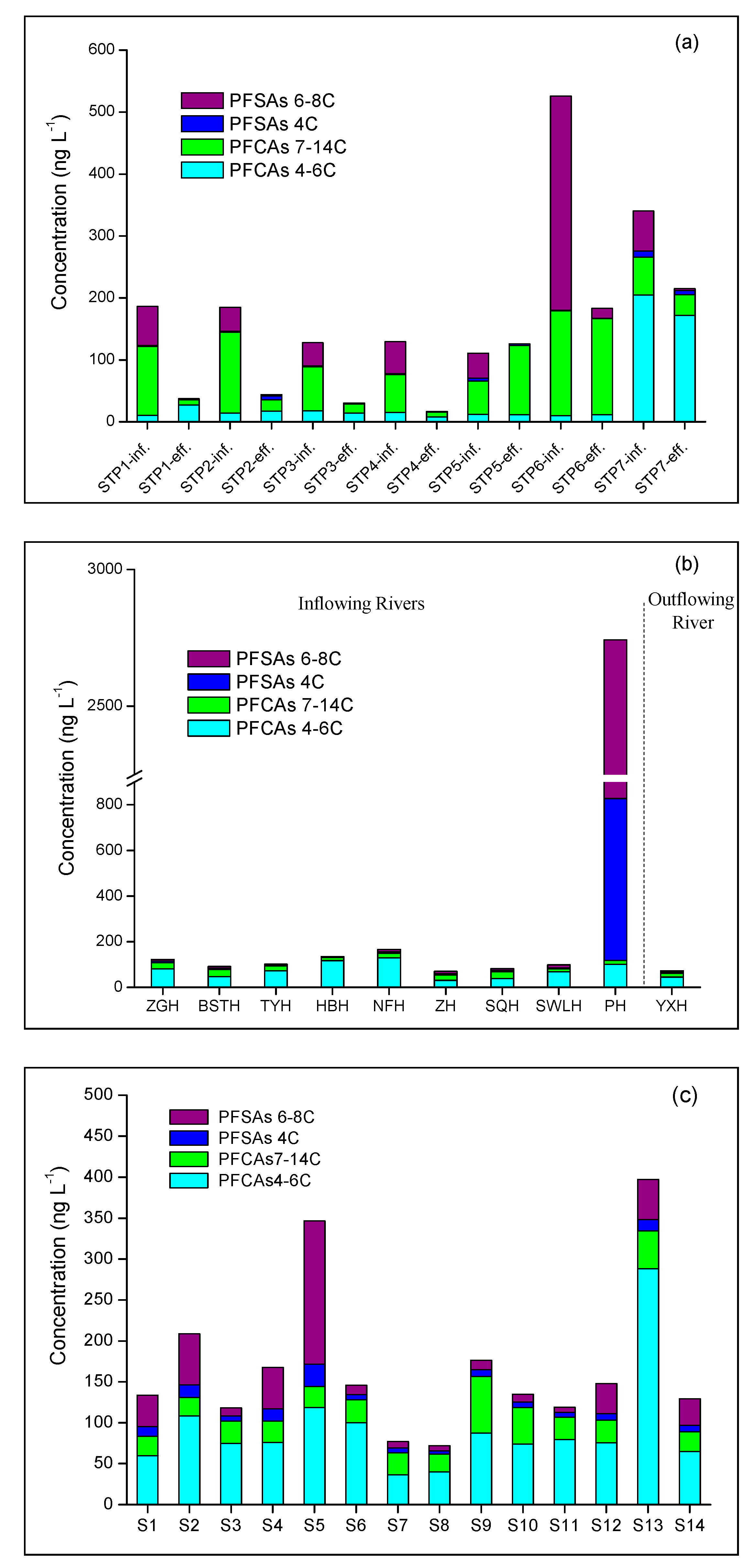
3.1. Occurrence, Removal and Flux of PFAAs from STPs
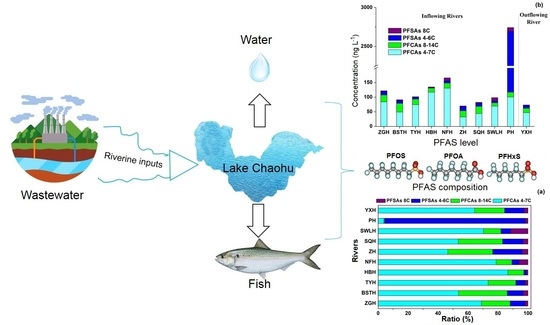
3.2. Occurrence and Distribution of PFAAs in The Rivers around Lake Chaohu
3.3. Occurrence and Distribution of PFAAs in Lake Chaohu
3.4. Composition of PFAAs in Lake Chaohu and Global Comparison
3.5. Occurrence of The Selected PFAAs in Biota Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Peer reviewed: Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 2002, 36, 146A–152A. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, O.; Snyder, S.A. Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environ. Sci. Technol. 2009, 43, 9089–9095. [Google Scholar] [CrossRef] [PubMed]
- Butt, C.M.; Berger, U.; Bossi, R.; Tomy, G.T. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci. Total Environ. 2010, 408, 2936–2965. [Google Scholar] [CrossRef]
- Harrad, S.; de Wit, C.A.; Abdallah, M.A.E.; Bergh, C.; Björklund, J.A.; Covaci, A.; Darnerud, P.O.; de Boer, J.; Diamond, M.; Huber, S.; et al. Indoor contamination with hexabromocyclododecanes, polybrominated diphenyl ethers, and perfluoroalkyl compounds: An important exposure pathway for people? Environ. Sci. Technol. 2010, 44, 3221–3231. [Google Scholar] [CrossRef]
- Filipovic, M.; Woldegiorgis, A.; Norström, K.; Bibi, M.; Lindberg, M.; Österås, A.H. Historical usage of aqueous film forming foam: A case study of the widespread distribution of perfluoroalkyl acids from a military airport to groundwater, lakes, soils and fish. Chemosphere 2015, 129, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yao, Y.; Zhao, Z.; Wang, Y.; Wang, Q.; Ren, C.; Wang, B.; Sun, H.; Alder, A.C.; Kannan, K. Multimedia distribution and transfer of per- and polyfluoroalkyl substances (PFASs) surrounding two fluorochemical manufacturing facilities in Fuxin, China. Environ. Sci. Technol. 2018, 52, 8263–8271. [Google Scholar] [CrossRef]
- Liu, W.; He, W.; Wu, J.; Qin, N.; He, Q.; Xu, F. Residues, bioaccumulations and biomagnification of perfluoroalkyl acids (PFAAs) in aquatic animals from Lake Chaohu, China. Environ. Pollut. 2018, 240, 607–614. [Google Scholar] [CrossRef]
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Surridge, B.; Hoover, D.; Grace, R.; Gobas, F.A.P.C. Perfluoroalkyl contaminants in an arctic marine food web: Trophic magnification and wildlife exposure. Environ. Sci. Technol. 2009, 43, 4037–4043. [Google Scholar] [CrossRef]
- Fang, S.; Chen, X.; Zhao, S.; Zhang, Y.; Jiang, W.; Yang, L.; Zhu, L. Trophic magnification and isomer fractionation of perfluoroalkyl substances in the food web of Taihu Lake, China. Environ. Sci. Technol. 2014, 48, 2173–2182. [Google Scholar] [CrossRef]
- Xu, J.; Guo, C.S.; Zhang, Y.; Meng, W. Bioaccumulation and trophic transfer of perfluorinated compounds in a eutrophic freshwater food web. Environ. Pollut. 2014, 184, 254–261. [Google Scholar] [CrossRef]
- Boudreau, T.M.; Sibley, P.K.; Mabury, S.A.; Muir, D.G.C.; Solomon, K.R. Laboratory evaluation of the toxicity of perfluorooctane sulfonate (PFOS) on Selenastrum capricornutum, Chlorella vulgaris, Lemna gibba, Daphnia magna, and Daphnia pulicaria. Arch. Environ. Contam. Toxicol. 2003, 44, 0307–0313. [Google Scholar] [CrossRef]
- Kumar, S.K. Fluorinated Organic Chemicals: A Review. Res. J. Chem. Environ. 2005, 9, 50–79. [Google Scholar]
- Wei, Y.; Dai, J.; Liu, M.; Wang, J.; Xu, M.; Zha, J.; Wang, Z. Estrogen-like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rarus). Environ. Toxicol. Chem. 2007, 26, 2440–2447. [Google Scholar] [CrossRef]
- Sinclair, E.; Kannan, K. Mass loading and fate of perfluoroalkyl surfactants in wastewater treatment plants. Environ. Sci. Technol. 2006, 40, 1408–1414. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Taniyasu, S.; Yeung, L.W.Y.; Lam, P.K.S.; Wang, J.; Li, X.; Yamashita, N.; Dai, J. Distribution and fate of perfluoroalkyl substances in municipal wastewater treatment plants in economically developed areas of China. Environ. Pollut. 2013, 176, 10–17. [Google Scholar] [CrossRef]
- Pan, C.G.; Liu, Y.S.; Ying, G.G. Perfluoroalkyl substances (PFASs) in wastewater treatment plants and drinking water treatment plants: Removal efficiency and exposure risk. Water Res. 2016, 106, 562–570. [Google Scholar] [CrossRef]
- Becker, A.M.; Gerstmann, S.; Frank, H. Perfluorooctanoic acid and perfluorooctane sulfonate in the sediment of the Roter Main river, Bayreuth, Germany. Environ. Pollut. 2008, 156, 818–820. [Google Scholar] [CrossRef]
- Sun, H.; Li, F.; Zhang, T.; Zhang, X.; He, N.; Song, Q.; Zhao, L.; Sun, L.; Sun, T. Perfluorinated compounds in surface waters and WWTPs in Shenyang, China: Mass flows and source analysis. Water Res. 2011, 45, 4483–4490. [Google Scholar] [CrossRef]
- Campo, J.; Masiá, A.; Picó, Y.; Farré, M.; Barceló, D. Distribution and fate of perfluoroalkyl substances in Mediterranean Spanish sewage treatment plants. Sci. Total Environ. 2014, 472, 912–922. [Google Scholar] [CrossRef]
- Houde, M.; Giraudo, M.; Douville, M.; Bougas, B.; Couture, P.; De Silva, A.O.; Spencer, C.; Lair, S.; Verreault, J.; Bernatchez, L.; et al. A multi-level biological approach to evaluate impacts of a major municipal effluent in wild St. Lawrence River yellow perch (Perca flavescens). Sci. Total Environ. 2014, 497–498, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Tokumura, M.; Islam, M.S.; Zushi, Y.; Oh, J.; Masunaga, S. Spatial distribution and importance of potential perfluoroalkyl acid precursors in urban rivers and sewage treatment plant effluent—Case study of Tama River, Japan. Water Res. 2014, 67, 77–85. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, H.; Li, F.; Zhou, Q. Occurrence and fate of perfluorinated acids in two wastewater treatment plants in Shanghai, China. Environ. Sci. Pollut. Res. 2015, 22, 1804–1811. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Bundschuh, M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef]
- Martin, J.W.; Whittle, D.M.; Muir, D.C.G.; Mabury, S.A. Perfluoroalkyl contaminants in a food web from Lake Ontario. Environ. Sci. Technol. 2004, 38, 5379–5385. [Google Scholar] [CrossRef]
- Loi, E.I.H.; Yeung, L.W.Y.; Taniyasu, S.; Lam, P.K.S.; Kannan, K.; Yamashita, N. Trophic magnification of poly- and perfluorinated compounds in a subtropical food Web. Environ. Sci. Technol. 2011, 45, 5506–5513. [Google Scholar] [CrossRef]
- Munoz, G.; Budzinski, H.; Babut, M.; Drouineau, H.; Lauzent, M.; Menach, K.L.; Lobry, J.; Selleslagh, J.; Simonnet-Laprade, C.; Labadie, P. Evidence for the trophic transfer of perfluoroalkylated substances in a temperate macrotidal estuary. Environ. Sci. Technol. 2017, 51, 8450–8459. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Liu, H.; Yan, Z. Tetrabromobisphenol A: Tissue distribution in fish, and seasonal variation in water and sediment of Lake Chaohu, China. Environ. Sci. Pollut. Res. 2012, 19, 4090–4096. [Google Scholar] [CrossRef]
- Tang, J.; Shi, T.; Wu, X.; Cao, H.; Li, X.; Hua, R.; Tang, F.; Yue, Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere 2015, 122, 154–161. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Q.; Shao, S.; Zhang, N.; Shen, Q.; Liu, C. Heavy Metal Pollution, Fractionation, and Potential Ecological Risks in Sediments from Lake Chaohu (Eastern China) and the Surrounding Rivers. Int. J. Environ. Res. Public Health 2015, 122, 14115–14131. [Google Scholar] [CrossRef]
- He, W.; Bai, Z.L.; Liu, W.X.; Kong, X.Z.; Yang, B.; Yang, C.; Jørgensen, S.E.; Xu, F.L. Occurrence, spatial distribution, sources, and risks of polychlorinated biphenyls and heavy metals in surface sediments from a large eutrophic Chinese lake (Lake Chaohu). Environ. Sci. Pollut. Res. 2016, 23, 10335–10348. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Zhang, F.; Nkoom, M.; Yan, Z.; Wu, D. Polybrominated Diphenyl Ethers (PBDEs) in a Large, Highly Polluted Freshwater Lake, China: Occurrence, Fate, and Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 1529. [Google Scholar] [CrossRef]
- Liu, W.X.; He, W.; Qin, N.; Kong, X.Z.; He, Q.S.; Yang, B.; Yang, C.; Jorgensen, S.E.; Xu, F.L. Temporal-spatial distributions and ecological risks of perfluoroalkyl acids (PFAAs) in the surface water from the fifth-largest freshwater lake in China (Lake Chaohu). Environ. Pollut. 2015, 200, 24–34. [Google Scholar] [CrossRef]
- Lu, Z.; Song, L.; Zhao, Z.; Ma, Y.; Wang, J.; Yang, H.; Ma, H.; Cai, M.; Codling, G.; Ebinghaus, R.; et al. Occurrence and trends in concentrations of perfluoroalkyl substances (PFASs) in surface waters of eastern China. Chemosphere 2015, 119, 820–827. [Google Scholar] [CrossRef]
- Wang, P.; Lu, Y.; Wang, T.; Zhu, Z.; Li, Q.; Meng, J.; Su, H.; Johnson, A.C.; Sweetman, A.J. Coupled production and emission of short chain perfluoroalkyl acids from a fast developing fluorochemical industry: Evidence from yearly and seasonal monitoring in Daling River Basin, China. Environ. Pollut. 2016, 218, 1234–1244. [Google Scholar] [CrossRef]
- Ma, X.; Shan, G.; Chen, M.; Zhao, J.; Zhu, L. Riverine inputs and source tracing of perfluoroalkyl substances (PFASs) in Taihu Lake, China. Sci. Total. Environ. 2018, 612, 18–25. [Google Scholar] [CrossRef]
- ECHA. One New Substance Added to the Candidate List, Several Entries Updated; ECHA: Helsinki, Finland, 2017. [Google Scholar]
- FAO Headquarters. Stockholm Convention. In Proceedings of the Thirteenth Meeting of the Persistent Organic Pollutants Review Committee (POPRC.13), Rome, Italy, 17–20 October 2017. [Google Scholar]
- Guo, L.; Xie, P.; Ni, L.; Hu, W.; Li, H. The status of fishery resources of Lake Chaohu and its response to eutrophication. Acta Hydrobiol. Sin. 2007, 31, 700–705. (In Chinese) [Google Scholar]
- Institute of Hydrobiology, Chinese Academy of Sciences, Shanghai Museum of Nature. Atlas of Freshwater Fish in China; Shanghai Science and Technology Publishers: Shanghai, China, 1982. (In Chinese) [Google Scholar]
- Taniyasu, S.; Kannan, K.; Yeung, L.W.Y.; Kwok, K.Y.; Lam, P.K.S.; Yamashita, N. Analysis of trifluoroacetic acid and other short-chain perfluorinated acids (C2–C4) in precipitation by liquid chromatography–tandem mass spectrometry: Comparison to patterns of long-chain perfluorinated acids (C5–C18). Anal. Chim. Acta 2008, 619, 221–230. [Google Scholar] [CrossRef]
- He, X.M.; Chen, H. Determination of eight perfluorinated compounds in fish muscles by isotope dilution ultra-performance liquid chromatography tandem mass spectrometry. Food Sci. 2014, 35, 193–197. (In Chinese) [Google Scholar]
- Higgins, C.P.; Luthy, R.G. Sorption of Perfluorinated Surfactants on Sediments. Environ. Sci. Technol. 2006, 40, 7251–7256. [Google Scholar] [CrossRef]
- Ort, C.; Lawrence, M.G.; Reungoat, J.; Mueller, J.F. Sampling for PPCPs in Wastewater Systems: Comparison of different sampling modes and optimization strategies. Environ. Sci. Technol. 2010, 44, 6289–6296. [Google Scholar] [CrossRef]
- Ort, C.; Lawrence, M.G.; Rieckermann, J.; Joss, A. Sampling for pharmaceuticals and personal care products (PPCPs) and illicit drugs in wastewater systems: Are your conclusions valid? A critical review. Environ. Sci. Technol. 2010, 44, 6024–6035. [Google Scholar] [CrossRef]
- Murakami, M.; Kuroda, K.; Sato, N.; Fukushi, T.; Takizawa, S.; Takada, H. Groundwater pollution by perfluorinated surfactants in Tokyo. Environ. Sci. Technol. 2009, 43, 3480–3486. [Google Scholar] [CrossRef]
- Vierke, L.; Möller, A.; Klitzke, S. Transport of perfluoroalkyl acids in a water-saturated sediment column investigated under near-natural conditions. Environ. Pollut. 2014, 186, 7–13. [Google Scholar] [CrossRef]
- Wang, X.; Xi, B.; Huo, S.; Deng, L.; Pan, H.; Xia, X.; Zhang, J.; Ren, Y.; Liu, H. Polybrominated diphenyl ethers occurrence in major inflowing rivers of Lake Chaohu (China): Characteristics, potential sources and inputs to lake. Chemosphere 2013, 93, 1624–1631. [Google Scholar] [CrossRef]
- Cui, Q.; Pan, Y.; Zhang, H.; Sheng, N.; Dai, J. Elevated concentrations of perfluorohexanesulfonate and other per- and polyfluoroalkyl substances in Baiyangdian Lake (China): Source characterization and exposure assessment. Environ. Pollut. 2018, 241, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Y.; Zhao, X.; Du, P.; Liu, S.; Lv, J.; Xu, F.; Meng, W.; Xu, J. Distribution, source characterization and inventory of perfluoroalkyl substances in Taihu Lake, China. Chemosphere 2015, 12, 201–207. [Google Scholar] [CrossRef]
- Takemine, S.; Matsumura, C.; Yamamoto, K.; Suzuki, M.; Tsurukawa, M.; Imaishi, H.; Nakano, T.; Kondo, A. Discharge of perfluorinated compounds from rivers and their influence on the coastal seas of Hyogo prefecture, Japan. Environ. Pollut. 2014, 184, 397–404. [Google Scholar] [CrossRef]
- Chen, H.; Sun, R.; Zhang, C.; Han, J.; Wang, X.; Han, G.; He, X. Occurrence, spatial and temporal distributions of perfluoroalkyl substances in wastewater, seawater and sediment from Bohai Sea, China. Environ. Pollut. 2016, 219, 389–398. [Google Scholar] [CrossRef]
- Nguyen, M.; Wiberg, K.; Ribeli, E.; Josefsson, S.; Futter, M.; Gustavsson, J.; Ahrens, L. Spatial distribution and source tracing of per-and polyfluoroalkyl substances (PFASs) in surface water in Northern Europe. Environ. Pollut. 2017, 220, 1438–1446. [Google Scholar] [CrossRef]
- Campo, J.; Perez, F.; Masia, A.; Pico, Y.; Farre, M.; Barcelo, D. Perfluoroalkyl substance contamination of the Llobregat River ecosystem (Mediterranean area, NE Spain). Sci. Total Environ. 2014, 503–504, 48–57. [Google Scholar]
- Wang, J.; Zhang, Y.; Zhang, F.; Yeung, L.; Taniyasu, S.; Yamazaki, E.; Wang, R.; Lam, P.; Yamashita, N.; Dai, J. Age- and gender-related accumulation of perfluoroalkyl substances in captive Chinese alligators (Alligator sinensis). Environ. Pollut. 2013, 179, 61–67. [Google Scholar] [CrossRef]
- Kannan, K.; Tao, L.; Sinclair, E.; Pastva, S.D.; Jude, D.J.; Giesy, J.P. Perfluorinated compounds in aquatic organisms at various trophic levels in a great lakes food chain. Arch. Environ. Contam. Toxicol. 2005, 48, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadou, I.; Costopoulou, D.; Kalogeropoulos, N.; Karavoltsos, S.; Sakellari, A.; Zafeiraki, E.; Dassenakis, M.; Leondiadis, L. Levels of perfluorinated compounds in raw and cooked Mediterranean finfish and shellfish. Chemosphere 2015, 127, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liang, Y.; Shi, Y.; Xu, L.; Cai, Y. Occurrence and transport of perfluoroalkyl acids (PFAAs), including short-chain PFAAs in Tangxun Lake, China. Environ. Sci. Technol. 2013, 47, 9249–9257. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Conder, J.M.; Hoke, R.A.; Wolf, W.D.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef] [PubMed]

| STP | Flow (m3 day−1) | Concentrations (ng L−1) | Flux (g) | ||
|---|---|---|---|---|---|
| ΣShort-Chain PFASs | ΣLong-Chain PFASs | ΣShort-Chain PFASs | ΣLong-Chain PFASs | ||
| STP1 | 180,000 | 28.4 | 8.9 | 5.11 | 1.6 |
| STP2 | 300,000 | 23.3 | 20.6 | 6.98 | 6.19 |
| STP3 | 200,000 | 15.8 | 14.6 | 3.16 | 2.91 |
| STP4 | 110,000 | 8.95 | 8.01 | 0.98 | 0.88 |
| STP5 | 600,000 | 14.2 | 112 | 8.5 | 67.1 |
| STP6 | 50,000 | 12 | 171 | 0.6 | 8.56 |
| STP7 | 600,000 | 179 | 36.4 | 107 | 21.8 |
| Sum. | 2,040,000 | ||||
| Analytes | Inflowing Rivers | Lake Sites | ||||||
|---|---|---|---|---|---|---|---|---|
| Detection Frequency (%) | Range | Median | Mean | Detection Frequency (%) | Range | Median | Mean | |
| PFBA | 100 | 15.2–52.2 | 28.3 | 31.1 | 100 | 12.7–46.2 | 30.7 | 29.5 |
| PFPeA | 100 | 11.1–67.5 | 41.5 | 37.7 | 100 | 9.06–236 | 44.5 | 57.5 |
| PFHxA | 100 | 2.9–16.9 | 3.86 | 7.26 | 100 | 2.42–6.55 | 4.46 | 4.73 |
| PFHpA | 89 | ND–5.21 | 1.92 | 2.05 | 100 | 0.92–5.69 | 2.5 | 2.71 |
| PFOA | 100 | 8.68–25.4 | 16.9 | 16.9 | 100 | 17.1–33.3 | 20.82 | 21.6 |
| PFNA | 89 | ND–2.29 | 1.15 | 1.15 | 100 | 0.64–3 | 1.3 | 1.53 |
| PFDA | 100 | 0.64–2.29 | 1.43 | 1.50 | 100 | 0.66–3.11 | 1.77 | 1.76 |
| PFUnDA | 56 | ND– <LOQ | LOQ | LOQ | 100 | ND–7.07 | ND | 7.07 |
| PFDoDA | 56 | ND– <LOQ | LOQ | LOQ | 100 | ND–9.95 | ND | 9.95 |
| PFTriDA | 33 | ND– <LOQ | ND | LOQ | 71 | ND–18 | ND | 15 |
| PFTDA | 33 | ND– <LOQ | ND | LOQ | 79 | ND–2.85 | ND | 2.14 |
| PFBS | 100 | 1.66–710 | 5.9 | 83.2 | 100 | 4.39–27 | 7.92 | 10.2 |
| PFHXS | 100 | 0.77–1866 | 4.89 | 211 | 100 | 3.44–168 | 18.50 | 32 |
| PFOS | 100 | 1.38–49.4 | 2.79 | 9.42 | 100 | 2.37–6.81 | 4.33 | 4.29 |
| Compound | Lake Chaohu, China (This Study) | Lake Chaohu, China [33] | Lake Taihu, China [50] | Bohai Sea, China [52] | Rivers, Swedish [53] | Rivers, Japan [51] |
|---|---|---|---|---|---|---|
| PFBA | 12.7–46.2 | 0.31–6.77 | ND–4.06 | ND–2.9 | 0.47–3.7 | ND–18 |
| PFPA | 9.06–236 | 0.03–8.12 | ND–6.08 | ND–7.91 | ND–16 | |
| PFHxA | 2.42–6.55 | 0.19–15.9 | ND–22.2 | ND–17.4 | 0.51–4.2 | ND–16000 |
| PFHpA | 0.92–5.69 | 0.14–1.47 | 1.28–4.53 | ND–1.46 | 0.36–1.7 | ND–27 |
| PFOA | 17.1–33.3 | 1.32–23.5 | 2.15–73.9 | ND–83.4 | 0.21–4.2 | ND–360 |
| PFNA | 0.64–2.69 | 0.05–1.74 | 0.55–5.04 | ND–0.53 | 0.09–5.8 | ND–39 |
| PFDA | 0.66–3.11 | 0.02–0.7 | ND–2.93 | ND–0.93 | 0.02–4.4 | ND–47 |
| PFUnDA | ND–7.07 | ND–0.12 | ND–3.27 | ND–1.4 | 0.02–1.8 | ND–39 |
| PFDoDA | ND–9.95 | ND–0.89 | ND–0.46 | 0.02–0.82 | ND–4.1 | |
| PFTriDA | ND–18 | ND | ||||
| PFTDA | ND–2.85 | ND–0.21 | 0.09–1.5 | |||
| PFBS | 4.39–27 | 0.03–6.14 | ND–1.46 | 0.03–19 | ND–49 | |
| PFHxS | 3.44–168 | 0.01–0.96 | ND–6.92 | ND–0.28 | 0.05–18 | ND–8.4 |
| PFOS | 2.37–6.81 | ND–0.82 | ND–10.5 | ND–6.8 | 0.04–6.9 | ND–97 |
| Analytes | Detection Frequency (%) | Concentrations (ng/g DW) | BAF | logBAF | logBAF [11] | logBAF [54] | logBAF [55] | ||
|---|---|---|---|---|---|---|---|---|---|
| Min–Max | Mean | Min–Max | Mean | ||||||
| PFBA | 100 | 1.42–19.3 | 5.12 | 48.2–655 | 174 | 2.24 | 0.95–3.58 | ||
| PFPeA | 100 | 0.64–12.4 | 3.18 | 11.4–128 | 55.3 | 1.74 | 3.53–3.94 | ||
| PFHxA | 46 | 0.12–4.76 | 1.02 | 25.2–1007 | 469 | 2.67 | |||
| PFHpA | 54 | 0.12–4.88 | 1.03 | 52.3–1801 | 706 | 2.85 | |||
| PFOA | 100 | 0.26–4.17 | 1.34 | 12.1–193 | 61.9 | 1.79 | 0.99–1.94 | 2.91 | 1.32–2.08 |
| PFNA | 85 | 1.19–5.83 | 2.64 | 778–3813 | 2043 | 3.31 | 1.69–2.97 | 2.15–3.53 | |
| PFDA | 100 | 0.33–13.9 | 5.6 | 186–7867 | 3183 | 3.5 | 1.48–3.75 | 4.3 | 2.20–3.98 |
| PFUnDA | 100 | 1.09–10.1 | 4.83 | 155–1427 | 684 | 2.83 | 2.50–4.17 | 2.79–4.45 | |
| PFDoDA | 100 | 0.33–2.78 | 1.49 | 39.4–279 | 150 | 2.17 | 2.89–4.06 | 0.04–1.23 | |
| PFTriDA | 92 | 0.44–2.78 | 1.23 | 29.2–186 | 93.3 | 1.97 | |||
| PFTDA | 85 | 0.14–1.81 | 0.49 | 102–844 | 289 | 2.46 | |||
| PFBS | 46 | 0.60–4.05 | 1.11 | 58.5–398 | 235 | 2.37 | |||
| PFHXS | 46 | 0.14–1.79 | 0.41 | 4.33–55.7 | 27.9 | 1.45 | |||
| PFOS | 100 | 0.44–50.6 | 9.73 | 102–11,794 | 2267 | 3.35 | 2.23–3.77 | 3.51–5.02 | 2.26–3.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Ye, J.; Zhang, H.; Tang, J.; Pan, D. Occurrence, Removal and Bioaccumulation of Perfluoroalkyl Substances in Lake Chaohu, China. Int. J. Environ. Res. Public Health 2019, 16, 1692. https://doi.org/10.3390/ijerph16101692
Pan X, Ye J, Zhang H, Tang J, Pan D. Occurrence, Removal and Bioaccumulation of Perfluoroalkyl Substances in Lake Chaohu, China. International Journal of Environmental Research and Public Health. 2019; 16(10):1692. https://doi.org/10.3390/ijerph16101692
Chicago/Turabian StylePan, Xu, Jing Ye, Hui Zhang, Jun Tang, and Dandan Pan. 2019. "Occurrence, Removal and Bioaccumulation of Perfluoroalkyl Substances in Lake Chaohu, China" International Journal of Environmental Research and Public Health 16, no. 10: 1692. https://doi.org/10.3390/ijerph16101692
APA StylePan, X., Ye, J., Zhang, H., Tang, J., & Pan, D. (2019). Occurrence, Removal and Bioaccumulation of Perfluoroalkyl Substances in Lake Chaohu, China. International Journal of Environmental Research and Public Health, 16(10), 1692. https://doi.org/10.3390/ijerph16101692