The Relationship between Nkx2.1 and DNA Oxidative Damage Repair in Nickel Smelting Workers: Jinchang Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Nickel Smelter Group Sampling
2.3. Administrative Officer Group Sampling
2.4. Detection Method
2.5. Statistical Analysis
3. Results
3.1. Oxidative Damage (MDA) Differences between the Nickel Smelter Group and Administrative Officer Group
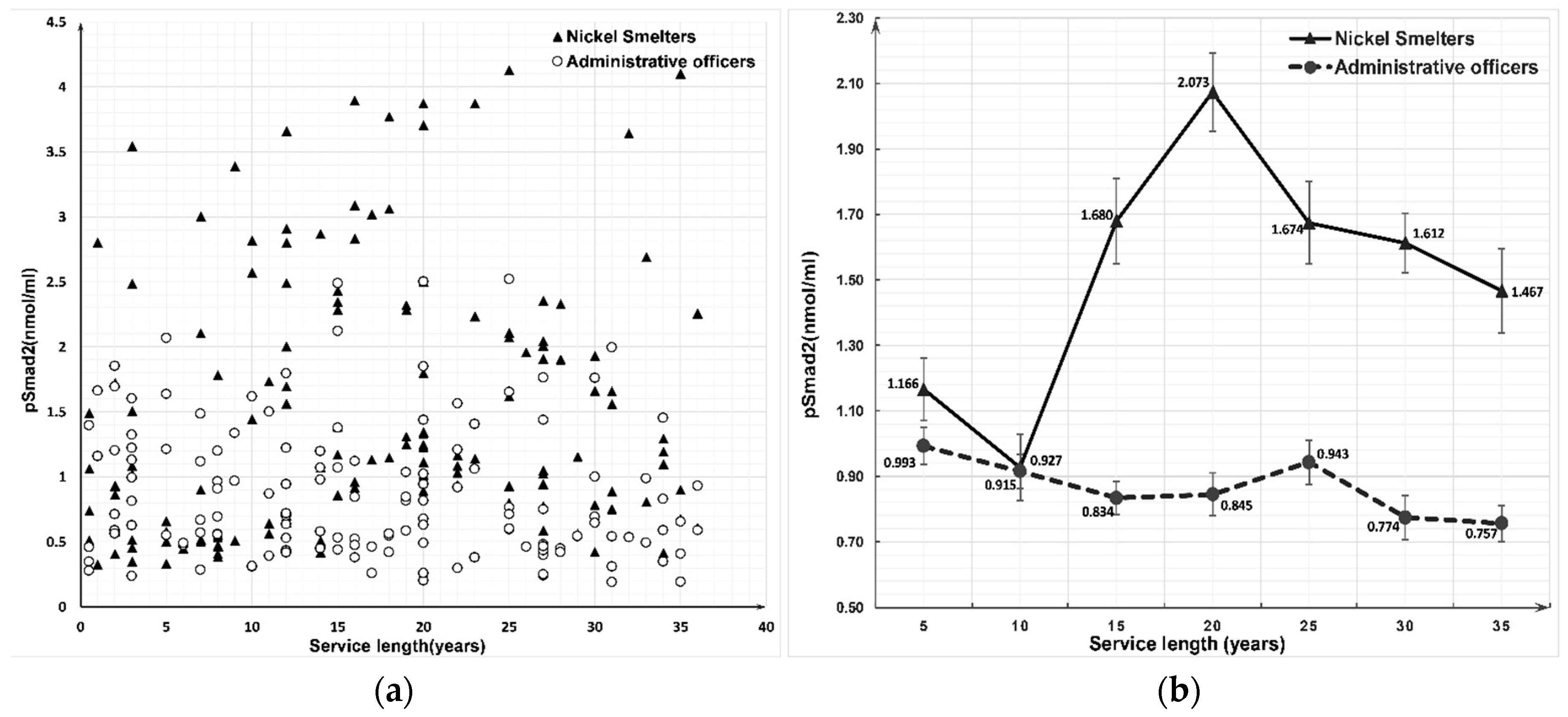
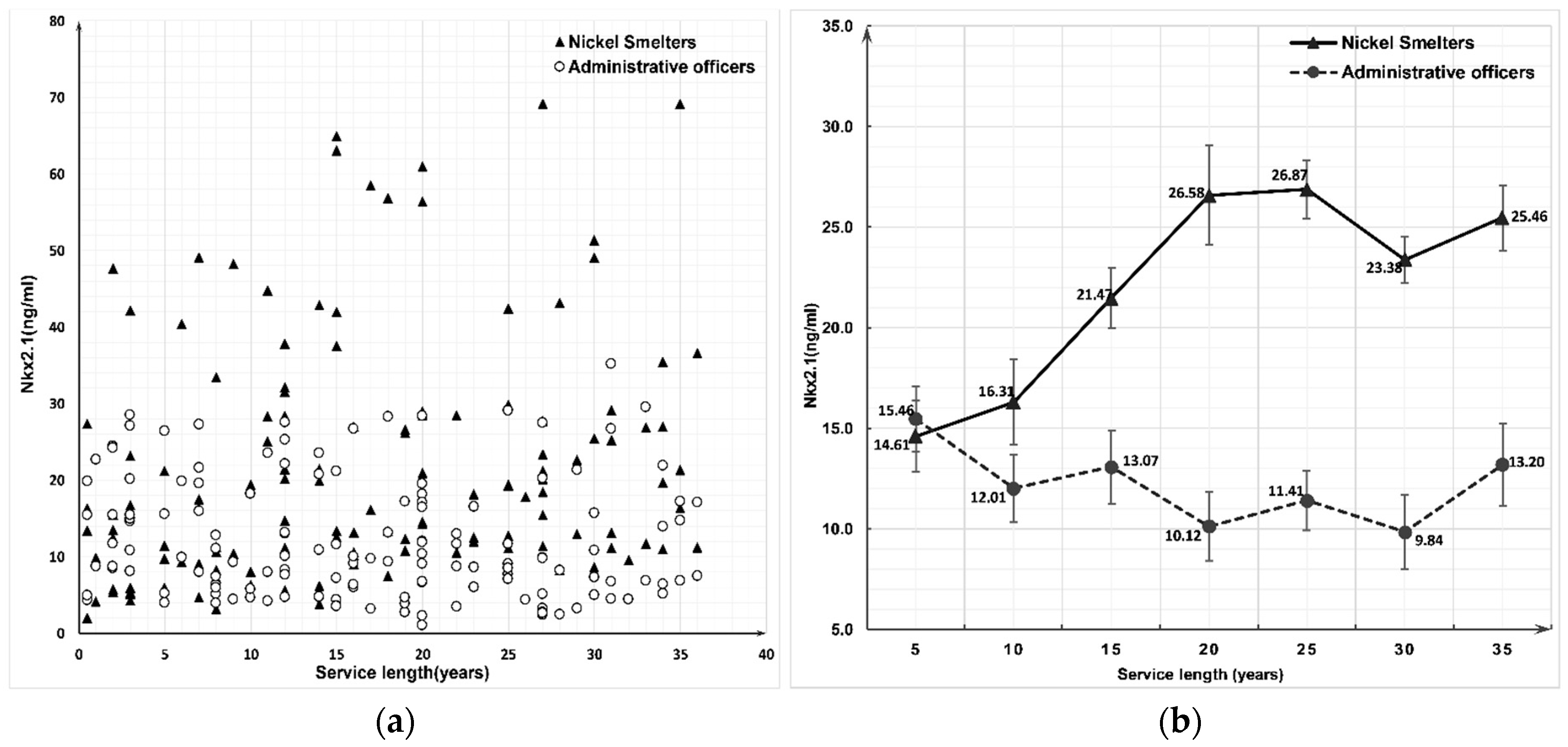
3.2. Expression of Nkx2.1 and pSmad2 Proteins between the Nickel Smelters and Administrative Officers Groups
3.3. Canonical Correlation Analysis of DNA Damage, Repair, and Embryonic Lung Development Biomarkers
4. Discussion
4.1. Occupational Nickel Exposure Could Enhances the LPO Processes and DNA Oxidative Damage
4.2. Nickel Exposure Could Decrease the Expression of hOGG1 but Amplifies the Expression of PARP
4.3. Nickel Exposure Could Significantly Increase the Expression of Nkx2.1 and pSmad2; Oxidative Stress and DNA Repair Capability Were Correlated with the Expression of Nkx2.1 and pSmad2
5. Conclusions
6. Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hostynek, J.J. Sensitization to nickel: Etiology, epidemiology, immune reactions, prevention, and therapy. Rev. Environ. Health 2006, 21, 253–280. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC monographs on the evaluation of the carcinogenic risk of chemicals to man: Cadmium, nickel, some epoxides, miscellaneous industrial chemicals and general consideration on volatile anaesthetics. IARC Monogr. Eval. Carcinog. Risk Chem. Man 1976, 11, 1–293. [Google Scholar]
- Lightfoot, N.; Berriault, C.; Semenciw, R. Mortality and cancer incidence in a nickel cohort. Occup. Med. 2010, 60, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Bai, Y.N.; Pu, H.Q.; He, J.; Bassig, B.A.; Dai, M.; Zhang, Y.W.; Zheng, T.Z.; Cheng, N. A retrospective cohort mortality study in Jinchang, the largest nickel production enterprise in China. Biomed. Environ. Sci. BES 2014, 27, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.N.; Yang, A.M.; Pu, H.Q.; He, J.; Cheng, N.; Zheng, T.Z.; Dai, M.; Zhang, Y.W.; Bassing, B.A.; Wang, Q.Y. Nickel-exposed workers in China: A cohort study. Biomed. Environ. Sci. BES 2014, 27, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, K.A.; Muir, B.; Reinhardt, F.; Carpenter, A.E.; Sgroi, D.C.; Weinberg, R.A. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc. Natl. Acad. Sci. USA 2006, 103, 18969–18974. [Google Scholar] [CrossRef]
- Winslow, M.M.; Dayton, T.L.; Verhaak, R.G.; Kim-Kiselak, C.; Snyder, E.L.; Feldser, D.M.; Hubbard, D.D.; DuPage, M.J.; Whittaker, C.A.; Hoersch, S.; et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature 2011, 473, 101–104. [Google Scholar] [CrossRef]
- Garnis, C.; Lockwood, W.W.; Vucic, E.; Ge, Y.; Girard, L.; Minna, J.D.; Gazdar, A.F.; Lam, S.; MacAulay, C.; Lam, W.L. High resolution analysis of non-small cell lung cancer cell lines by whole genome tiling path array CGH. Int. J. Cancer 2006, 118, 1556–1564. [Google Scholar] [CrossRef]
- Kendall, J.; Liu, Q.; Bakleh, A.; Krasnitz, A.; Nguyen, K.C.; Lakshmi, B.; Gerald, W.L.; Powers, S.; Mu, D. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 16663–16668. [Google Scholar] [CrossRef]
- Inoue, Y.; Matsuura, S.; Kurabe, N.; Kahyo, T.; Mori, H.; Kawase, A.; Karayama, M.; Inui, N.; Funai, K.; Shinmura, K.; et al. Clinicopathological and Survival Analysis of Japanese Patients with Resected Non-Small-Cell Lung Cancer Harboring NKX2-1, SETDB1, MET, HER2, SOX2, FGFR1, or PIK3CA Gene Amplification. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 1590–1600. [Google Scholar] [CrossRef]
- Clarke, N.; Biscocho, J.; Kwei, K.A.; Davidson, J.M.; Sridhar, S.; Gong, X.; Pollack, J.R. Integrative Genomics Implicates EGFR as a Downstream Mediator in NKX2-1 Amplified Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0142061. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Yanagisawa, K.; Sugiyama, R.; Hosono, Y.; Shimada, Y.; Arima, C.; Kato, S.; Tomida, S.; Suzuki, M.; Osada, H.; et al. NKX2-1/TITF1/TTF-1-Induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell 2012, 21, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Boggaram, V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin. Sci. 2009, 116, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Yang, G.Y.; Yang, J.H.; Li, J. Analysis of clinical characteristics and differential diagnosis of the lung biopsy specimens in 99 adenocarcinoma cases and 111 squamous cell carcinoma cases: Utility of an immunohistochemical panel containing CK5/6, CK34betaE12, p63, CK7 and TTF-1. Pathol. Res. Pract. 2014, 210, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kwei, K.A.; Kim, Y.H.; Girard, L.; Kao, J.; Pacyna-Gengelbach, M.; Salari, K.; Lee, J.; Choi, Y.L.; Sato, M.; Wang, P.; et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene 2008, 27, 3635–3640. [Google Scholar] [CrossRef]
- Tanaka, H.; Yanagisawa, K.; Shinjo, K.; Taguchi, A.; Maeno, K.; Tomida, S.; Shimada, Y.; Osada, H.; Kosaka, T.; Matsubara, H.; et al. Lineage-specific dependency of lung adenocarcinomas on the lung development regulator TTF-1. Cancer Res. 2007, 67, 6007–6011. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hosono, Y.; Yanagisawa, K.; Takahashi, T. NKX2-1/TTF-1: An Enigmatic Oncogene that Functions as a Double-Edged Sword for Cancer Cell Survival and Progression. Cancer Cell 2013, 23, 718–723. [Google Scholar] [CrossRef]
- Bai, X.; Jing, L.; Li, Y.; Li, Y.; Luo, S.; Wang, S.; Zhou, J.; Liu, Z.; Diao, A. TMEPAI inhibits TGF-beta signaling by promoting lysosome degradation of TGF-beta receptor and contributes to lung cancer development. Cell Signal. 2014, 26, 2030–2039. [Google Scholar] [CrossRef]
- Du, S.; Bouquet, S.; Lo, C.H.; Pellicciotta, I.; Bolourchi, S.; Parry, R.; Barcellos-Hoff, M.H. Attenuation of the DNA damage response by transforming growth factor-beta inhibitors enhances radiation sensitivity of non-small-cell lung cancer cells in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 91–99. [Google Scholar] [CrossRef]
- Jakubowska, K.; Naumnik, W.; Niklinska, W.; Chyczewska, E. Clinical Significance of HMGB-1 and TGF-beta Level in Serum and BALF of Advanced Non-Small Cell Lung Cancer. Adv. Exp. Med. Biol. 2015, 852, 49–58. [Google Scholar] [CrossRef]
- Park, C.Y.; Min, K.N.; Son, J.Y.; Park, S.Y.; Nam, J.S.; Kim, D.K.; Sheen, Y.Y. An novel inhibitor of TGF-beta type I receptor, IN-1130, blocks breast cancer lung metastasis through inhibition of epithelial-mesenchymal transition. Cancer Lett. 2014, 351, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Wang, J.; An, Y.; Gong, Q.; Gregg, E.W.; Yang, W.; Zhang, B.; Shuai, Y.; Hong, J.; et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study. Lancet Diabetes Endocrinol. 2014, 2, 474–480. [Google Scholar] [CrossRef]
- Witkowska, M.; Smolewski, P. SMAD family proteins: The current knowledge on their expression and potential role in neoplastic diseases. Postepy Hig. I Med. Dosw. (Online) 2014, 68, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bai, Y.N.; Pu, H.Q.; He, J.; Zheng, T.Z.; Li, H.Y.; Dai, M.; Cheng, N. Dynamic Changes in DNA Damage and Repair Biomarkers with Employment Length among Nickel Smelting Workers. Biomed. Environ. Sci. 2015, 28, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Yang, A.; Pu, H.; Dai, M.; Cheng, N.; Ding, J.; Li, J.; Li, H.; Hu, X.; Ren, X.; et al. Cohort Profile: The China Metal-Exposed Workers Cohort Study (Jinchang Cohort). Int. J. Epidemiol. 2016. [Google Scholar] [CrossRef]
- Yang, A.; Liu, S.; Cheng, N.; Pu, H.; Dai, M.; Ding, J.; Li, J.; Li, H.; Hu, X.; Ren, X.; et al. Multiple metals exposure, elevated blood glucose and dysglycemia among Chinese occupational workers. J. Diabetes Complicat. 2017, 31, 101–107. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Lin, T.H. Nickel toxicity to human term placenta: In vitro study on lipid peroxidation. J. Toxicol. Environ. Healt. Part A 1998, 54, 37–47. [Google Scholar] [CrossRef]
- Lin, M.H.; Yen, J.H.; Weng, C.Y.; Wang, L.; Ha, C.L.; Wu, M.J. Lipid peroxidation end product 4-hydroxy-trans-2-nonenal triggers unfolded protein response and heme oxygenase-1 expression in PC12 cells: Roles of ROS and MAPK pathways. Toxicology 2014, 315, 24–37. [Google Scholar] [CrossRef]
- Borella, P.; Manni, S.; Giardino, A. Cadmium, nickel, chromium and lead accumulate in human lymphocytes and interfere with PHA-induced proliferation. J. Trace Elem. Electrolytes Health Dis. 1990, 4, 87–95. [Google Scholar] [PubMed]
- Pilger, A.; Rudiger, H.W. 8-Hydroxy-2’-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Gupta, A.D.; Dhundasi, S.A.; Patil, A.M.; Das, S.N.; Ambekar, J.G. Protective role of L-ascorbic acid on antioxidant defense system in erythrocytes of albino rats exposed to nickel sulfate. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2007, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, P.; Saranya, S.; Poornima, P.; Prabhakaran, R.; Dallemer, F.; Vijaya Padma, V.; Natarajan, K. Biological evaluation of new nickel(II) metallates: Synthesis, DNA/protein binding and mitochondrial mediated apoptosis in human lung cancer cells (A549) via ROS hypergeneration and depletion of cellular antioxidant pool. Eur. J. Med. Chem. 2014, 82, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Ali, D.; Alhadlaq, H.A.; Akhtar, M.J. Nickel oxide nanoparticles exert cytotoxicity via oxidative stress and induce apoptotic response in human liver cells (HepG2). Chemosphere 2013, 93, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Oikawa, S.; Inoue, S.; Nishino, K. Distinct mechanisms of oxidative DNA damage induced by carcinogenic nickel subsulfide and nickel oxides. Environ. Health Perspect. 2002, 110 (Suppl. 5), 789–791. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Klungland, A.; Bjelland, S. Oxidative damage to purines in DNA: Role of mammalian Ogg1. DNA Repair 2007, 6, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Liu, W.; Cooper, K.L.; Qin, X.J.; de Souza Bergo, P.L.; Hudson, L.G.; Liu, K.J. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J. Biol. Chem. 2009, 284, 6809–6817. [Google Scholar] [CrossRef]
- Vogel, U.; Moller, P.; Dragsted, L.; Loft, S.; Pedersen, A.; Sandstrom, B. Inter-individual variation, seasonal variation and close correlation of OGG1 and ERCC1 mRNA levels in full blood from healthy volunteers. Carcinogenesis 2002, 23, 1505–1509. [Google Scholar] [CrossRef]
- Nishioka, K.; Ohtsubo, T.; Oda, H.; Fujiwara, T.; Kang, D.; Sugimachi, K.; Nakabeppu, Y. Expression and differential intracellular localization of two major forms of human 8-oxoguanine DNA glycosylase encoded by alternatively spliced OGG1 mRNAs. Mol. Biol. Cell 1999, 10, 1637–1652. [Google Scholar] [CrossRef] [PubMed]
- Stedeford, T.; Cardozo-Pelaez, F.; Hover, C.; Harbison, R.D.; Sanchez-Ramos, J. Organ-specific differences in 8-oxoguanosine glycosylase (OGG1) repair following acute treatment with benzo[a]pyrene. Res. Commun. Mol. Pathol. Pharm. 2001, 109, 73–85. [Google Scholar]
- Sakumi, K.; Tominaga, Y.; Furuichi, M.; Xu, P.; Tsuzuki, T.; Sekiguchi, M.; Nakabeppu, Y. Ogg1 knockout-associated lung tumorigenesis and its suppression by Mth1 gene disruption. Cancer Res. 2003, 63, 902–905. [Google Scholar] [PubMed]
- Noren Hooten, N.; Kompaniez, K.; Barnes, J.; Lohani, A.; Evans, M.K. Poly(ADP-ribose) polymerase 1 (PARP-1) binds to 8-oxoguanine-DNA glycosylase (OGG1). J. Biol. Chem. 2011, 286, 44679–44690. [Google Scholar] [CrossRef]
- Boiteux, S.; Radicella, J.P. The human OGG1 gene: Structure, functions, and its implication in the process of carcinogenesis. Arch. Biochem. Biophys. 2000, 377, 1–8. [Google Scholar] [CrossRef]
- Wilson, D.M., 3rd; Kim, D.; Berquist, B.R.; Sigurdson, A.J. Variation in base excision repair capacity. Mutat. Res. 2011, 711, 100–112. [Google Scholar] [CrossRef]
- Hill, J.W.; Evans, M.K. A novel R229Q OGG1 polymorphism results in a thermolabile enzyme that sensitizes KG-1 leukemia cells to DNA damaging agents. Cancer Detect. Prev. 2007, 31, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.; Lynch, C.; Ruff, V.; Reynolds, M. Co-exposure to nickel and cobalt chloride enhances cytotoxicity and oxidative stress in human lung epithelial cells. Toxicol. Appl. Pharmacol. 2012, 258, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.; Mateo, J.; Olmos, D.; de Bono, J.S. Targeting DNA Repair: The Role of PARP Inhibition in the Treatment of Castration-Resistant Prostate Cancer. Cancer J. 2016, 22, 353–356. [Google Scholar] [CrossRef]
- Somlo, G.; Frankel, P.H.; Arun, B.K.; Ma, C.X.; Garcia, A.A.; Cigler, T.; Cream, L.V.; Harvey, H.A.; Sparano, J.A.; Nanda, R.; et al. Efficacy of the PARP Inhibitor Veliparib with Carboplatin or as a Single Agent in Patients with Germline BRCA1- or BRCA2-Associated Metastatic Breast Cancer: California Cancer Consortium Trial NCT01149083. Clin. Cancer Res. 2017, 23, 4066–4076. [Google Scholar] [CrossRef]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Zander, S.A.; Derksen, P.W.; de Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef]
- Wang, M.; Saha, J.; Cucinotta, F.A. Smad7 foci are present in micronuclei induced by heavy particle radiation. Mutat. Res. 2013, 756, 108–114. [Google Scholar] [CrossRef]
- Radha, K.S.; Sugiki, M.; Harish Kumar, M.; Omura, S.; Maruyama, M. Post-transcriptional regulation of plasminogen activator inhibitor-1 by intracellular iron in cultured human lung fibroblasts--interaction of an 81-kDa nuclear protein with the 3’-UTR. J. Thromb. Haemost. 2005, 3, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.M.; Ali, S.; Philip, P.A.; Wozniak, A.; Sarkar, F.H. Genistein enhances the effect of epidermal growth factor receptor tyrosine kinase inhibitors and inhibits nuclear factor kappa B in nonsmall cell lung cancer cell lines. Cancer 2009, 115, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ren, T.; Xiao, C.; Li, H.; Wu, T. Nickel promotes the invasive potential of human lung cancer cells via TLR4/MyD88 signaling. Toxicology 2011, 285, 25–30. [Google Scholar] [CrossRef]
- Wesselkamper, S.C.; Case, L.M.; Henning, L.N.; Borchers, M.T.; Tichelaar, J.W.; Mason, J.M.; Dragin, N.; Medvedovic, M.; Sartor, M.A.; Tomlinson, C.R.; et al. Gene expression changes during the development of acute lung injury: Role of transforming growth factor beta. Am. J. Respir. Crit. Care Med. 2005, 172, 1399–1411. [Google Scholar] [CrossRef]
- Massague, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Bohinski, R.J.; Di Lauro, R.; Whitsett, J.A. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol. Cell. Biol. 1994, 14, 5671–5681. [Google Scholar] [CrossRef]
- Yatabe, Y.; Mitsudomi, T.; Takahashi, T. TTF-1 expression in pulmonary adenocarcinomas. Am. J. Surg. Pathol. 2002, 26, 767–773. [Google Scholar] [CrossRef]
- Berghmans, T.; Mascaux, C.; Haller, A.; Meert, A.P.; Van Houtte, P.; Sculier, J.P. EGFR, TTF-1 and Mdm2 expression in stage III non-small cell lung cancer: A positive association. Lung Cancer (Amst. Neth.) 2008, 62, 35–44. [Google Scholar] [CrossRef]
- Ji, H.; Ramsey, M.R.; Hayes, D.N.; Fan, C.; McNamara, K.; Kozlowski, P.; Torrice, C.; Wu, M.C.; Shimamura, T.; Perera, S.A.; et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007, 448, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Takahashi, M.; Ariki, S.; Asakawa, D.; Tajiri, M.; Wada, Y.; Yamaguchi, Y.; Nishitani, C.; Takamiya, R.; Saito, A.; et al. Surfactant protein D suppresses lung cancer progression by downregulation of epidermal growth factor signaling. Oncogene 2015, 34, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Riis, B.; Risom, L.; Loft, S.; Poulsen, H.E. OGG1 mRNA expression and incision activity in rats are higher in foetal tissue than in adult liver tissue while 8-oxo-2’-deoxyguanosine levels are unchanged. DNA Repair 2002, 1, 709–717. [Google Scholar] [CrossRef]
| Gender | Nickel Exposure Level (μg/L Creatinine) | n | Mean (95% CI) | St.d * | Selected Percentiles | Kruskal-Wallis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | H | p | |||||
| Male | Low (Administrative stuff) | 25 | 4.61 (3.27–5.94) | 0.65 | 1.62 | 2.33 | 3.43 | 5.87 | 12.96 | 8.767 | 0.003 |
| Medium (Technician) | 125 | 6.88 (5.28–8.48) | 0.85 | 1.79 | 3.15 | 5.10 | 7.28 | 16.39 | |||
| High (Smelter & Miner) | 101 | 8.75 (7.44–10.05) | 0.66 | 2.08 | 4.43 | 6.56 | 12.23 | 21.32 | |||
| Sum | 251 | 7.40 (6.44–8.37) | 0.51 | 1.79 | 3.24 | 5.27 | 9.12 | 19.13 | |||
| Female | Low (Administrative stuff) | 25 | 6.12 (3.60–8.65) | 1.22 | 2.10 | 3.14 | 4.31 | 6.04 | 24.16 | 0.688 | 0.407 |
| Medium (Technician) | 125 | 7.88 (6.25–9.52) | 2.24 | 2.09 | 3.72 | 6.12 | 8.88 | 20.02 | |||
| High (Smelter & Miner) | 99 | 12.65 (8.99–16.32) | 2.04 | 1.93 | 3.63 | 5.91 | 12.97 | 43.66 | |||
| Sum | 249 | 9.61 (7.90–11.31) | 1.40 | 2.05 | 3.59 | 5.78 | 9.28 | 27.52 | |||
| Characteristic | Categories | Nickel Smelters | Administrative Officers | ||
|---|---|---|---|---|---|
| n = 140 | % | n = 140 | % | ||
| Gender | Male | 140 | 100 | 140 | 100 |
| Female | 0 | 0 | 0 | 0 | |
| Age | 20–24 | 2 | 1.4 | 2 | 1.4 |
| 25–29 | 29 | 20.7 | 29 | 20.7 | |
| 30–34 | 26 | 18.6 | 23 | 16.4 | |
| 35–39 | 23 | 16.4 | 26 | 18.6 | |
| 40–44 | 20 | 14.3 | 20 | 14.3 | |
| 45–49 | 20 | 14.3 | 20 | 14.3 | |
| 50–55 | 20 | 14.3 | 20 | 14.3 | |
| Nationality | Han nationality | 138 | 98.6 | 140 | 100 |
| Man nationality | 1 | 0.7 | 0 | 0 | |
| Other | 1 | 0.7 | 0 | 0 | |
| Occupation | Cadre | 0 | 0 | 63 | 45 |
| Worker | 140 | 100 | 3 | 2.1 | |
| Technician | 0 | 0 | 7 | 5 | |
| Service | 0 | 0 | 67 | 47.9 | |
| Marital status | Not married | 17 | 12.1 | 21 | 15 |
| Married | 118 | 84.3 | 118 | 84.3 | |
| Other | 5 | 3.6 | 1 | 0.7 | |
| Residence years | <20 | 17 | 12.1 | 32 | 22.9 |
| 20–39 | 100 | 71.4 | 79 | 56.4 | |
| >40 | 23 | 16.4 | 29 | 20.7 | |
| Education status | Primary and below | 4 | 2.9 | 1 | 0.7 |
| Junior high school | 34 | 24.3 | 6 | 4.3 | |
| Senior high school | 52 | 37.1 | 22 | 15.7 | |
| Undergraduate and above | 50 | 35.8 | 111 | 79.3 | |
| Family income (RMB/month/person) | <1000 | 10 | 7.1 | 10 | 7.1 |
| 1000–2000 | 80 | 57.1 | 34 | 24.3 | |
| ≥2000 | 50 | 35.7 | 96 | 68.6 | |
| Cancer family history | Non | 106 | 75.7 | 101 | 72.1 |
| Case | 32 | 22.9 | 38 | 27.1 | |
| Unknown | 2 | 1.4 | 1 | 0.7 | |
| Behavioral Factors | Categories | Nickel Smelters | Administrative Officers | Chi-sq * | p | ||
|---|---|---|---|---|---|---|---|
| n = 140 | % | n = 140 | % | ||||
| Smoking | Non | 43 | 30.71 | 46 | 32.86 | 0.37 | 0.83 |
| Past | 18 | 12.86 | 15 | 10.71 | |||
| Current | 79 | 56.43 | 79 | 56.43 | |||
| Smoking index | 0–400 | 108 | 77.10 | 120 | 85.70 | 3.4 | 0.07 |
| >400 | 32 | 22.90 | 20 | 14.30 | |||
| Alcohol drinking | Non | 84 | 60.00 | 76 | 53.60 | 1.80 | 0.41 |
| Past | 9 | 6.40 | 7 | 5.00 | |||
| Current | 47 | 33.60 | 58 | 41.40 | |||
| Drinking index | 0 | 85 | 60.71 | 77 | 55.00 | 1.49 | 0.68 |
| <5200 | 32 | 22.86 | 34 | 24.29 | |||
| 5200–10,400 | 10 | 7.14 | 15 | 10.71 | |||
| >10400 | 13 | 9.29 | 14 | 10.00 | |||
| Tea consumption | Non | 42 | 30.00 | 44 | 31.40 | 0.2 | 0.91 |
| Past | 4 | 2.90 | 3 | 2.10 | |||
| Current | 94 | 67.10 | 93 | 66.40 | |||
| Tea index | 0 | 42 | 30.00 | 45 | 32.10 | 1.19 | 0.76 |
| <600 | 55 | 39.30 | 60 | 42.90 | |||
| 600–1200 | 21 | 15.00 | 18 | 12.90 | |||
| >1200 | 22 | 15.70 | 17 | 12.10 | |||
| Service Length (Years) | MDA (nmol/mL) | PARP (ng/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Nickel Smelters | Administrative Officers | Nickel Smelters | Administrative Officers | |||||
| n | x ± st.d * | n | x ± st.d | n | x ± st.d * | n | x ± st.d | |
| ≤4 | 20 | 11.3668 ± 5.0144 | 20 | 6.1012 ± 3.8763 | 20 | 0.0405 ± 0.0017 | 20 | 0.0402 ± 0.0010 |
| 5–9 | 20 | 13.7679 ± 6.1174 | 20 | 7.5872 ± 4.2609 | 20 | 0.0406 ± 0.0015 | 20 | 0.0398 ± 0.0008 |
| 10–14 | 20 | 14.8466 ± 3.6641 | 20 | 9.6055 ± 5.9680 | 20 | 0.0414 ± 0.0028 | 20 | 0.0402 ± 0.0013 |
| 15–19 | 20 | 16.0179 ± 4.6807 | 20 | 10.8349 ± 4.8698 | 20 | 0.0405 ± 0.0012 | 20 | 0.0401 ± 0.0012 |
| 20–24 | 20 | 17.6983 ± 2.9618 | 20 | 13.7706 ± 5.7772 | 20 | 0.0405 ± 0.0021 | 20 | 0.0394 ± 0.0002 |
| 25–29 | 20 | 17.1786 ± 3.0584 | 20 | 14.5487 ± 4.7357 | 20 | 0.0399 ± 0.0004 | 20 | 0.0397 ± 0.0009 |
| ≥30 | 20 | 21.5776 ± 29.7619 | 20 | 15.0885 ± 8.9044 | 20 | 0.0397 ± 0.0006 | 20 | 0.0396 ± 0.0006 |
| Total | 140 | 16.0648 ± 12.0839 | 140 | 11.0766 ± 6.4684 | 140 | 0.0405 ± 0.0017 | 140 | 0.0398 ± 0.0009 |
| Z (Wilcoxon) | 6.125 | 4.853 | ||||||
| p | <0.001 | <0.001 | ||||||
| F (ANOVA) | 1.47 | 7.644 | 2.217 | 2.255 | ||||
| p | 0.193 | 0 | 0.045 | 0.042 | ||||
| rs (Spearman) | 0.273 | 0.569 | −0.175 | −0.376 | ||||
| p | <0.001 | <0.001 | 0.039 | <0.001 | ||||
| Service Length (Years) | Nkx2.1 (ng/mL) | pSmad2 (nmol/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Nickel Smelters | Administrative Officers | Nickel Smelters | Administrative Officers | |||||
| n | x ± st.d * | n | x ± st.d | n | x ± st.d | n | x ± st.d | |
| ≤4 | 20 | 14.6062 ± 12.3940 | 20 | 15.4643 ± 7.3406 | 20 | 1.1658 ± 0.8780 | 20 | 0.9929 ± 0.5046 |
| 5–9 | 20 | 16.3074 ± 14.4587 | 20 | 12.0121 ± 7.5392 | 20 | 0.9272 ± 0.9011 | 20 | 0.9149 ± 0.4639 |
| 10–14 | 20 | 21.4685 ± 12.4842 | 20 | 13.0714 ± 8.1059 | 20 | 1.6796 ± 1.0290 | 20 | 0.8335 ± 0.4476 |
| 15–19 | 20 | 26.5803 ± 19.9658 | 20 | 10.1228 ± 7.6858 | 20 | 2.0731 ± 0.9835 | 20 | 0.8448 ± 0.5806 |
| 20–24 | 20 | 26.8712 ± 15.4844 | 20 | 11.4143 ± 6.6340 | 20 | 1.6744 ± 1.0137 | 20 | 0.9429 ± 0.5985 |
| 25–29 | 20 | 23.3837 ± 14.1935 | 20 | 9.8430 ± 8.2613 | 20 | 1.6122 ± 0.8516 | 20 | 0.7739 ± 0.5957 |
| ≥30 | 20 | 25.4599 ± 16.2387 | 20 | 13.198 ± 9.1051 | 20 | 1.4666 ± 1.0241 | 20 | 0.7567 ± 0.4891 |
| Total | 140 | 22.0967 ± 15.5792 | 140 | 12.1608 ± 7.8845 | 140 | 1.5141 ± 0.9988 | 140 | 0.8657 ± 0.5240 |
| Z (Wilcoxon) | −6.123 | −5.845 | ||||||
| p | <0.001 | <0.001 | ||||||
| F (ANOVA) | 2.099 | 1.242 | 3.069 | 0.552 | ||||
| p | 0.057 | 0.289 | 0.008 | 0.768 | ||||
| rs (Spearman) | 0.312 | −0.146 | 0.232 | −0.169 | ||||
| p | <0.001 | 0.085 | 0.006 | 0.046 | ||||
| Group V | Canonical Coefficient | |
| χ1 | χ2 | |
| Service length | 0.2784 | 0.875 |
| MDA | 0.0369 | 0.1734 |
| 8-OHdG | 0.764 | −0.6255 |
| PARP | 0.5858 | −0.2695 |
| hOGG1 | −0.4531 | −0.0561 |
| Proportion explained by own var % | 28.39 | 25.26 |
| Group W | η1 | η2 |
| Nkx2.1 | 0.9886 | 0.1507 |
| pSmad2 | −0.0083 | 1 |
| Proportion explained by own var % | 48.87 | 51.13 |
| R2 | 0.518 | 0.033 |
| R | 0.72 | 0.18 |
| Adjusted R | 0.709 | 0.124 |
| p | <0.0001 | 0.347 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Cheng, N.; Shi, D.; Ren, X.; Gan, T.; Bai, Y.; Yang, K. The Relationship between Nkx2.1 and DNA Oxidative Damage Repair in Nickel Smelting Workers: Jinchang Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 120. https://doi.org/10.3390/ijerph16010120
Cheng Z, Cheng N, Shi D, Ren X, Gan T, Bai Y, Yang K. The Relationship between Nkx2.1 and DNA Oxidative Damage Repair in Nickel Smelting Workers: Jinchang Cohort Study. International Journal of Environmental Research and Public Health. 2019; 16(1):120. https://doi.org/10.3390/ijerph16010120
Chicago/Turabian StyleCheng, Zhiyuan, Ning Cheng, Dian Shi, Xiaoyu Ren, Ting Gan, Yana Bai, and Kehu Yang. 2019. "The Relationship between Nkx2.1 and DNA Oxidative Damage Repair in Nickel Smelting Workers: Jinchang Cohort Study" International Journal of Environmental Research and Public Health 16, no. 1: 120. https://doi.org/10.3390/ijerph16010120
APA StyleCheng, Z., Cheng, N., Shi, D., Ren, X., Gan, T., Bai, Y., & Yang, K. (2019). The Relationship between Nkx2.1 and DNA Oxidative Damage Repair in Nickel Smelting Workers: Jinchang Cohort Study. International Journal of Environmental Research and Public Health, 16(1), 120. https://doi.org/10.3390/ijerph16010120





