About One in Five Novice Vapers Buying Their First E-Cigarette in a Vape Shop Are Smoking Abstinent after Six Months
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Questionnaires
2.2.2. Biochemical Verification of Smoking Status
2.3. Procedure
2.4. Statistical Analyses
3. Results
3.1. Smoking Profile at Intake
3.2. E-Cigarette Profile at Intake
3.3. Predictors Smoking Status at FU
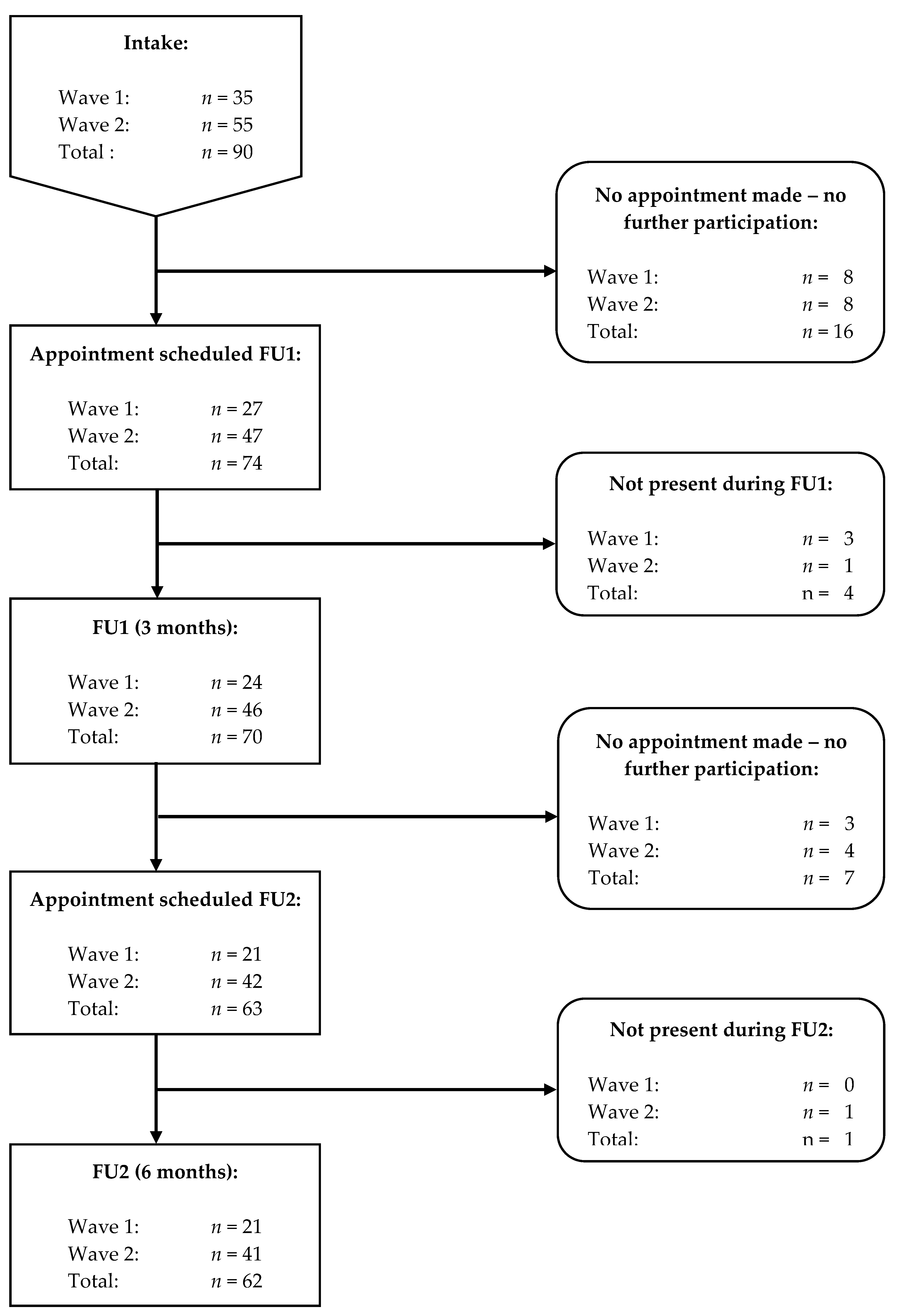
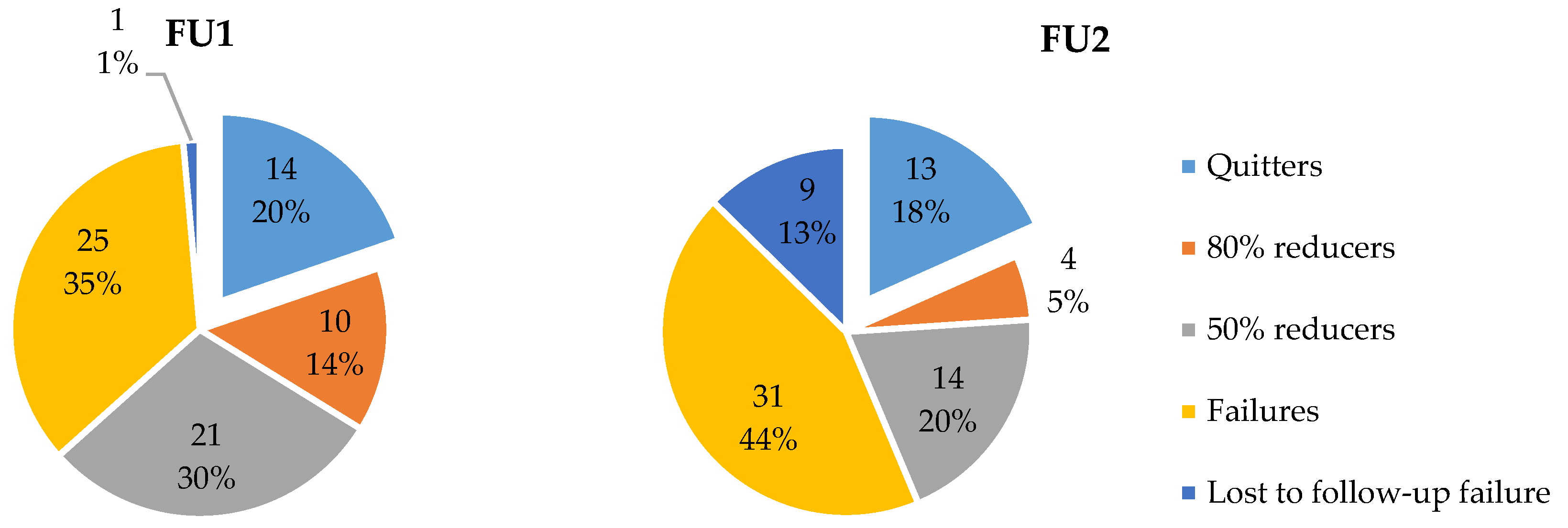
3.4. Smoking Status and Profile at FU
3.4.1. Smoking Status at FU
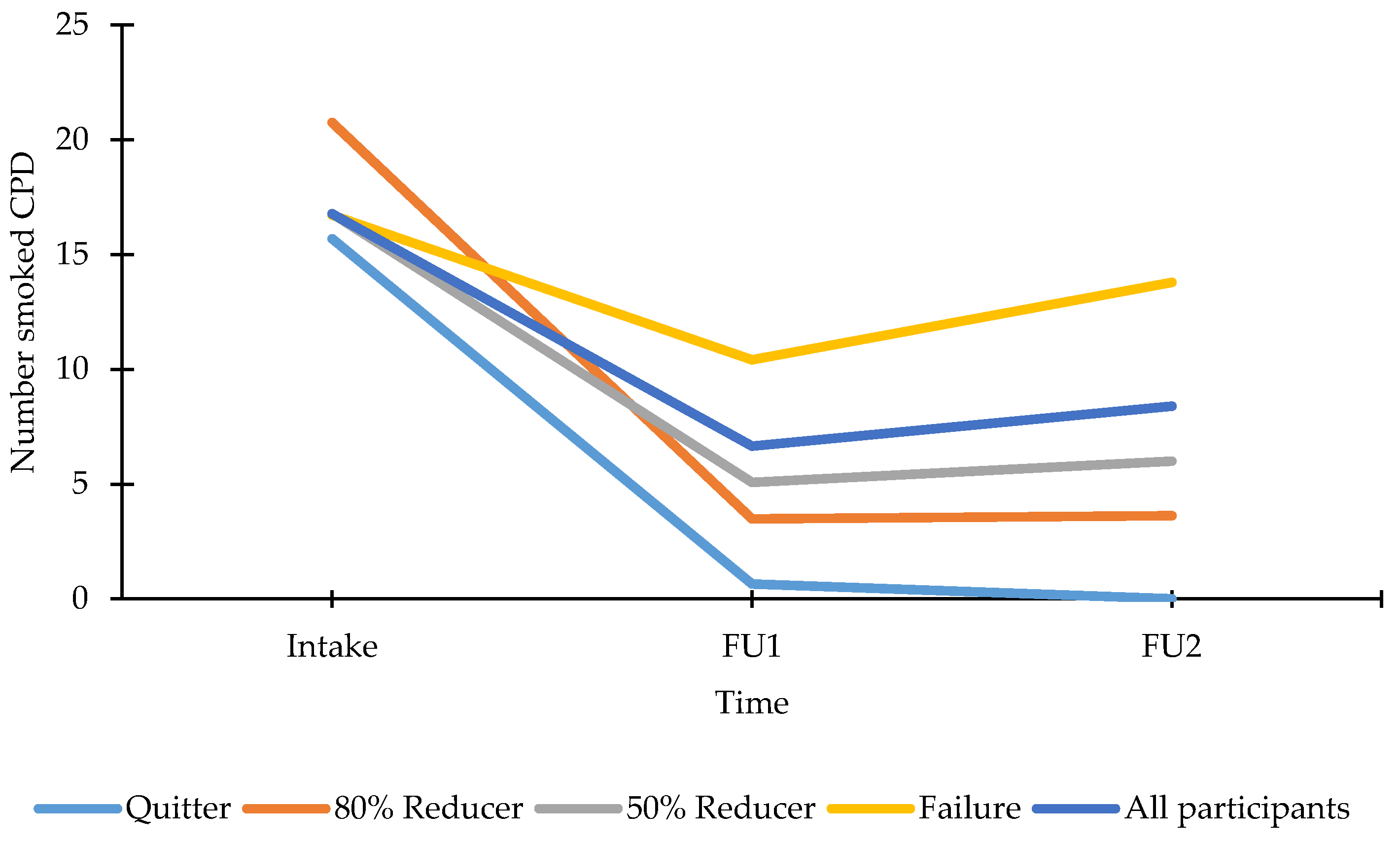
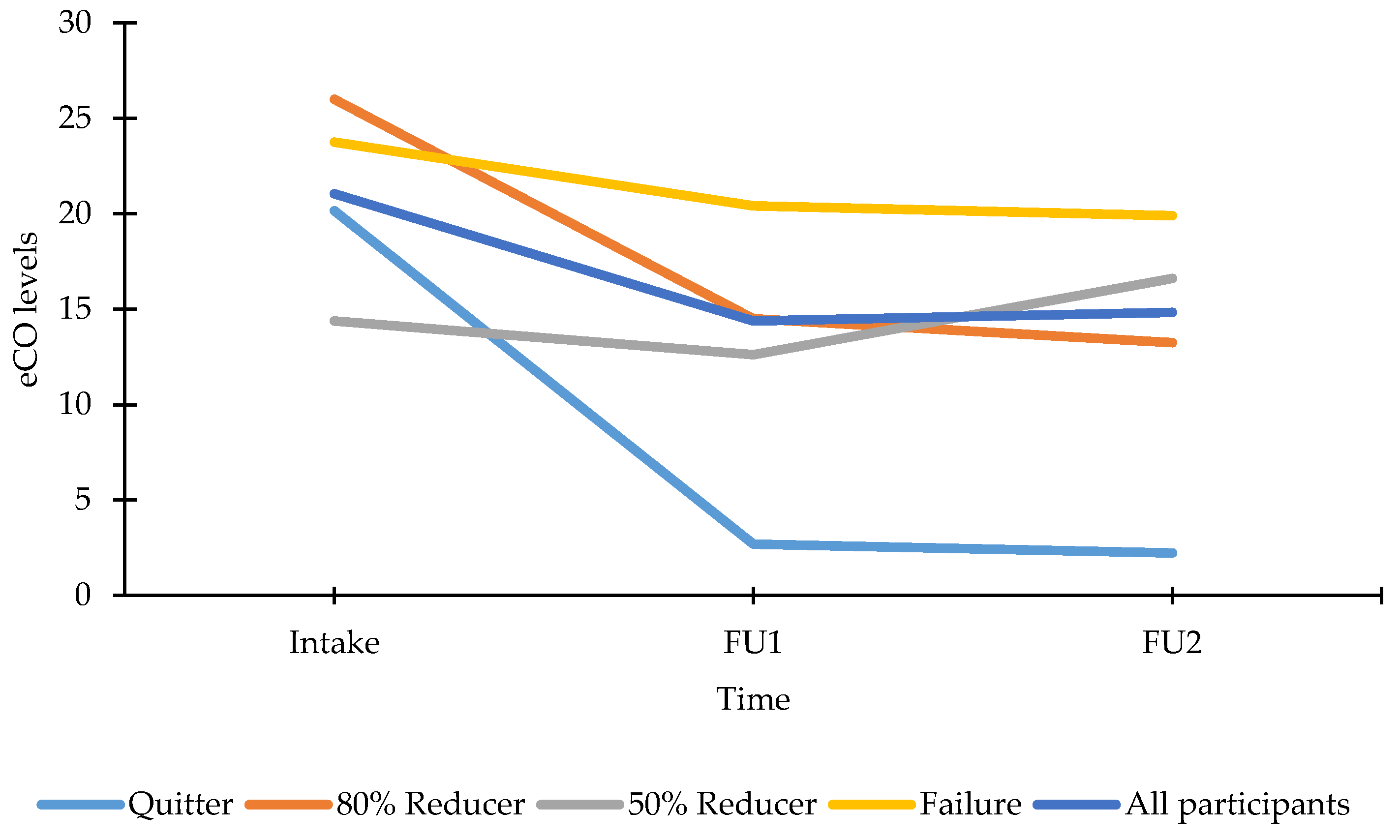
3.4.2. Smoking Profile at FU
3.5. Vaping Status and E-Cigarette Profile at FU
3.5.1. Vaping Status at FU
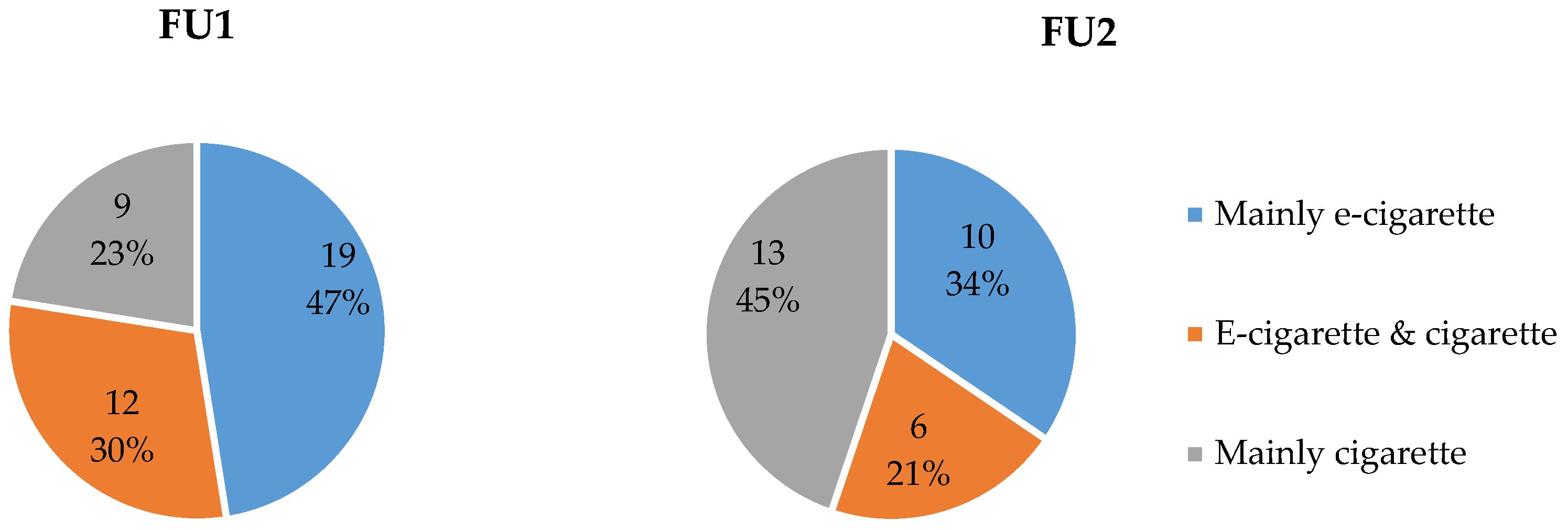
3.5.2. E-Cigarette Profile at FU
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | Moment | Never n (%) | Seldom n (%) | Occasionally n (%) | Often n (%) | Always n (%) | n M (SD) |
|---|---|---|---|---|---|---|---|
| Increased weight | IN | 44 (62.86) | 10 (14.29) | 11 (15.71) | 4 (5.71) | 1 (1.43) | 70 0.69 (1.03) |
| FU1 | 31 (56.57) | 12 (35.71) | 8 (25.00) | 2 (3.64) | 2 (3.64) | 55 0.76 (1.07) | |
| FU2 | 23 (46.94) | 14 (28.57) | 8 (16.33) | 0 (0.00) | 4 (8.16) | 49 0.94 (1.18) | |
| Headaches | IN | 32 (45.07) | 20 (28.17) | 12 (16.90) | 7 (9.86) | 0 (0.00) | 71 0.92 (1.01) |
| FU1 | 16 (28.57) | 26 (46.43) | 11 (19.64) | 3 (5.36) | 0 (0.00) | 56 1.02 (0.84) | |
| FU2 | 17 (34.69) | 14 (28.57) | 17 (34.69) | 1 (2.04) | 0 (0.00) | 49 1.04 (0.89) | |
| Sleeping problems | IN | 37 (52.11) | 13 (18.31) | 10 (14.08) | 7 (9.86) | 4 (5.63) | 71 0.99 (1.26) |
| FU1 | 23 (41.07) | 19 (33.93) | 5 (8.93) | 8 (14.29) | 1 (1.79) | 56 1.02 (1.12) | |
| FU2 | 22 (44.90) | 16 (32.65) | 8 (16.33) | 3 (6.122) | 0 (0.00) | 49 0.84 (0.92) | |
| Increased palpitations | IN | 20 (28.17) | 21 (29.58) | 19 (26.76) | 8 (11.27) | 3 (4.23) | 71 1.34 (1.13) |
| FU1 | 16 (28.57) | 20 (35.71) | 14 (25.00) | 5 (8.93) | 1 (1.79) | 56 1.20 (1.02) | |
| FU2 | 12 (24.49) | 22 (44.90) | 12 (24.49) | 3 (6.12) | 0 (0.00) | 49 1.12 (0.86) | |
| Poor sense of smell | IN | 20 (28.57) | 15 (21.43) | 21 (30.00) | 13 (18.57) | 1 (1.43) | 70 1.43 (1.34) |
| FU1 | 14 (25.00) | 20 (35.71) | 9 (16.07) | 12 (21.43) | 1 (1.79) | 56 1.39 (1.14) | |
| FU2 | 12 (24.49) | 20 (40.82) | 13 (26.53) | 3 (6.12) | 1 (2.04) | 49 1.20 (0.96) | |
| Poor sense of taste | IN | 12 (17.39) | 19 (27.54) | 26 (37.68) | 12 (17.39) | 0 (0.00) | 69 1.55 (0.98) |
| FU1 | 10 (17.86) | 23 (41.07) | 16 (28.57) | 6 (10.71) | 1 (1.79) | 56 1.38 (0.96) | |
| FU2 | 7 (14.29) | 17 (34.69) | 22 (44.90) | 2 (4.08) | 1 (2.04) | 49 1.45 (0.87) | |
| Poor taste sensation | IN | 16 (22.86) | 14 (20.00) | 25 (35.71) | 13 (18.57) | 2 (2.86) | 70 1.59 (1.22) |
| FU1 | 11 (19.64) | 21 (37.50) | 16 (28.57) | 6 (10.71) | 2 (3.57) | 56 1.41 (1.04) | |
| FU2 | 6 (12.25) | 22 (44.90) | 18 (36.73) | 2 (4.08) | 1 (2.04) | 49 1.39 (0.84) | |
| Unpleasant smelling | IN | 12 (17.14) | 21 (30.00) | 16 (22.86) | 17 (24.29) | 4 (5.71) | 70 1.71 (1.18) |
| FU1 | 4 (7.28) | 24 (43.64) | 12 (21.82) | 12 (21.82) | 3 (5.45) | 55 1.75 (1.06) | |
| FU2 | 6 (12.50) | 21 (43.75) | 13 (27.08) | 5 (10.42) | 3 (6.25) | 48 1.54 (1.05) | |
| Dry throat | IN | 12 (16.90) | 18 (25.35) | 23 (32.39) | 14 (19.72) | 4 (5.63) | 71 1.71 (1.14) |
| FU1 | 7 (12.50) | 20 (35.71) | 21 (37.50) | 7 (12.50) | 1 (1.79) | 56 1.55 (0.93) | |
| FU2 | 6 (12.25) | 17 (34.69) | 19 (38.78) | 7 (14.29) | 0 (0.00) | 49 1.55 (0.89) | |
| Dry mouth | IN | 8 (11.27) | 21 (29.58) | 24 (33.80) | 13 (18.31) | 5 (7.04) | 71 1.80 (1.09) |
| FU1 | 7 (12.50) | 20 (35.71) | 17 (30.36) | 11 (19.64) | 1 (1.79) | 56 1.63 (1.00) | |
| FU2 | 7 (14.29) | 15 (30.61) | 21 (42.86) | 6 (12.25) | 0 (0.00) | 49 1.53 (0.89) | |
| Difficult breathing | IN | 8 (11.43) | 14 (20.00) | 31 (44.29) | 16 (22.86) | 1 (1.43) | 70 1.83 (0.96) |
| FU1 | 6 (10.71) | 15 (26.79) | 24 (42.86) | 9 (16.07) | 2 (3.57) | 56 1.75 (0.98) | |
| FU2 | 6 (12.25) | 11 (22.45) | 23 (46.94) | 8 (16.33) | 1 (2.04) | 49 1.73 (0.95) | |
| Cough tendencies | IN | 5 (7.14) | 17 (24.29) | 24 (34.29) | 19 (27.14) | 5 (7.14) | 70 2.03 (1.05) |
| FU1 | 4 (7.27) | 14 (25.45) | 21 (38.18) | 15 (27.27) | 1 (1.82) | 55 1.91 (0.95) | |
| FU2 | 4 (8.16) | 16 (32.65) | 16 (32.65) | 12 (24.49) | 1 (2.04) | 49 1.80 (0.98) | |
| Pondering health | IN | 10 (14.08) | 10 (14.08) | 19 (26.76) | 22 (30.99) | 10 (14.08) | 71 2.17 (1.25) |
| FU1 | 6 (10.71) | 12 (21.43) | 18 (32.14) | 15 (26.79) | 5 (8.93) | 56 2.02 (1.14) | |
| FU2 | 5 (10.20) | 9 (18.37) | 20 (40.82) | 13 (26.53) | 2 (4.08) | 49 1.96 (1.02) | |
| Bad condition | IN | 1 (1.41) | 9 (12.68) | 20 (28.17) | 33 (46.48) | 8 (11.27) | 71 2.54 (0.91) |
| FU1 | 2 (3.64) | 8 (14.55) | 18 (32.73) | 20 (36.36) | 7 (12.73) | 55 2.40 (1.01) | |
| FU2 | 2 (4.08) | 6 (12.25) | 18 (36.73) | 16 (32.65) | 7 (14.29) | 49 2.41 (1.02) |
| Variable | Moment | Never n (%) | Seldom n (%) | Occasionally n (%) | Often n (%) | Always n (%) | n M (SD) |
|---|---|---|---|---|---|---|---|
| Headaches | FU1 | 39 (70.91) | 14 (25.45) | 2 (3.64) | 0 (0.00) | 0 (0.00) | 55 0.33 (0.55) |
| FU2 | 27 (64.29) | 9 (21.43) | 6 (14.29) | 0 (0.00) | 0 (0.00) | 42 0.50 (0.74) | |
| Poor sense of smell | FU1 | 37 (67.27) | 17 (30.91) | 1 (1.82) | 0 (0.00) | 0 (0.00) | 55 0.35 (0.52) |
| FU2 | 26 (61.90) | 16 (38.10) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 42 0.38 (0.49) | |
| Unpleasant smelling | FU1 | 35 (63.64) | 17 (30.91) | 3 (5.46) | 0 (0.00) | 0 (0.00) | 55 0.42 (0.60) |
| FU2 | 28 (66.67) | 13 (30.95) | 1 (2.38) | 0 (0.00) | 0 (0.00) | 42 0.36 (0.53) | |
| Sleeping problems | FU1 | 36 (65.46) | 15 (27.27) | 3 (5.46) | 1 (1.82) | 0 (0.00) | 55 0.44 (0.69) |
| FU2 | 27 (64.29) | 12 (28.57) | 2 (4.76) | 1 (2.38) | 0 (0.00) | 42 0.45 (0.71) | |
| Increased palpitations | FU1 | 35 (63.64) | 14 (25.46) | 4 (7.27) | 2 (3.64) | 0 (0.00) | 55 0.51 (0.79) |
| FU2 | 25 (59.52) | 12 (28.57) | 4 (9.52) | 1 (2.38) | 0 (0.00) | 42 0.55 (0.77) | |
| Poor sense of taste | FU1 | 32 (58.18) | 17 (30.91) | 5 (9.09) | 0 (0.00) | 1 (1.82) | 55 0.56 (0.81) |
| FU2 | 23 (54.76) | 16 (38.10) | 2 (4.76) | 1 (2.38) | 0 (0.00) | 42 0.55 (0.71) | |
| Difficult breathing | FU1 | 27 (50.00) | 22 (40.74) | 4 (7.40) | 1 (1.85) | 0 (0.00) | 54 0.61 (0.71) |
| FU2 | 23 (54.76) | 13 (30.95) | 3 (7.14) | 3 (7.14) | 0 (0.00) | 42 0.67 (0.90) | |
| Bad condition | FU1 | 27 (49.09) | 23 (41.82) | 3 (5.45) | 2 (3.63) | 0 (0.00) | 55 0.64 (0.75) |
| FU2 | 20 (47.62) | 17 (40.48) | 3 (7.14) | 0 (0.00) | 2 (4.76) | 42 0.74 (0.96) | |
| Poor taste sensation | FU1 | 27 (49.09) | 18 (32.73) | 9 (16.36) | 1 (1.82) | 0 (0.00) | 55 0.71 (0.81) |
| FU2 | 15 (35.71) | 22 (52.38) | 3 (7.14) | 2 (4.76) | 0 (0.00) | 42 0.81 (0.77) | |
| Increased weight | FU1 | 30 (54.55) | 16 (29.09) | 5 (9.09) | 3 (5.46) | 1 (1.82) | 55 0.71 (0.98) |
| FU2 | 23 (56.10) | 9 (21.95) | 4 (9.76) | 3 (7.32) | 2 (4.88) | 41 0.83 (1.18) | |
| Dry throat | FU1 | 27 (50.00) | 14 (25.93) | 10 (18.52) | 3 (5.56) | 0 (0.00) | 54 0.80 (0.94) |
| FU2 | 15 (35.71) | 15 (35.71) | 8 (19.05) | 2 (4.76) | 2 (4.76) | 42 1.07 (1.09) | |
| Dry mouth | FU1 | 28 (50.91) | 13 (23.64) | 10 (18.18) | 4 (7.27) | 0 (0.00) | 55 0.82 (0.98) |
| FU2 | 19 (45.24) | 12 (28.57) | 7 (16.67) | 2 (4.76) | 2 (4.76) | 42 0.95 (1.13) | |
| Pondering health | FU1 | 26 (47.27) | 16 (29.09) | 9 (16.36) | 3 (5.46) | 1 (1.82) | 55 0.85 (1.01) |
| FU2 | 17 (40.48) | 12 (28.57) | 9 (21.43) | 3 (7.41) | 1 (2.38) | 42 1.02 (1.07) | |
| Cough tendencies | FU1 | 16 (29.09) | 17 (30.91) | 14 (25.46) | 6 (10.91) | 2 (3.64) | 55 1.29 (1.12) |
| FU2 | 15 (35.71) | 14 (33.33) | 8 (19.05) | 3 (7.14) | 2 (4.76) | 42 1.12 (1.13) |
| Variable | Moment | Totally Disagree n (%) | Disagree n (%) | Neutral n (%) | Agree n (%) | Totally Agree n (%) | n M (SD) |
|---|---|---|---|---|---|---|---|
| Not disturbing others | FU1 | 4 (7.55) | 10 (18.87) | 15 (28.01) | 11 (20.75) | 13 (24.53) | 53 2.36 (1.26) |
| FU2 | 0 (0.00) | 12 (28.57) | 7 (16.67) | 13 (30.95) | 10 (23.81) | 42 2.50 (1.15) | |
| No technical problems | FU1 | 2 (3.64) | 12 (21.82) | 7 (12.73) | 19 (34.55) | 15 (27.27) | 55 2.60 (1.21) |
| FU2 | 0 (0.00) | 9 (21.95) | 8 (19.51) | 14 (34.15) | 10 (24.39) | 41 2.61 (1.09) | |
| Helps to quit smoking | FU1 | 1 (1.82) | 5 (9.09) | 12 (21.82) | 18 (32.73) | 19 (34.55) | 55 2.89 (1.05) |
| FU2 | 1 (2.50) | 2 (50.00) | 10 (25.00) | 13 (32.50) | 14 (35.00) | 40 2.93 (1.03) | |
| Helps to take away craving cigarette | FU1 | 2 (3.64) | 3 (5.46) | 9 (16.36) | 19 (34.55) | 22 (40.00) | 55 3.02 (1.06) |
| FU2 | 1 (2.44) | 4 (9.76) | 11 (26.83) | 9 (21.95) | 16 (39.02) | 41 2.85 (1.13) | |
| More places | FU1 | 5 (9.09) | 14 (25.46) | 13 (23.64) | 10 (18.18) | 13 (23.64) | 55 3.22 (1.32) |
| FU2 | 5 (11.90) | 8 (19.05) | 7 (16.67) | 14 (33.33) | 8 (19.05) | 42 2.29 (1.31) |
References
- ASH Factsheet: Use of E-Cigarettes (Vapourisers) among Adults in Great Britain. Available online: http://ash.org.uk/information-and-resources/fact-sheets/use-of-e-cigarettes-among-adults-in-great-britain-2017/ (accessed on 1 February 2018).
- Jamal, A.; Homa, D.M.; O’Connor, E.; Babb, S.D.; Caraballo, R.S.; Singh, T.; Hu, S.S.; King, B.A. Current cigarette smoking among adults—United States, 2005–2014. Morb. Mortal. Wkly. Rep. 2015, 64, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Special Eurobarometer 458: Attitudes of Europeans towards Tobacco and Electronic Cigarettes. Available online: http://ec.europa.eu/commfrontoffice/publicopinion/index.cfm/Survey/getSurveyDetail/search/458/surveyKy/2146 (accessed on 6 February 2018).
- West, R.; Beard, E.; Brown, J. Trends in Electronic Cigarette Use in England. Available online: http://www.smokinginengland.info/latest-statistics/ (accessed on 6 February 2018).
- Stichting Tegen Kanker. Rookgedrag in België 2017. Available online: https://www.kanker.be/sites/default/files/stichting_tegen_kanker_-_enquete_rookgedrag_2017_1.pdf (accessed on 6 February 2018).
- Lee, Y.O.; Kim, A.E. ‘Vape shops’ and ‘e-cigarette lounges’ open across the USA to promote ENDS. Tob. Control 2015, 24, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Pattinson, J.; Lewis, S.; Bains, M.; Britton, J.; Langley, T. Vape shops: Who uses them and what do they do? BMC Public Health 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.; Cox, S.; Dawkins, L.; Jakes, S.; Holland, R.; Notley, C. A qualitative exploration of the role of vape shop environments in supporting smoking abstinence. Int. J. Environ. Res. Public Health 2018, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Klassieke Sigaret Ruimt Baan Voor Dampshops. Available online: https://www.hln.be/regio/antwerpen/klassieke-sigaret-ruimt-baan-voor-dampshops~ab1a5a26/ (accessed on 1 February 2018).
- Federale Overheidsdienst Volksgezondheid, Veiligheid van de Voedselketen en Leefmilieu. Koninklijk Besluit Betreffende Het Fabriceren en Het in de Handel Brengen van Elektronische Sigaretten. Available online: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/2016_10_28_ar_ecigarette_2.pdf (accessed on 1 February 2018).
- Berry, K.M.; Reynolds, L.M.; Collins, J.M.; Siegel, M.B.; Fetterman, J.L.; Hamburg, N.M.; Bhatnagar, A.; Benjamin, E.J.; Stokes, A. E-cigarette initiation and associated changes in smoking cessation and reduction: The Population Assessment of Tobacco and Health Study, 2013–2015. Tob. Control 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Biener, L.; Hargraves, J.L. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: Association with smoking cessation and motivation to quit. Nicotine Tob. Res. 2015, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hitchman, S.C.; Brose, L.S.; Brown, J.; Robson, D.; McNeill, A. Associations between e-cigarette type, frequency of use, and quitting smoking: Findings from a longitudinal online panel survey in Great Britain. Nicotine Tob. Res. 2015, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Cummins, S.E.; Sun, J.Y.; Zhu, S. Long-term e-cigarette use and smoking cessation: A longitudinal study with US population. Tob. Control 2016, 25, i90–i95. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Poulas, K.; Voudris, V.; Le Houezec, J. Electronic cigarette use in the European Union: Analysis of a representative sample of 27 460 Europeans from 28 countries. Addiction 2016, 111, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Poulas, K.; Voudris, V.; Le Houezec, J. Prevalence and correlates of current daily use of electronic cigarettes in the European Union: Analysis of the 2014 Eurobarometer survey. Intern. Emerg. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pasquereau, A.; Gautier, A.; Andler, R.; Guignard, R.; Richard, J.-B.; Nguyen-Thanh, V. Tabac et e-cigarette en France: Niveaux d’usage d’après les premiers résultats du Baromètre Santé 2016. Bull. Epidémiol. Hebd. 2017, 12, 214–222. [Google Scholar]
- Polosa, R.; Caponnetto, P.; Cibella, F.; Le-Houezec, J. Quit and smoking reduction rates in vape shop consumers: A prospective 12-month survey. Int. J. Environ. Res. Public Health 2015, 12, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Tackett, A.P.; Lechner, W.V.; Meier, E.; Grant, D.M.; Driskill, L.M.; Tahirkheli, N.N.; Wagener, T.L. Biochemically verified smoking cessation and vaping beliefs among vape store customers. Addiction 2015, 110, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Wagener, T.L.; Shaikh, R.A.; Meier, E.; Tackett, A.P.; Tahirkheli, N.N.; Leavens, E.L.; Driskill, L. Examining the smoking and vaping behaviors and preferences of vape shop customers. Tob. Prev. Cessat. 2016, 2. [Google Scholar] [CrossRef]
- piCOTM Smokerlyzer® Apparatus and Software; Bedfont® Scientific Ltd.: Maidstone, UK, 2017.
- TIBCO Software Inc. Statistica (Version 13) (Computer Software); TIBCO Software Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Goniewicz, M.L.; Smith, D.M. Are some e-cigarette users “blowing smoke”? Assessing the accuracy of self-reported smoking abstinence in exclusive e-cigarette users. Nicotine Tob. Res. 2018, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Keely, J.; Naud, S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2003, 99, 29–38. [Google Scholar] [CrossRef]
- Schlam, T.R.; Baker, T.B. Interventions for tobacco smoking. Annu. Rev. Clin. Psychol. 2013, 9, 675–702. [Google Scholar] [CrossRef] [PubMed]
- Cahill, K.; Stevens, S.; Lancaster, T. Pharmacological treatments for smoking cessation. JAMA 2014, 311, 193–194. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Coyle, K.; Owen, L.; Coyle, D.; Pokhrel, S. Estimates of effectiveness and reach for ‘return on investment’ modelling of smoking cessation interventions using data from England. Addiction 2017, 113, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bauld, L.; Hiscock, R.; Dobbie, F.; Aveyard, P.; Coleman, T.; Leonardi-Bee, J.; McRobbie, H.; McEwen, A. English stop-smoking services: One-year outcomes. Int. J. Environ. Res. Public Health 2016, 13, 1175. [Google Scholar] [CrossRef] [PubMed]
- Rodu, B. CDC Data Shows E-Cigarette Use Declines Again. Available online: https://rodutobaccotruth.blogspot.com/2017/09/2016-cdc-data-shows-e-cigarette-use.html (accessed on 6 February 2018).
- Inoue-Choi, M.; Liao, L.M.; Reyes-Guzman, C.; Hartge, P.; Caporaso, N.; Freedman, N.D. Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the national institutes of health-AARP diet and health study. JAMA 2016, 177, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Hartge, P.; Liao, L.M.; Caporaso, N.; Freedman, N.D. Association between long-term low-intensity cigarette smoking and incidence of smoking-related cancer in the national institutes of health-AARP cohort. Int. J. Cancer 2018, 142, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Pirie, K.; Peto, R.; Reeves, G.K.; Green, J.; Beral, V. The 21st century hazards of smoking and benefits of stopping: A prospective study of one million women in the UK. Lancet 2013, 381, 133–141. [Google Scholar] [CrossRef]
- Shahab, L.; Goniewicz, M.L.; Blount, B.C.; Brown, J.; McNeill, A.; Alwis, U.; Feng, J.; Wang, L.; West, R. Nicotine, carcinogen, and toxin exposures in long-term e-cigarette and nicotine replacement therapy users: A cross sectional study. Ann. Intern. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Notley, C.; Ward, E.; Dawkins, L.; Holland, R. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduct. J. 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Begh, R.; Lindson-Hawley, N.; Aveyard, P. Does reduced smoking if you can’t stop make any difference? BMC Med. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.L.; Walker, K.L.; Sears, C.G.; Lee, A.S.; Smith, C.; Siu, A.; Keith, R.; Ridner, S.L. Vape shop employees: Public health advocates? Tob. Prev. Cessat. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- McKeganey, N.; Dickson, T. Why don’t more smokers switch to using e-cigarettes: The views of confirmed smokers. Int. J. Environ. Res. Public Health 2017, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, K.; Van Gucht, D.; Baeyens, F. Differences between dual users and switchers center around vaping behavior and its experiences rather than beliefs and attitudes. Int. J. Environ. Res. Public Health 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Van Gucht, D.; Adriaens, K.; Baeyens, F. Online vape shop customers who use e-cigarettes report abstinence from smoking and improved quality of life, but a substantial minority still have vaping-related health concerns. Int. J. Environ. Res. Public Health 2017, 14, 798. [Google Scholar] [CrossRef]
- Smets, J.; Van Gucht, D.; Chaumont, M.; Adriaens, K.; Baeyens, F. High-wattage big-volume low-nicotine vaping: Subculture or global trend. in preparation.
- Etter, J.-F. Electronic cigarette: A longitudinal study of regular vapers. Nicotine Tob. Res. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.V. Science Lesson: Clinical Trials Are a Terrible Way to Assess Vaping. Available online: http://dailyvaper.com/2017/12/11/science-lesson-clinical-trials-are-a-terrible-way-to-assess-vaping/ (accessed on 5 July 2018).
| Variable | n | M (SD) or % |
|---|---|---|
| Demographic characteristics | 71 | |
| Age (years) | 71 | 38.25 (14.06) |
| Gender (men/women) | 44/27 | 61.97/38.03 |
| Highest educational degree | 70 | |
| None | 2 | 2.86 |
| Elementary school | 4 | 5.71 |
| High school | 40 | 57.14 |
| Non-academic bachelor | 16 | 22.86 |
| University | 5 | 7.14 |
| Other | 3 | 4.29 |
| Occupation | 71 | |
| Student | 7 | 9.86 |
| Part-time job | 2 | 2.82 |
| Full-time job | 52 | 73.24 |
| Housewife/-man | 1 | 1.41 |
| Job seeker | 1 | 1.41 |
| Retired | 6 | 8.45 |
| Invalidity | 2 | 2.82 |
| Net income per month (in €) | 70 | |
| <1000 € | 10 | 14.29 |
| 1000–1500 € | 13 | 18.57 |
| 1500–2000 € | 30 | 42.86 |
| 2000–2500 € | 13 | 18.57 |
| 2500–3000 € | 3 | 4.29 |
| >3000 € | 1 | 1.43 |
| Ethnicity | 71 | |
| Caucasian | 70 | 98.59 |
| Other | 1 | 1.41 |
| Variable | nintake (%) | nFU1 (%) | nFU2 (%) |
|---|---|---|---|
| Situations when smoking * | 71 | 56 | 49 |
| After a meal | 59 (83.10) | 34 (60.71) | 31 (63.27) |
| When drinking (coffee, alcohol) | 52 (73.24) | 30 (53.57) | 29 (59.19) |
| At home | 47 (66.20) | 26 (46.43) | 24 (48.98) |
| With friends | 45 (63.38) | 19 (33.93) | 17 (34.69) |
| At work or school | 38 (53.52) | 20 (35.71) | 19 (38.78) |
| Alone | 37 (52.11) | 18 (32.14) | 16 (32.65) |
| Reasons why smoking * | 71 | 56 | 49 |
| Routine | 52 (73.24) | 24 (42.86) | 22 (44.90) |
| Craving | 47 (66.20) | 37 (66.07) | 28 (57.14) |
| Stress reduction | 43 (60.56) | 26 (46.43) | 26 (53.06) |
| Atmosphere | 33 (46.48) | 17 (30.36) | 20 (40.82) |
| Nicotine craving | 29 (40.85) | 15 (26.79) | 12 (24.49) |
| Relaxation | 29 (40.85) | 14 (25.00) | 14 (28.57) |
| Pastime | 20 (28.17) | 9 (16.07) | 11 (22.45) |
| Motivation to quit smoking | 71 | 56 | 48 |
| I do not want to quit smoking | 2 (2.82) | 5 (8.93) | 5 (10.42) |
| I think about quitting smoking but not in the next 6 months | 11 (15.49) | 12 (21.43) | 14 (29.17) |
| I think about quitting smoking, in the next 6 months | 23 (32.39) | 21 (37.50) | 17 (35.42) |
| I think about quitting smoking, in the next month | 12 (16.90) | 6 (10.71) | 8 (16.67) |
| I want to quit smoking now | 23 (32.39) | 12 (21.43) | 4 (8.33) |
| Experienced negative health effects from smoking ** | 46 | 46 | 46 |
| 1.63 (0.09) | 1.52 (0.08) | 1.46 (0.08) |
| Variable | nintake (%) | nFU1 (%) | nFU2 (%) |
|---|---|---|---|
| Most important reasons e-cigarette purchase | 71 | / | / |
| To quit smoking–gradually reducing smoking | 37 (52.11) | / | / |
| To quit smoking–immediately switching | 19 (26.76) | / | / |
| To reduce smoking (dual use) | 8 (11.27) | / | / |
| Curiosity | 3 (4.23) | / | / |
| To vape where smoking is prohibited | 1 (1.41) | / | / |
| Financial reasons | 0 (0.00) | / | / |
| No reason indicated | 3 (4.23) | / | / |
| Future plans e-cigarette in case reduced/quit smoking | 71 | 54 | 43 |
| Continue using e-cigarette, without reducing nicotine concentration | 4 (5.63) | 8 (14.81) | 10 (23.26) |
| Continue using e-cigarette and reducing nicotine concentration | 32(45.07) | 32 (39.51) | 16 (37.21) |
| Reducing e-cigarette use | 17 (23.94) | 7 (12.96) | 10 (23.26) |
| Quitting e-cigarette use | 18 (25.35) | 7 (12.96) | 7 (16.28) |
| Reasons why quit vaping * | / | 16 | 20 |
| No satisfaction | / | 5 (31.25) | 6 (30.00) |
| Uncomfortable practical use | / | 4 (25.00) | 7 (35.00) |
| Little similarities with cigarettes | / | 4 (25.00) | 3 (15.00) |
| Physical complaints from e-cigarette use | / | 2 (12.50) | 3 (15.00) |
| No confidence in e-cigarettes | / | 2 (12.50) | 0 (0.00) |
| No reason reported | / | 1 (6.25) | 3 (15.00) |
| Dual use–preference e-cigarette * | / | 41 | 29 |
| Health | / | 18 (43.90) | 13 (44.83) |
| Context | / | 9 (21.95) | 6 (20.69) |
| For others | / | 5 (12.20) | 2 (6.90) |
| Financial aspect | / | 2 (4.88) | 0 (0.00) |
| Taste/smell | / | 11 (26.83) | 3 (10.34) |
| Smoking cessation | / | 1 (2.44) | 5 (17.24) |
| Other or no reason | / | 6 (14.63) | 2 (6.90) |
| Dual use–preference cigarette * | / | 41 | 29 |
| Sensorial aspects of smoking cigarettes | / | 6 (14.63) | 9 (31.03) |
| Context | / | 11 (26.83) | 5 (17.24) |
| Stress | / | 5 (12.20) | 2 (6.90) |
| Addiction | / | 10 (24.39) | 8 (27.59) |
| Habit | / | 9 (21.95) | 9 (31.03) |
| Technical problems e-cigarette | / | 2 (4.88) | 1 (3.45) |
| Other or no reason | / | 4 (9.76) | 0 (0.00) |
| Situations when vaping * | / | 55 | 42 |
| At home | / | 35 (63.64) | 26 (61.90) |
| At work/school | / | 26 (47.27) | 21 (50.00) |
| With friends | / | 20 (36.36) | 18 (42.86) |
| After a meal | / | 18 (32.73) | 7 (16.67) |
| Everywhere | / | 15 (27.27) | 9 (21.43) |
| When drinking (coffee, alcohol) | / | 14 (25.46) | 11 (26.19) |
| Alone | / | 14 (25.46) | 12 (28.57) |
| Reasons why vaping * | / | 55 | 42 |
| Craving for e-cigarette | / | 29 (52.73) | 19 (45.24) |
| Nicotine craving | / | 28 (50.91) | 18 (42.86) |
| Routine | / | 22 (40.00) | 11 (26.19) |
| Stress reduction | / | 19 (34.55) | 10 (23.81) |
| Relaxation | / | 12 (21.82) | 12 (28.57) |
| Atmosphere | / | 11 (20.00) | 11 (26.19) |
| Pastime | / | 9 (16.36) | 8 (19.05) |
| Variable | na | Intake | FU1 | FU2 |
|---|---|---|---|---|
| Puffs per day | 36 | / | 77.67 (10.75) | 83.30 (27.20) |
| Quitters | 11 | / | 126.93 (17.20) | 142.05 (47.67) |
| 80% reducers | 4 | / | 30.38 (28.52) | 66.63 (79.05) |
| 50% reducers | 8 | / | 60.06 (20.17) | 165.13 (55.89) |
| Failures | 13 | / | 61.38 (15.82) | 24.37 (43.85) |
| Amount of e-liquid used (mL/week) | 37 | / | 12.95 (1.60) | 13.14 (1.68) |
| Quitters | 11 | / | 21.36 (2.50) | 22.45 (2.51) |
| 80% reducers | 4 | / | 6.50 (4.14) | 13.75 (4.16) |
| 50% reducers | 9 | / | 9.50 (2.76) | 9.89 (2.77) |
| Failures | 13 | / | 10.19 (2.30) | 7.21 (2.31) |
| Nicotine concentration (mg/mL) | 39 | 7.01 (0.41) | 6.01 (0.51) | 5.79 (0.56) |
| Quitters | 12 | 7.00 (0.75) | 5.63 (0.95) | 4.50 (0.98) |
| 80% reducers | 3 | 6.00 (1.49) | 5.00 (1.89) | 3.50 (1.96) |
| 50% reducers | 10 | 6.35 (0.82) | 5.60 (1.04) | 5.90 (1.07) |
| Failures | 14 | 7.71 (0.69) | 6.86 (0.88) | 7.29 (0.91) |
| Flavour e-liquid | 71 a | 55 a | 41 a | |
| Tobacco b | 33 (46.48) | 15 (27.27) | 14 (34.15) | |
| Mint b | 18 (25.35) | 15 (27.27) | 9 (21.95) | |
| Coffee b | 6 (8.45) | 3 (5.55) | 2 (4.88) | |
| Sweet b | 46 (64.79) | 40 (72.73) | 28 (68.29) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adriaens, K.; Van Gucht, D.; Baeyens, F. About One in Five Novice Vapers Buying Their First E-Cigarette in a Vape Shop Are Smoking Abstinent after Six Months. Int. J. Environ. Res. Public Health 2018, 15, 1886. https://doi.org/10.3390/ijerph15091886
Adriaens K, Van Gucht D, Baeyens F. About One in Five Novice Vapers Buying Their First E-Cigarette in a Vape Shop Are Smoking Abstinent after Six Months. International Journal of Environmental Research and Public Health. 2018; 15(9):1886. https://doi.org/10.3390/ijerph15091886
Chicago/Turabian StyleAdriaens, Karolien, Dinska Van Gucht, and Frank Baeyens. 2018. "About One in Five Novice Vapers Buying Their First E-Cigarette in a Vape Shop Are Smoking Abstinent after Six Months" International Journal of Environmental Research and Public Health 15, no. 9: 1886. https://doi.org/10.3390/ijerph15091886
APA StyleAdriaens, K., Van Gucht, D., & Baeyens, F. (2018). About One in Five Novice Vapers Buying Their First E-Cigarette in a Vape Shop Are Smoking Abstinent after Six Months. International Journal of Environmental Research and Public Health, 15(9), 1886. https://doi.org/10.3390/ijerph15091886





