Prevalence of Microbiological and Chemical Contaminants in Private Drinking Water Wells in Maryland, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Safe Drinking Water Clinics
2.2. Laboratory Analyses
2.3. Animal Feeding Operations Data
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Safe Drinking Water Clinic Participants
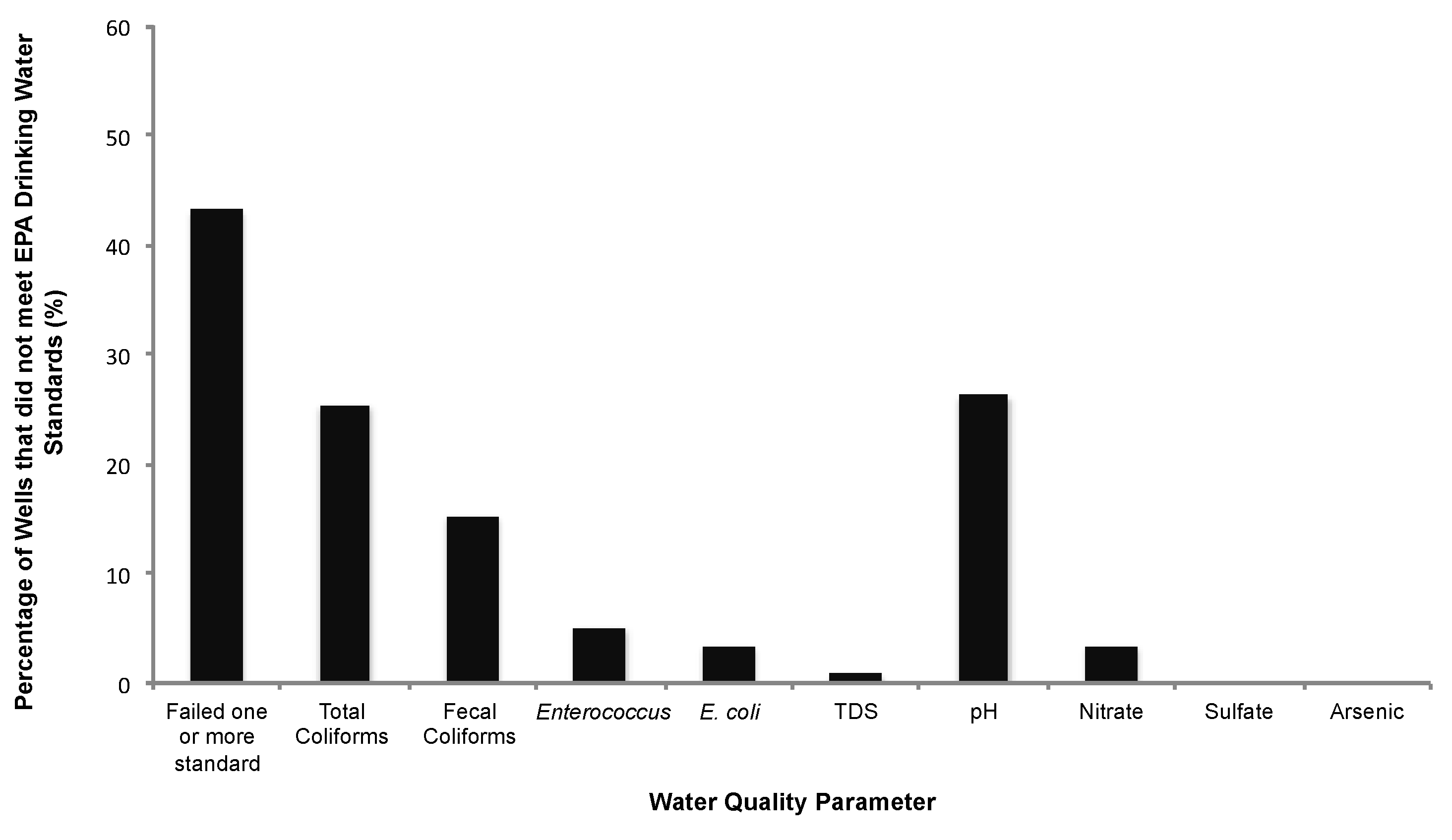
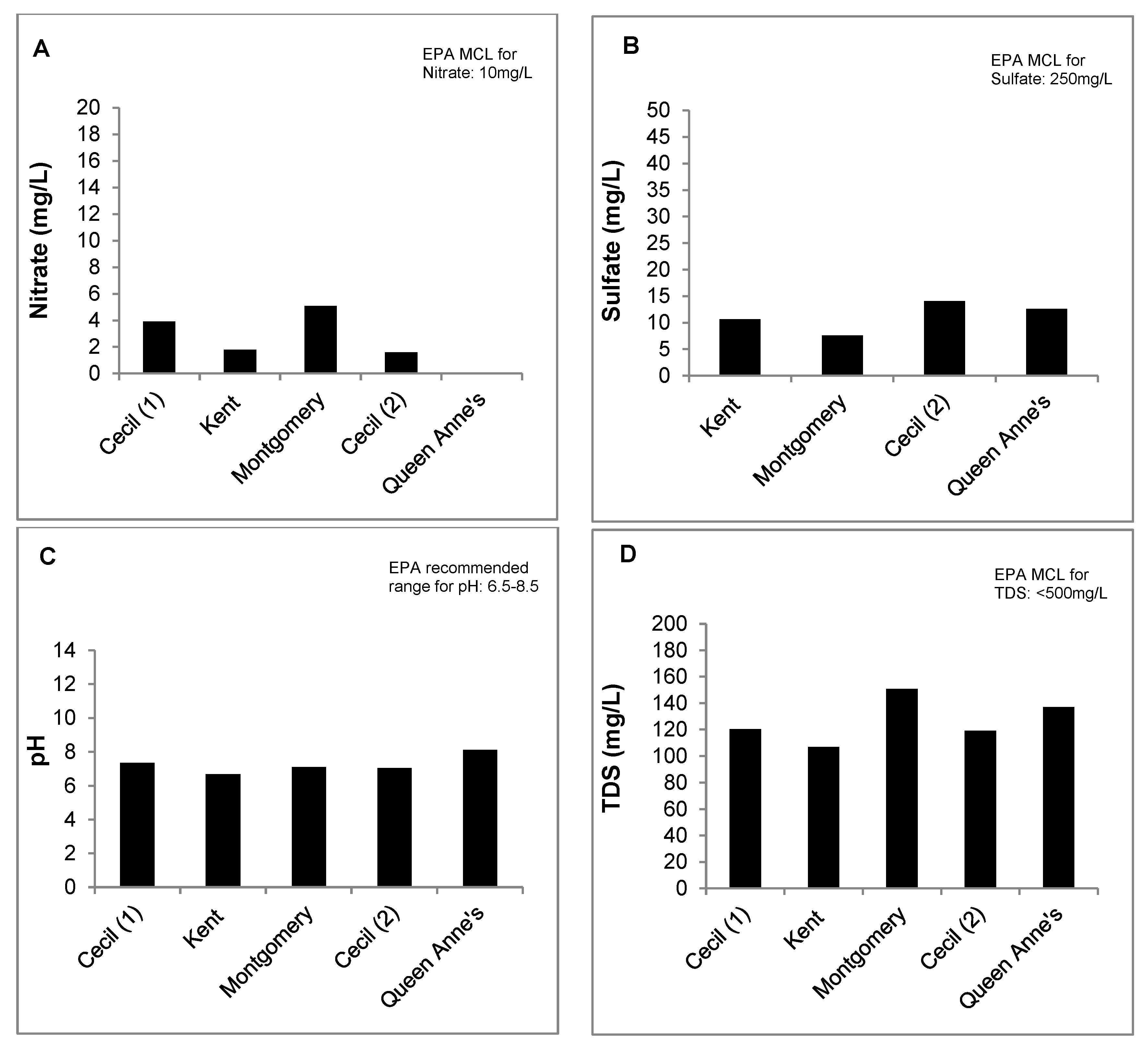
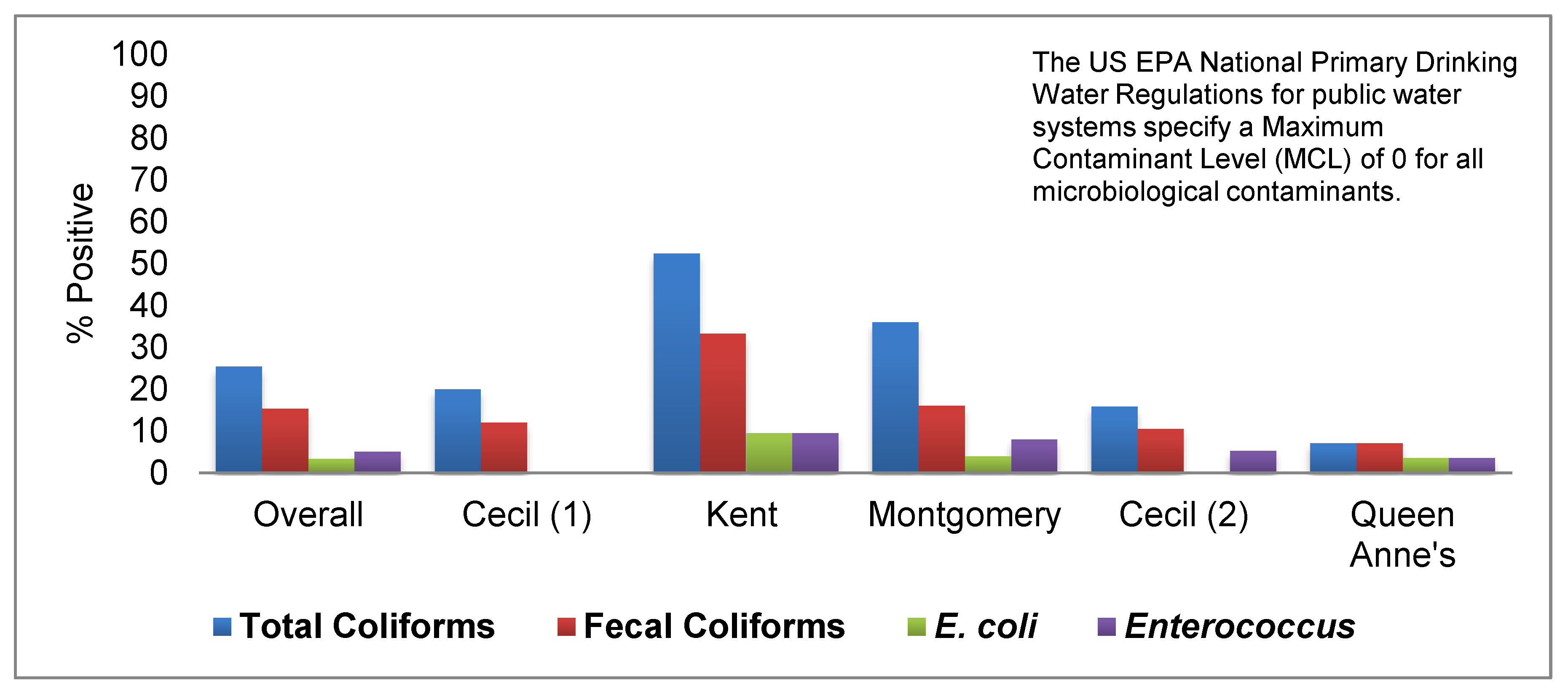
3.2. Well Water Quality
3.3. Influence of Animal Feeding Operations on Well Water Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- US Census Bureau American Housing Survey (AHS) for the United States. 2015. Available online: https://www.census.gov/programs-surveys/ahs.html (accessed on 27 August 2017).
- Maupin, M.A.; Kenny, J.F.; Hutson, S.S.; Lovelace, J.K.; Barber, N.L.; Linsey, K.S. Estimated Use of Water in the United States in 2010; U.S. Geological Survey: Reston, VA, USA, 2014; p. 56.
- U.S. Environmental Protection Agency. Title XIV of the Public Health Service Act (The Safe Drinking Water Act); U.S. Environmental Protection Agency: Washington, DC, USA, 1974.
- U.S. Environmental Protection Agency, Office of Water. Private Drinking Water Wells. Available online: https://www.epa.gov/privatewells (accessed on 27 March 2016).
- National Groundwater Association (NGA). Schedule Your Annual Water Well Checkup. Available online: http://www.ngwa.org/Events-Education/awareness/Pages/Schedule-your-annual-water-well-checkup.aspx (accessed on 21 October 2017).
- Gasteyer, S.P.; Vaswani, R. Still Living without the Basics in the 21st Century: Analyzing the Availability of Water and Sanitation Services in the United States; Rural Community Assistance Partnership: Washington, DC, USA, 2004. [Google Scholar]
- Wescoat, J.L.; Headington, L.; Theobald, R. Water and poverty in the United States. Geoforum 2007, 38, 801–814. [Google Scholar] [CrossRef]
- Craun, G.F.; Brunkard, J.M.; Yoder, J.S.; Roberts, V.A.; Carpenter, J.; Wade, T.; Calderon, R.L.; Roberts, J.M.; Beach, M.J.; Roy, S.L. Causes of Outbreaks Associated with Drinking Water in the United States from 1971 to 2006. Clin. Microbiol. Rev. 2010, 23, 507–528. [Google Scholar] [CrossRef] [PubMed]
- DeFelice, N.B.; Johnston, J.E.; Gibson, J.M. Reducing Emergency Department Visits for Acute Gastrointestinal Illnesses in North Carolina (USA) by Extending Community Water Service. Environ. Health Perspect. 2016, 124. [Google Scholar] [CrossRef] [PubMed]
- Swistock, B.R.; Clemens, S.; Sharpe, W.E.; Rummel, S. Water Quality and Management of Private Drinking Water Wells in Pennsylvania. J. Environ. Health 2013, 75, 60–66. [Google Scholar] [PubMed]
- Pieper, K.J.; Krometis, L.-A.H.; Gallagher, D.L.; Benham, B.L.; Edwards, M. Incidence of waterborne lead in private drinking water systems in Virginia. J. Water Health 2015, 13, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Knobeloch, L.; Gorski, P.; Christenson, M.; Anderson, H. Private Drinking Water Quality in Rural Wisconsin. J. Environ. Health 2013, 75, 16–20. [Google Scholar] [PubMed]
- Smith, T.; Krometis, L.-A.H.; Hagedorn, C.; Lawrence, A.H.; Benham, B.; Ling, E.; Ziegler, P.; Marmagas, S.W. Associations between fecal indicator bacteria prevalence and demographic data in private water supplies in Virginia. J. Water Health 2014, 12, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Stillo, F.; MacDonald Gibson, J. Exposure to Contaminated Drinking Water and Health Disparities in North Carolina. Am. J. Public Health 2017, 107, 180–185. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, L. Quality of Water from Domestic Wells in Principal Aquifers of the United States, 1991–2004; U.S. Geological Survey: Reston, VA, USA, 2009; p. 139.
- Pieper, K.J.; Krometis, L.-A.H.; Benham, B.L.; Gallagher, D.L. Simultaneous Influence of Geology and System Design on Drinking Water Quality in Private Systems. J. Environ. Health Denver 2016, 79, E1–E9. [Google Scholar]
- Swistock, B.R.; Sharpe, W.E.; Robillard, P.D. A survey of lead, nitrate and radon contamination of private individual water systems in Pennsylvania. J. Environ. Health 1993, 55, 6–13. [Google Scholar]
- Wallender, E.K.; Ailes, E.C.; Yoder, J.S.; Roberts, V.A.; Brunkard, J.M. Contributing Factors to Disease Outbreaks Associated with Untreated Groundwater. Groundwater 2014, 52, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Tuthill, A.; Meikle, D.B.; Alavanja, M.C.R. Coliform bacteria and nitrate contamination of wells in major soils of Frederick, Maryland. J. Environ. Health Denver 1998, 60, 16–20. [Google Scholar]
- Knobeloch, L. Use of the Behavioral Risk Factor Surveillance Survey to Assess the Safety of Private Drinking Water Supplies; Wisconsin Department of Health Services: Madison, WI, USA, 2010. [Google Scholar]
- Krometis, L.-A. Associations between homeowner perceptions of water quality and measures of contamination in private drinking water supplies. In Proceedings of the 142nd APHA Annual Meeting and Exposition, New Orleans, LA, USA, 15–19 November 2014. [Google Scholar]
- Li, X.; Atwill, E.R.; Antaki, E.; Applegate, O.; Bergamaschi, B.; Bond, R.F.; Chase, J.; Ransom, K.M.; Samuels, W.; Watanabe, N.; et al. Fecal Indicator and Pathogenic Bacteria and Their Antibiotic Resistance in Alluvial Groundwater of an Irrigated Agricultural Region with Dairies. J. Environ. Qual. 2015, 44, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Maryland State Archives Maryland at a Glance: Agriculture and Farming. Available online: http://msa.maryland.gov/msa/mdmanual/01glance/html/agri.html#poultry (accessed on 4 October 2016).
- Dance, S. As chicken industry booms, Eastern Shore farmers face not-in-my-backyard activism. The Baltimore Sun, 2 April 2016. [Google Scholar]
- Wheeler, T. Environmentalists call for moratorium on growth of Shore poultry industry. The Baltimore Sun, 8 September 2015. [Google Scholar]
- Environmental Integrity Project More Phosphorus, Less Monitoring. Available online: http://www.environmentalintegrity.org/wp-content/uploads/2016/11/Poultry-report_2013.pdf (accessed on 19 November 2016).
- U.S. Environmental Protection Agency. Method 1604: Total Coliforms and Escherichia coli in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium); U.S. Environmental Protection Agency: Washington, DC, USA, 2002.
- U.S. Environmental Protection Agency (USEPA). Manual for the Certification of Laboratories Analyzing Drinking Water: Criteria and Procedures Quality Assurance, 5th ed.; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2005.
- U.S. Environmental Protection Agency. Method 1600: Enterococci in Water by Membrane Filtration Using Membrane-Enterococcus Indoxyl-β-D-Glucoside Agar (mEI); U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- U.S. Environmental Protection Agency. Method 1603: Escherichia coli (E. coli) in Water by Membrane Filtration Using Modified Membrane—Thermotolerant Escherichia coli Agar (Modified mTEC); U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- Hach USA Stream Survey Test Kit—Hach USA. Available online: https://www.hach.com/stream-survey-test-kit/product?id=7640219501 (accessed on 12 July 2018).
- Hach USA Pocket Pro Low Range TDS Tester—Hach USA. Available online: https://www.hach.com/pocket-pro-low-range-tds-tester/product?id=17990686204&_bt=218809546514&_bk=&_bm=b&_bn=g&gclid=EAIaIQobChMIlN_F6Jua3AIVjYvICh1clwGYEAAYASAAEgIJ9_D_BwE (accessed on 12 July 2018).
- U.S. Environmental Protection Agency. Method 353.2, Revision 2.0: Determination of Nitrate-Nitrite Nitrogen by Automated Colorimetry; Environmental Monitoring Systems Laboratory Office of Research and Development, U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1993.
- U.S. Environmental Protection Agency. Method 375.2, Revision 2.0: Determination of Sulfate by Automated Colorimetry; Environmental Monitoring Systems Laboratory Office of Research and Development, U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1993.
- U.S. Environmental Protection Agency. Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1994.
- United States Department of Agriculture (USDA). USDA NASS QuickStats Query Tool. Available online: https://quickstats.nass.usda.gov/ (accessed on 19 February 2018).
- SAS Institute. The SAS System for Windows Copyright © 2014, version 9.4; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). CFR Part 141: National Primary Drinking Water Implementation Regulations; U.S. Environmental Protection Agency: Washington, DC, USA, 1974.
- Richardson, H.Y.; Nichols, G.; Lane, C.; Lake, I.R.; Hunter, P.R. Microbiological surveillance of private water supplies in England—The impact of environmental and climate factors on water quality. Water Res. 2009, 43, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Carrique-Mas, J.; Andersson, Y.; Hjertqvist, M.; Svensson, Å.; Torner, A.; Giesecke, J. Risk factors for domestic sporadic campylobacteriosis among young children in Sweden. Scand. J. Infect. Dis. 2005, 37, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kapperud, G.; Espeland, G.; Wahl, E.; Walde, A.; Herikstad, H.; Gustavsen, S.; Tveit, I.; Natås, O.; Bevanger, L.; Digranes, A. Factors Associated with Increased and Decreased Risk of Campylobacter Infection: A Prospective Case-Control Study in Norway. Am. J. Epidemiol. 2003, 158, 234–242. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, E.; White, R.; Mexia, R.; Bruun, T.; Kapperud, G.; Lange, H.; Nygård, K.; Vold, L. Risk Factors for Sporadic Domestically Acquired Campylobacter Infections in Norway 2010–2011: A National Prospective Case-Control Study. PLoS ONE 2015, 10, e0139636. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.M.; Santo Domingo, J.W.; Reasoner, D.J. Microbial Source Tracking: State of the Science. Environ. Sci. Technol. 2002, 36, 5279–5288. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.K.; Snyder, B.J.; Oladeinde, A.; Spidle, D.; Berrang, M.E.; Meinersmann, R.J.; Oakley, B.; Sidle, R.C.; Sullivan, K.; Molina, M. Characterizing relationships among fecal indicator bacteria, microbial source tracking markers, and associated waterborne pathogen occurrence in stream water and sediments in a mixed land use watershed. Water Res. 2016, 101, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Allevi, R.P.; Krometis, L.-A.H.; Hagedorn, C.; Benham, B.; Lawrence, A.H.; Ling, E.J.; Ziegler, P.E. Quantitative analysis of microbial contamination in private drinking water supply systems. J. Water Health 2013, 11, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Krolik, J.; Evans, G.; Belanger, P.; Maier, A.; Hall, G.; Joyce, A.; Guimont, S.; Pelot, A.; Majury, A. Microbial source tracking and spatial analysis of E. coli contaminated private well waters in southeastern Ontario. J. Water Health 2014, 12, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Krolik, J.; Maier, A.; Thompson, S.; Majury, A. Microbial source tracking of private well water samples across at-risk regions in southern Ontario and analysis of traditional fecal indicator bacteria assays including culture and qPCR. J. Water Health 2016, 14, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
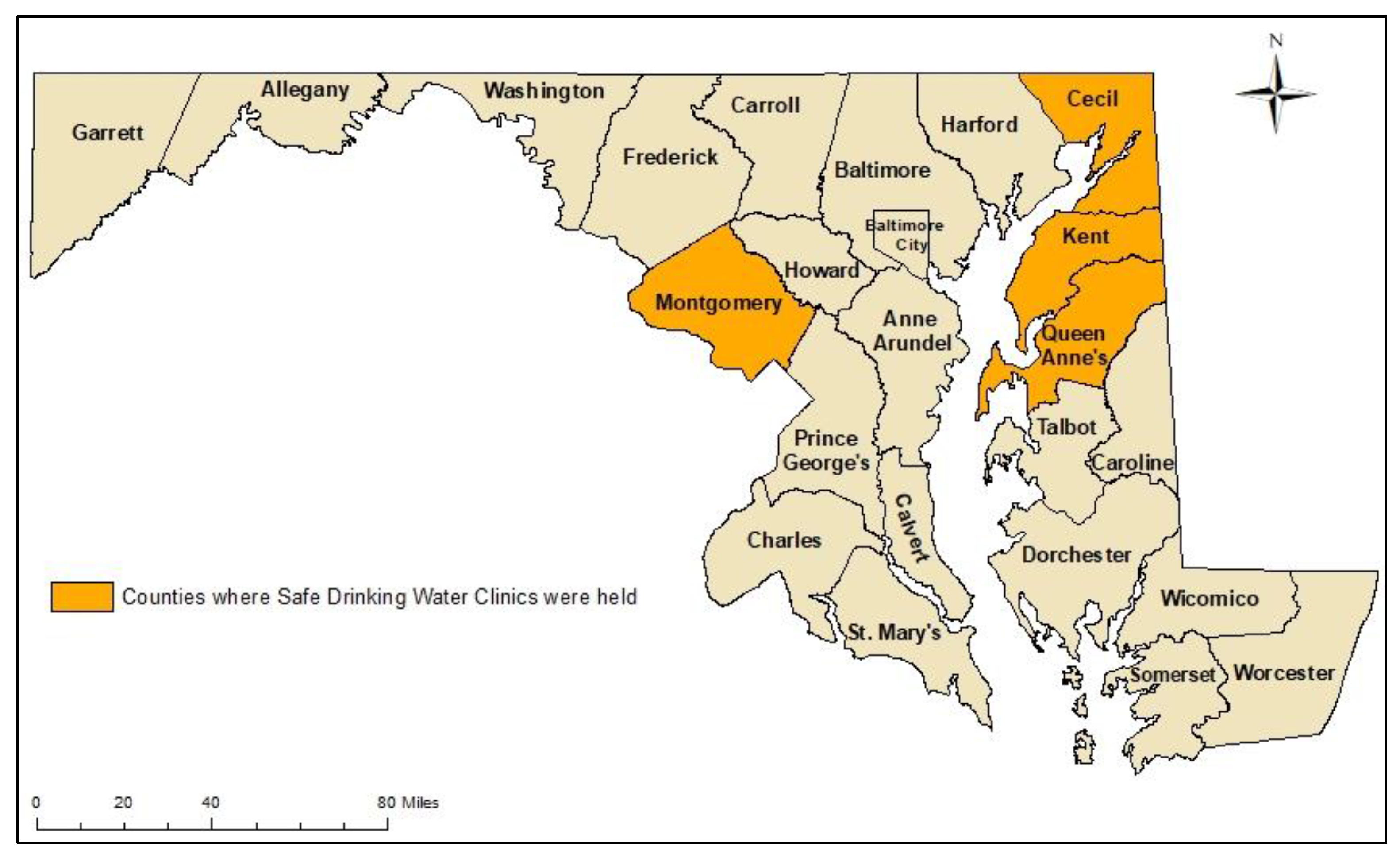
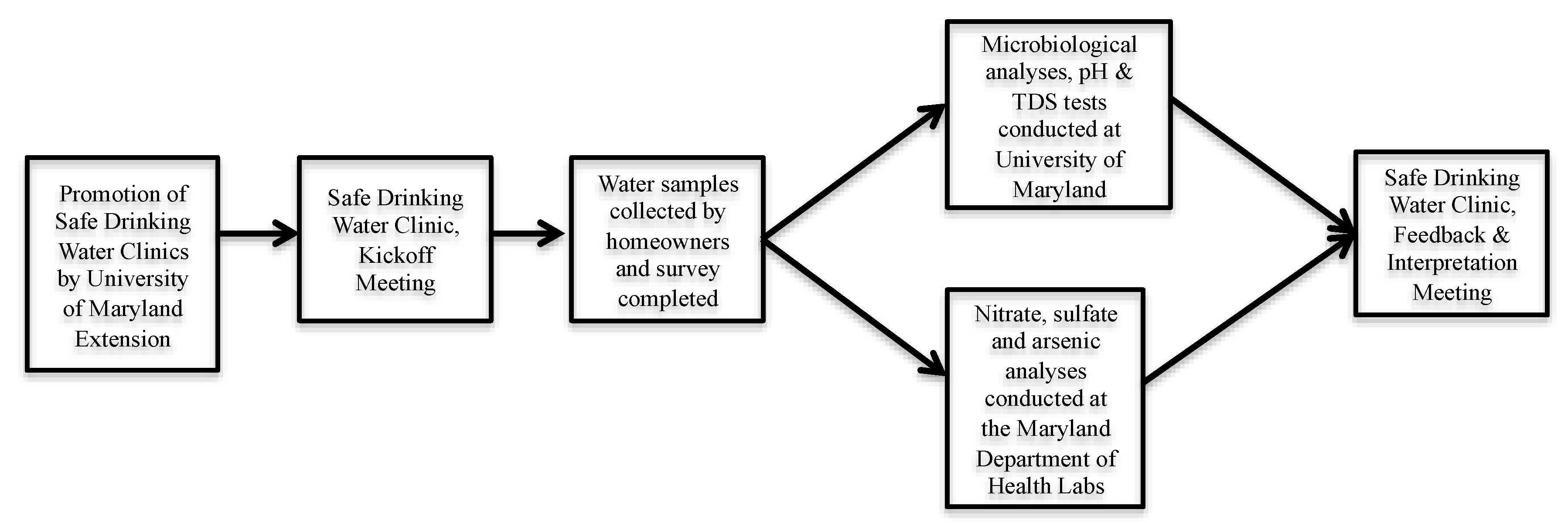
| Maryland County | Kick-Off Meeting | Interpretation Meeting |
|---|---|---|
| Cecil County I | March 2012 | May 2012 |
| Kent County | October 2012 | December 2012 |
| Montgomery | February 2013 | March 2013 |
| Cecil II | September 2013 | November 2013 |
| Queen Anne’s | February 2014 | March 2014 |
| Characteristic | Category | Number (%) (n = 118) |
|---|---|---|
| County | Cecil (1) | 25 (21.2) |
| Kent | 21 (17.8) | |
| Montgomery | 25 (21.2) | |
| Cecil (2) | 19 (16.1) | |
| Queen Anne’s | 28 (23.7) | |
| Age | 18–49 | 17 (14.4) |
| 50–59 | 29 (24.6) | |
| 60–69 | 40 (33.9) | |
| 70–79 | 23 (19.5) | |
| ≥80 | 9 (7.6) | |
| Race/Ethnicity | African American | 5 (4.2) |
| Hispanic | 1 (0.8) | |
| White | 103 (87.3) | |
| Other or Unspecified | 9 (7.6) | |
| Level of formal education | <High school | 1 (0.8) |
| High School | 10 (8.5) | |
| High school and some college | 16 (13.6) | |
| Associate’s degree | 9 (7.6) | |
| Bachelor’s degree | 35 (29.7) | |
| Graduate degree | 47 (39.8) | |
| Number of years living at current home | 1–10 years | 34 (28.8) |
| 10–20 years | 46 (39.0) | |
| More than 20 years | 34 (28.8) | |
| Unknown | 4 (3.4) | |
| Previous testing of well water quality | Never | 29 (24.6) |
| Once | 58 (49.2) | |
| Every few years | 11 (9.3) | |
| Every year | 4 (3.4) | |
| >Once per year | 1 (0.8) | |
| Other or Unsure | 12 (10.2) | |
| Experienced diarrhea within the last 30 days | Yes | 14 (11.9) |
| No | 104 (88.1) | |
| Experienced vomiting within the last 30 days | Yes | 0 (0%) |
| No | 118 (100%) |
| Total Coliforms | Fecal Coliforms | |
|---|---|---|
| Zip Code Variable | Odds Ratio (95% CI) | Odds Ratio (95% CI) |
| Cattle operations | 1.23 (0.89, 1.68) | 1.19 (0.82, 1.73) |
| Broiler operations | 0.93 (0.84, 1.03) | 1.10 (0.41, 3.00) |
| Hog operations | 0.76 (0.49, 1.17) | 0.81 (0.48, 1.37) |
| Dairy operations | 1.12 (0.96, 1.31) | 1.11 (0.93, 1.33) |
| Turkey operations | 0.92 (0.68, 1.24) | 1.24 (0.44, 3.47) |
| Aquaculture operations | 1.32 (0.59, 2.93) | 1.33 (0.52, 3.40) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murray, R.T.; Rosenberg Goldstein, R.E.; Maring, E.F.; Pee, D.G.; Aspinwall, K.; Wilson, S.M.; Sapkota, A.R. Prevalence of Microbiological and Chemical Contaminants in Private Drinking Water Wells in Maryland, USA. Int. J. Environ. Res. Public Health 2018, 15, 1686. https://doi.org/10.3390/ijerph15081686
Murray RT, Rosenberg Goldstein RE, Maring EF, Pee DG, Aspinwall K, Wilson SM, Sapkota AR. Prevalence of Microbiological and Chemical Contaminants in Private Drinking Water Wells in Maryland, USA. International Journal of Environmental Research and Public Health. 2018; 15(8):1686. https://doi.org/10.3390/ijerph15081686
Chicago/Turabian StyleMurray, Rianna T., Rachel E. Rosenberg Goldstein, Elisabeth F. Maring, Daphne G. Pee, Karen Aspinwall, Sacoby M. Wilson, and Amy R. Sapkota. 2018. "Prevalence of Microbiological and Chemical Contaminants in Private Drinking Water Wells in Maryland, USA" International Journal of Environmental Research and Public Health 15, no. 8: 1686. https://doi.org/10.3390/ijerph15081686
APA StyleMurray, R. T., Rosenberg Goldstein, R. E., Maring, E. F., Pee, D. G., Aspinwall, K., Wilson, S. M., & Sapkota, A. R. (2018). Prevalence of Microbiological and Chemical Contaminants in Private Drinking Water Wells in Maryland, USA. International Journal of Environmental Research and Public Health, 15(8), 1686. https://doi.org/10.3390/ijerph15081686





