Meta-Prediction of MTHFR Gene Polymorphism and Air Pollution on the Risks of Congenital Heart Defects Worldwide: A Transgenerational Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
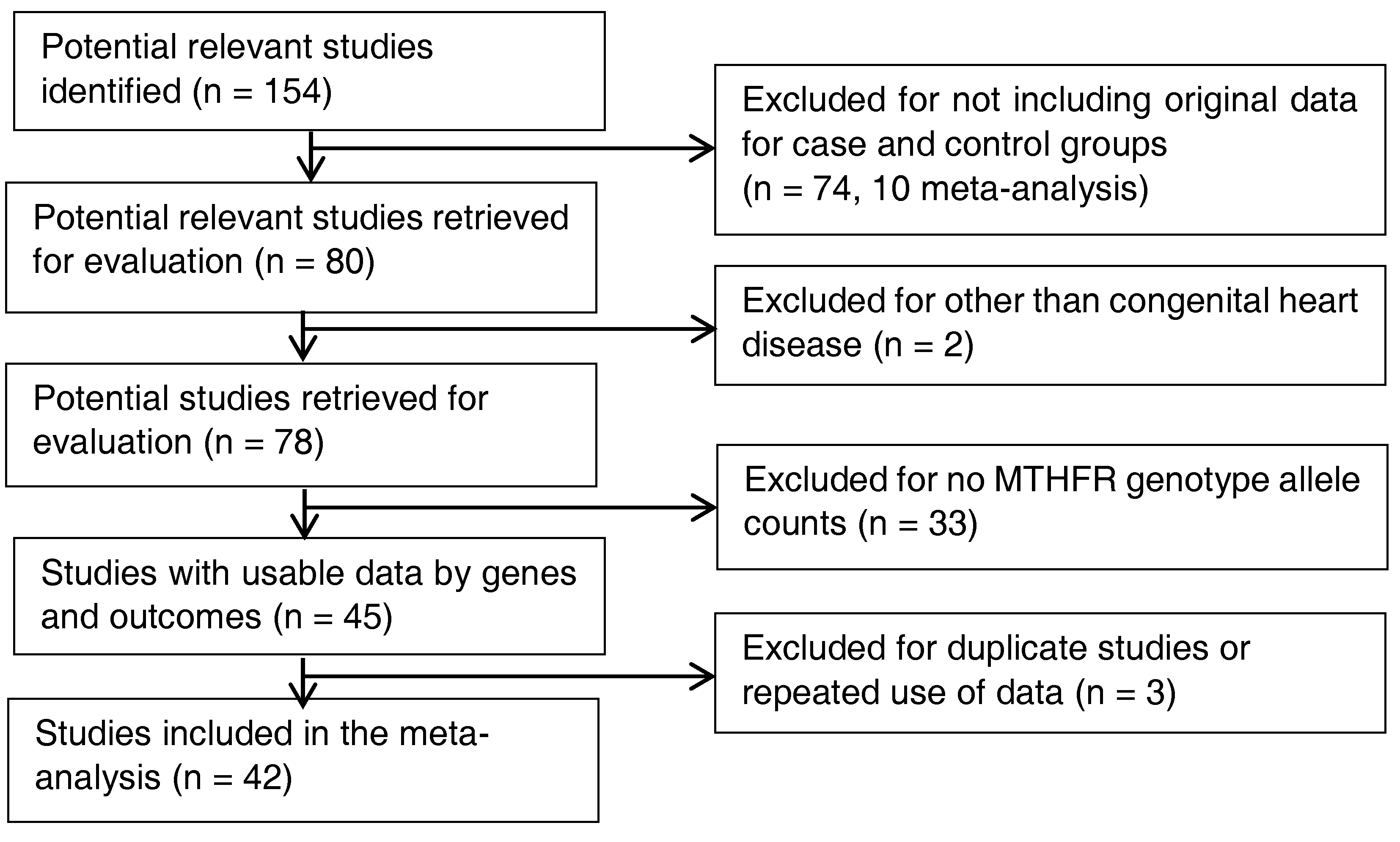
2.2. Inclusion and Exclusion Criteria-Study Identification
2.3. Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Characteristics of Original Studies
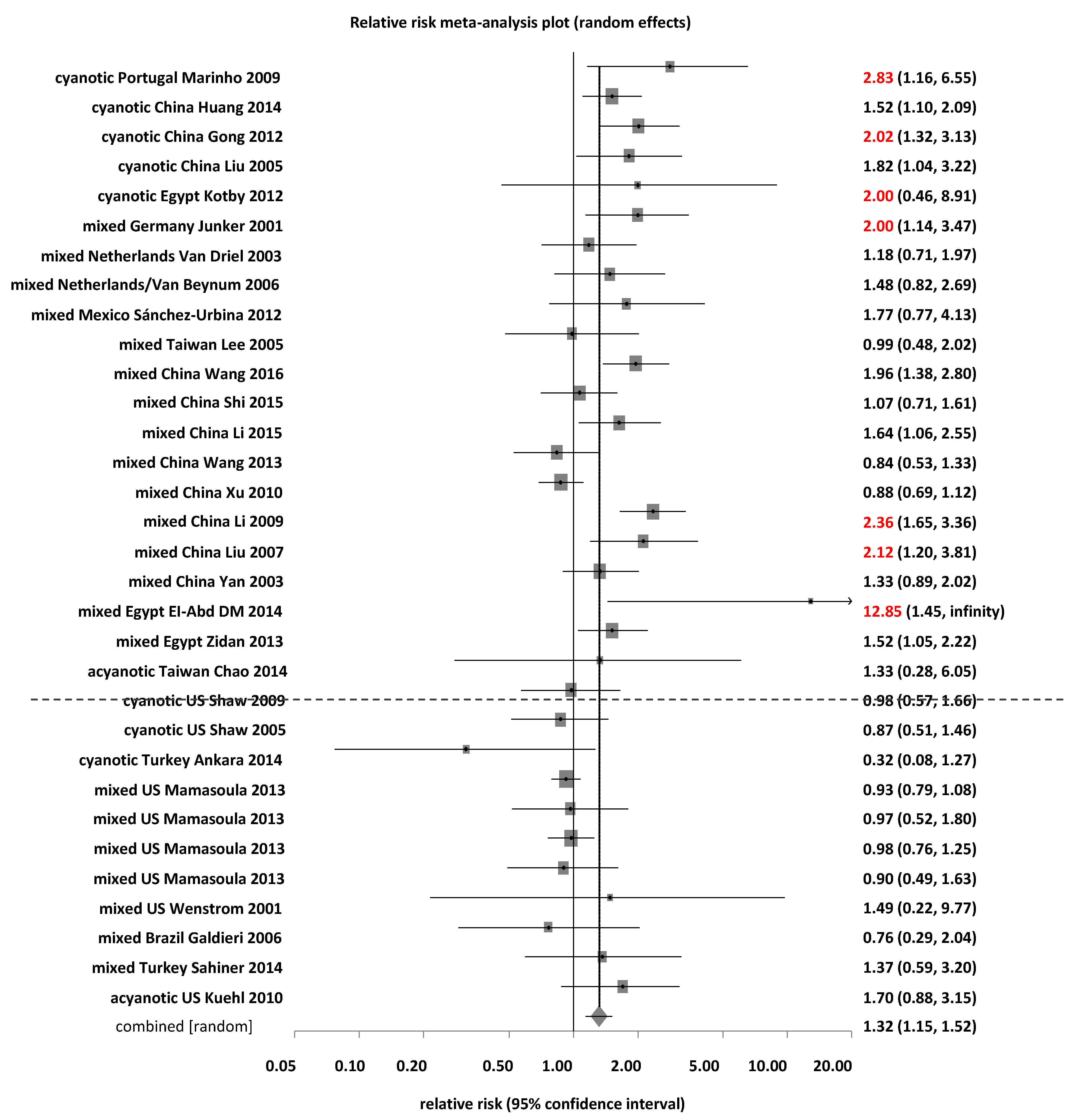
3.2. Association between MTHFR C677T and Risk of CHD
3.2.1. Subgroup Analyses by Ethnic Groups for MTHFR C677T
3.2.2. Subgroup Analyses by CHD Types for MTHFR C677T
3.2.3. Subgroup Analyses by Countries for MTHFR C677T
3.3. Association between MTHFR A1298C and Risk of CHD
3.3.1. Subgroup Analyses by Ethnic Groups and CHD Types for MTHFR A1298C
3.3.2. Subgroup Analysis by Countries for MTHFR A1298C
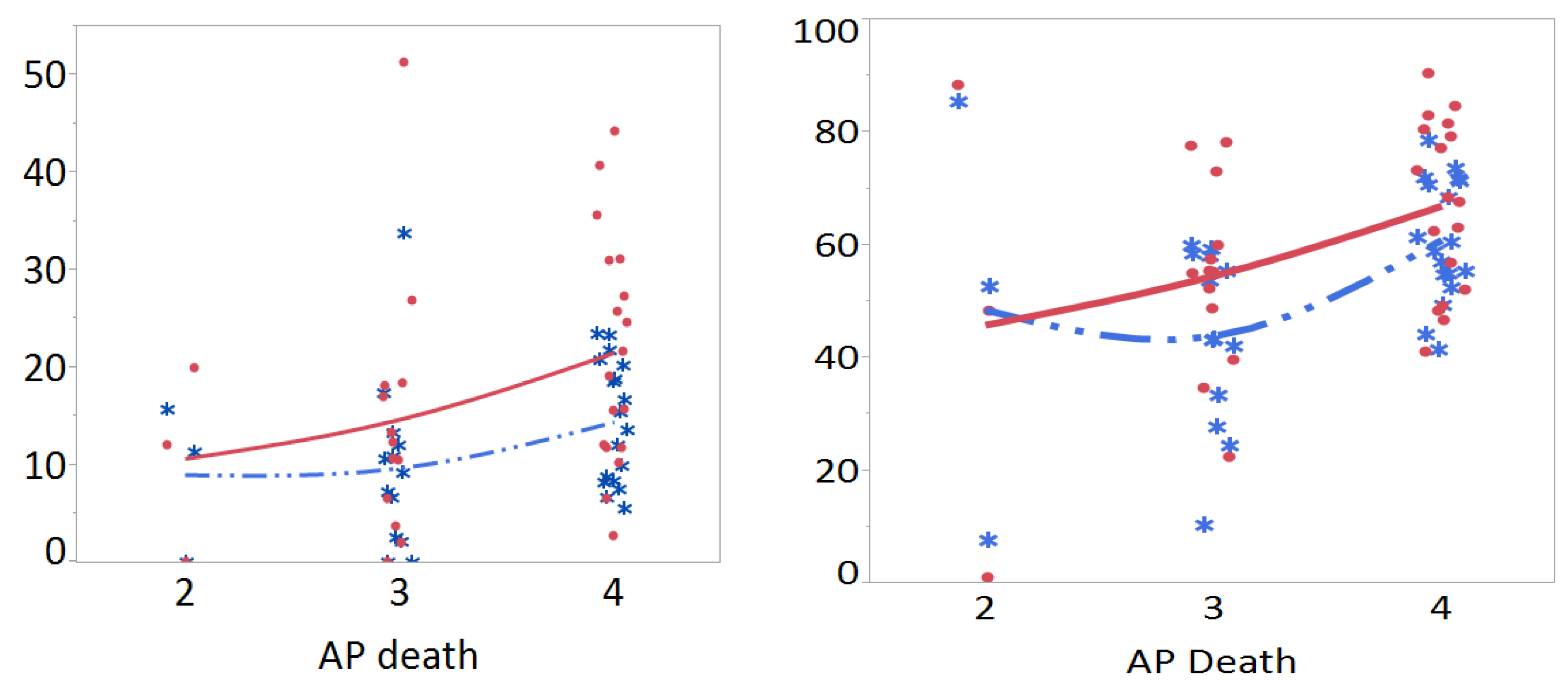
3.4. Meta-Prediction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dolk, H.; Loane, M.; Garne, E. The prevalence of congenital anomalies in Europe. Rare Dis. Epidemiol. 2010, 686, 349–364. [Google Scholar] [CrossRef]
- Van der Linde, D.; Konings, E.E.; Slager, M.A.; Witsenburg, M.; Helbing, W.A.; Takkenberg, J.J.; Roos-Hesselink, J.W. Birth prevalence of congenital heart disease worldwide. J. Am. Coll. Cardiol. 2011, 58, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Moons, P.; Bovijn, L.; Budts, W.; Belmans, A.; Gewillig, M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010, 122, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Blue, G.M.; Kirk, E.P.; Sholler, G.F.; Harvey, R.P.; Winlaw, D.S. Congenital heart disease: Current knowledge about causes and inheritance. Med. J. Aust. 2012, 197, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Fahed, A.C.; Gelb, B.D.; Seidman, J.; Seidman, C.E. Genetics of congenital heart disease: The glass half empty. Circ. Res. 2013, 112, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Vrijheid, M.; Martinez, D.; Manzanares, S.; Dadvand, P.; Schembari, A.; Rankin, J.; Nieuwenhuijsen, M. Ambient air pollution and risk of congenital anomalies: A systematic review and meta-analysis. Environ. Health Perspect. 2011, 119, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Botto, L.D.; Yang, Q. 5, 10-methylenetetrahydrofolate reductase gene variants and congenital anomalies: A HuGE review. Am. J. Epidemiol. 2000, 151, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Balion, C.; Kapur, B. Folate. Clinical utility of serum and red blood cell analysis. Clin. Lab. News 2011, 37, 8–10. [Google Scholar]
- Limpach, A.; Dalton, M.; Miles, R.; Gadson, P. Homocysteine inhibits retinoic acid synthesis: A mechanism for homocysteine-induced congenital defects. Exp. Cell Res. 2000, 260, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Frosst, P.; Blom, H.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.; Boers, G.; den Heijer, M.; Kluijtmans, L.; van den Heuve, L. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Lievers, K.J.; Boers, G.H.; Verhoef, P.; Heijer, M.; Kluijtmans, L.A.; Put, N.M.; Trijbels, F.J.; Blom, H.J. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J. Mol. Med. 2001, 79, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.; Bostom, A.; Williams, R.; Ellison, R.; Eckfeldt, J.; Rosenberg, I.; Selhub, J.; Rozen, R. Relation between folate status, a common mutation in methylenetetrahydrofolate reductase, and plasma homocysteine concentrations. Circulation 1996, 93, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Li, H.; Zhao, J.-X.; Wang, H.-W.; Wang, Y.; Ning, C.P.; Liu, Z.; Zhang, B.B.; He, G.W.; Lun, L.M. Association between MTHFR polymorphisms and congenital heart disease: A meta-analysis based on 9329 cases and 15,076 controls. Sci. Rep. 2014, 4, 7311. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jun, Y.; Zhong-Bao, R.; Jie, L.; Jian-Ming, L. Association between MTHFR C677T polymorphism and congenital heart disease. Herz 2015, 40, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hou, Z.; Wang, C.; Wei, C.; Li, Y.; Jiang, L. Association between 5, 10-methylenetetrahydrofolate reductase (MTHFR) polymorphisms and congenital heart disease: A meta-analysis. Meta Gene 2013, 1, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Dong, L.; Zheng, J.; Zhang, H.; Liu, J.; Xu, Z. Meta analysis of the association between MTHFR C677T polymorphism and the risk of congenital heart defects. Ann. Hum. Genet. 2012, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Q. MTHFR C677T and A1298C polymorphisms are not related to ventricular or atrial septal defect: A meta-analysis of 1272 cases and 1386 controls. Int. J. Clin. Exp. Med. 2016, 9, 10673–10683. [Google Scholar]
- Wang, W.; Wang, Y.; Gong, F.; Zhu, W.; Fu, S. MTHFR C677T polymorphism and risk of congenital heart defects: Evidence from 29 case-control and TDT studies. PLoS ONE 2013, 8, e58041. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Gu, H.; Gong, J.; Wang, J.; Gong, D.; Cong, X.; Chen, X.; Hu, S. Methylenetetrahydrofolate reductase C677T polymorphism and congenital heart disease: A meta-analysis. Clin. Chem. Lab. Med. 2011, 49, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Van Beynum, I.; Den Heijer, M.; Blom, H.; Kapusta, L. The MTHFR 677C→ T polymorphism and the risk of congenital heart defects: A literature review and meta-analysis. QJM 2007, 100, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Verkleij-Hagoort, A.; Bliek, J.; Sayed-Tabatabaei, F.; Ursem, N.; Steegers, E.; Steegers-Theunissen, R. Hyperhomocysteinemia and MTHFR polymorphisms in association with orofacial clefts and congenital heart defects: A meta-analysis. Am. J. Med. Genet. A 2007, 143, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chen, L.; Li, W.; Fang, Y.; Huang, G. Maternal MTHFR C677T polymorphism and congenital heart defect risk in the Chinese Han population: A meta-analysis. Genet. Mol. Res. 2013, 12, 6212–6219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Yang, H.-L.; Shiao, S.P.K. Meta-Prediction of MTHFR Gene Polymorphisms and Air Pollution on the Risk of Hypertensive Disorders in Pregnancy Worldwide. Int. J. Environ. Res. Public Health 2018, 15, 326. [Google Scholar] [CrossRef] [PubMed]
- Agay-Shay, K.; Friger, M.; Linn, S.; Peled, A.; Amitai, Y.; Peretz, C. Air pollution and congenital heart defects. Environ. Res. 2013, 124, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Proietti, E.; Röösli, M.; Frey, U.; Latzin, P. Air pollution during pregnancy and neonatal outcome: A review. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Vecoli, C.; Pulignani, S.; Andreassi, M.G. Genetic and Epigenetic Mechanisms Linking Air Pollution and Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2016, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Clark, E. Growth, morphogenesis, and function: The dynamics cardiac development. In Fetal, Neonatal and Infant Cardiac Disease; Moller, J.H., Neal, W.A., Eds.; Appleton and Lange: Norwalk, CT, USA, 1990; pp. 3–23. [Google Scholar]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Byun, H.M.; Zhong, J.; Motta, V.; Barupal, J.; Zheng, Y.; Dou, C.; Zhang, F.; McCracken, J.P.; Diaz, A. Effects of short-term exposure to inhalable particulate matter on DNA methylation of tandem repeats. Environ. Mol. Mutagen. 2014, 55, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Kloog, I.; Ridgway, B.; Koutrakis, P.; Coull, B.A.; Schwartz, J.D. Long-and short-term exposure to PM 2.5 and mortality: Using novel exposure models. Epidemiology 2013, 24, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.; Yu, C. Meta-prediction of MTHFR gene polymorphism mutations and associated risk for colorectal cancer. Biol. Res. Nurs. 2016, 18, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liang, S.; Zhao, J.; Qian, Z.; Bassig, B.A.; Yang, R.; Zhang, Y.; Hu, K.; Xu, S.; Zheng, T. Maternal exposure to air pollutant PM 2.5 and PM 10 during pregnancy and risk of congenital heart defects. J. Exp. Sci. Environ. Epidemiol. 2016, 26, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.P.; Salemi, J.L.; Stuart, A.L.; Yu, H.; Jordan, M.M.; DuClos, C.; Cavicchia, P.; Correia, J.A.; Watkins, S.M.; Kirby, R.S. Associations between exposure to ambient benzene and PM 2.5 during pregnancy and the risk of selected birth defects in offspring. Environ. Res. 2015, 142, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISAM statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. Cochrane handbook for systematic reviews of interventions. In The Cochrane Library; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Margulis, A.V.; Pladevall, M.; Riera-Guardia, N.; Varas-Lorenzo, C.; Hazell, L.; Berkman, N.D.; Viswanathan, M.; Perez-Gutthann, S. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: The Newcastle-Ottawa Scale and the RTI item bank. Clin. Epidemiol. 2014, 10, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-M.; Chen, Z.-F.; Young, L.; Shiao, S.P.K. Meta-prediction of the effect of methylenetetrahydrofolate reductase polymorphisms and air pollution on Alzheimer’s disease risk. Int. J. Environ. Res. Public Heath 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.C.; Yu, P.-J.; Shiao, S.P.K. Meta-prediction of MTHFR gene polymorphism-mutations and air pollution as risk factors for breast cancer. Nurs. Res. 2017, 66, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.-Y.A.; Young, L.; Gau, B.-S.; Shiao, S.P.K. Meta-prediction of MTHFR gene polymorphism-mutations, air pollution, and risks of leukemia among world populations. Oncotarget 2017, 8, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Wittke-Thompson, J.K.; Pluzhnikov, A.; Cox, N.J. Rational inferences about departures from hardy-weinberg equilibrium. Am. J. Hum. Genet. 2005, 76, 967–986. [Google Scholar] [CrossRef] [PubMed]
- Nurk, E.; Tell, G.S.; Refsum, H.; Ueland, P.M.; Vollset, S.E. Associations between maternal methylenetetrahydrofolate reductase polymorphisms and adverse outcomes of pregnancy: The Hordaland homocysteine study. Am. J. Med. 2004, 117, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, M.; Gong, D.; Man, C.; Chen, Y. Cardiac troponin for predicting all-cause mortality in patients with acute ischemic stroke: A meta-analysis. Biosci. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Viera, A.J. Odds ratios and risk ratios: What’s the difference and why does it matter? Southern Med. J. 2008, 101, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Higgins, J.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane handbook for Systematic Reviews of Interventions; Cochrane Book Series; Green, S., Higgens, J., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 243–296. [Google Scholar]
- WCRF-AICR Continuous Update Project. Diet, Nutrition, Physical Activity and Colorectal Cancer. World Cancer Research Fund International/American Institute for Cancer Research, 2017. Available online: http://www.aicr.org/continuous-update-project/reports/colorectal-cancer-2017-report.pdf (accessed on 1 July 2018).
- Speybroeck, N. Classification and regression trees. Int. J. Public Health 2012, 57, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In International Symposium on Information Theory; Petrov, B., Csaki, F., Eds.; Akademia Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Symonds, M.R.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar] [CrossRef]
- Mao, W.; Mu, X.; Zheng, Y.; Yan, G. Leave-one-out cross-validation-based model selection for multi-input multi-output support vector machine. Neural Comput. Appl. 2014, 24, 441–451. [Google Scholar] [CrossRef]
- Kenworthy, J.; Laube, F. Urban transport patterns in a global sample of cities & their linkages to transport infrastructure, land use, economics & environment. In World Transport. Policy & Practice; Eco-Logica Limited: Lancaster, UK, 2002; Volume 8, pp. 5–20. [Google Scholar]
- World Health Organization. The Urban Environment. 2015. Available online: http://www.who.int/heli/risks/urban/urbanenv/en/ (accessed on 27 February 2018).
- US Environmental Protection Agency, Air Quality Index Basics. Available online: https://www.airnow.gov/index.cfm?action=aqibasics.aqi (accessed on 2 January 2018).
- World Health Organization. Deaths Attributable to Urban Air Pollution. 2004. Available online: http://www.who.int/heli/risks/urban/en/uapmap.1.pdf?ua¼1 (accessed on 2 January 2018).
- World Health Organization. Global Health Risks. 2009. Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (accessed on 2 January 2018).
- World Health Organization. Global Health Risks. 2012. Available online: https://commons.wikimedia.org/wiki/File:Deaths_from_air_pollution.png (accessed on 2 January 2018).
- Tam, W.W.; Wong, T.W.; Wong, A.H.; Hui, D.S. Effect of dust storm events on daily emergency admissions for respiratory diseases. Respirology 2012, 17, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.-W. Public health risks of prolonged fine particle events associated with stagnation and air quality index based on fine particle matter with a diameter <2.5 μm in the Kaoping region of Taiwan. Int. J. Biometeorol. 2016, 60, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, L.M.; Verkleij-Hagoort, A.C.; de Jonge, R.; Uitterlinden, A.G.; Steegers, E.A.; van Duijn, C.M.; Steegers-Theunissen, R.P. Two MTHFR polymorphisms, maternal B-vitamin intake, and CHDs. Birth Defects Res. A 2008, 82, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Mamasoula, C.; Prentice, R.R.; Pierscionek, T.; Pangilinan, F.; Mills, J.L.; Druschel, C.; Pass, K.; Russell, M.W.; Hall, D.; Töpf, A. Association between c677t polymorphism of methylene tetrahydrofolate reductase and congenital heart disease: Meta-analysis of 7697 cases and 13,125 controls. Circ. Cardiovasc. Genet. 2013, 6, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Bruneau, B.G. The developmental genetics of congenital heart disease. Nature 2008, 451, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.W.-L.; Jak, S. Analyzing big data in psychology: A split/analyze/meta-analyze approach. Front. Psychol. 2016, 7, 738. [Google Scholar] [CrossRef] [PubMed]
- Padula, A.M.; Tager, I.B.; Carmichael, S.L.; Hammond, S.K.; Yang, W.; Lurmann, F.; Shaw, G.M. Ambient air pollution and traffic exposures and congenital heart defects in the San Joaquin valley of California. Paediatr. Perinat. Epidemiol. 2013, 27, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Weldy, C.S.; Liu, Y.; Liggitt, H.D.; Chin, M.T. In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice. PLoS ONE 2014, 9, e88582. [Google Scholar] [CrossRef] [PubMed]
- Anscombe, F. Graphs in statistical analysis. Am. Stat. 1973, 27, 17–21. [Google Scholar]
- Xiao, X.; White, E.P.; Hooten, M.B.; Durham, S.L. On the use of log-transformation vs. nonlinear regression for analyzing biological power laws. Ecology 2011, 92, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Packard, G. Review article: On the use of log-transformation vs. nonlinear regression for analyzing biological powerlaws. Biol. J. Linn. Soc. 2014, 113, 1167–1178. [Google Scholar] [CrossRef]
- Yu, C.H.; Lee, H.S.; Gan, S.; Brown, E. Nonlinear modeling with big data in SAS and JMP. Presented at the Western Users of SAS Software Conference, Long Beach, CA, USA, 20–22 September 2017. [Google Scholar]
- Lu, Y.; Fang, T.B. Examining personal air pollution exposure, intake, and health danger zone using time geography and 3D geovisualization. ISPRS Int. J. Geo-Inf. 2015, 4, 32–46. [Google Scholar] [CrossRef]
- Xuan, C.; Wang, B.-B.; Gao, G.; Bai, X.-Y.; Yang, Q.; Liu, X.-C.; Jing, W.-B.; Ma, X.; He, G.-W. A novel variation of plagl1 in Chinese patients with isolated ventricular septal defect. Genet. Test. Mol. Biomark. 2012, 16, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, S.; Chen, R.; Tong, X.; Wu, Z.; Mo, X. Maternal folic acid supplementation and the risk of congenital heart defects in offspring: A meta-analysis of epidemiological observational studies. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
| MTHFR 677 | All | Children | Parents | Mothers | Fathers |
|---|---|---|---|---|---|
| 58 studies (n Case/n Control) (12,347/18,106) | 35 Studies (9751/15,050) | 23 Studies (2596/3056) | 19 Studies (2038/2560) | 4 Studies (558/496) | |
| Overall (58 Studies) | Risk Type: TT, TT + CT Protective: CC | Risk Type: TT, TT + CT Protective: CC | Risk Type: TT, TT + CT Protective: CC | Risk Type: TT, TT + CT Protective: CC | Risk Type: TT |
| Subgroups | |||||
| Caucasian (14 Studies) | 14 Studies (5923/9998) Risk Type: TT, TT + CT | 7 Studies (5096/8626) Risk Type: TT + CT | 7 studies (827/1372) NS | 5 studies (567/1091) NS | 2 studies (260/281) Risk Type: TT |
| East Asian (20 Studies) | 20 Studies (3139/3113) Risk Type: TT, TT + CT Protective: CC | 13 Studies (2259/2345) Risk Type: TT, TT + CT Protective: CC | 7 studies (880/768) Risk Type: TT | 5 studies (582/553) Risk Type: TT | 2 studies (298/215) NS |
| South Asian (1 Study) | 1 Study (96/90) -- | 1 Study (96/90) -- | -- | -- | -- |
| Mixed (7 Studies) | 7 Studies (1268/1634) NS | 5 Studies (668/1252) NS | 2 studies (600/382) NS | 2 studies (600/382) NS | -- |
| Middle Eastern (3 Studies) | 3 Studies (334/313) NS | 3 Studies (334/313) NS | -- | -- | -- |
| Hispanic (5 Studies) | 5 Studies (626/1326) NS | 2 Studies (508/982) NS | 3 studies (118/344) Risk Type: TT | 3 studies (118/344) Risk Type: TT | -- |
| African (8 Studies) | 8 Studies (961/1632) Risk Type: TT, TT + CT Protective: CC | 4 Studies (790/1442) Risk Type: TT | 4 studies (171/190) NS | 4 studies (171/190) NS | -- |
| Genotypes by Race or Ethnicity (Number of Studies) | Case (N = 9751) n (%) | Control (N = 15,050) n (%) | Test of Heterogeneity | Statistical Model | Test of Association | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | p | I2 % | Risk Ratio (95% Cl) | p | |||||||
| TT (35) | 1402 | (14.4) | 1791 | (12.9) | 85.2 | <0.0001 | 62.4 | Random | 1.30 | (1.14–1.48) | 0.0001 |
| Caucasian (7) | 605 | (11.9) | 1005 | (11.7) | 16.3 | 0.0123 | 63.1 | Random | 1.23 | (0.98–1.53) | 0.069 |
| East Asian (13) | 566 | (25.1) | 429 | (18.3) | 39.4 | <0.0001 | 69.6 | Random | 1.46 | (1.17–1.82) | 0.0008 |
| South Asian (1) | 0 | (0.0) | 0 | (0.0) | - | - | - | - | 0 | - | - |
| Mixed (5) | 61 | (9.1) | 117 | (9.3) | 0.39 | 0.9831 | 0 | Fixed | 0.91 | (0.67–1.23) | 0.54 |
| Middle Eastern (3) | 16 | (4.8) | 15 | (4.8) | 2.7 | 0.0989 | 63.6 | Fixed | 0.89 | (0.43–1.81) | 0.74 |
| Hispanic (2) | 88 | (17.3) | 167 | (17.0) | 1.7 | 0.1923 | 41.2 | Fixed | 1.03 | (0.81–1.30) | 0.84 |
| African (4) | 66 | (8.4) | 58 | (4.0) | 3.7 | 0.2997 | 18.2 | Fixed | 1.44 | (1.05–1.98) | 0.026 |
| CT (35) | 4180 | (42.9) | 6311 | (41.9) | 52.3 | 0.0023 | 35.0 | Random | 1.00 | (0.96–1.05) | 0.98 |
| Caucasian (7) | 2318 | (45.5) | 3837 | (44.5) | 10.2 | 0.1154 | 41.3 | Fixed | 1.03 | (0.99–1.07) | 0.16 |
| East Asian (13) | 1065 | (47.1) | 1108 | (47.2) | 18.8 | 0.0936 | 36.1 | Fixed | 0.99 | (0.93–1.05) | 0.70 |
| South Asian (1) | 1 | (1.0) | 7 | (7.8) | - | - | - | - | 0.13 | - | - |
| Mixed (5) | 258 | (28.6) | 474 | (37.6) | 10.5 | 0.0333 | 61.8 | Random | 1.04 | (0.84–1.31) | 0.70 |
| Middle Eastern (3) | 146 | (43.7) | 137 | (43.8) | 1.0 | 0.5939 | 0 | Fixed | 1.01 | (0.85–1.21) | 0.89 |
| Hispanic (2) | 213 | (41.9) | 421 | (42.9) | 0.0 | 0.8641 | 0 | Fixed | 0.94 | (0.83–1.06) | 0.32 |
| African (4) | 179 | (22.7) | 327 | (22.7) | 4.8 | 0.1884 | 37.3 | Fixed | 0.96 | (0.82–1.13) | 0.65 |
| CC (35) | 4169 | (42.8) | 6948 | (46.2) | 124.0 | <0.0001 | 72.6 | Random | 0.90 | (0.85–0.96) | 0.0014 |
| Caucasian (7) | 2173 | (42.6) | 3784 | (43.9) | 17.8 | 0.0066 | 66.4 | Random | 0.93 | (0.84–1.03) | 0.16 |
| East Asian (13) | 628 | (27.8) | 808 | (34.5) | 50.6 | <0.0001 | 76.3 | Random | 0.75 | (0.62–0.91) | 0.0028 |
| South Asian (1) | 95 | (99.0) | 83 | (92.2) | - | - | - | - | 1.07 | - | - |
| Mixed (5) | 349 | (52.5) | 661 | (52.8) | 5.9 | 0.2079 | 32 | Fixed | 1.02 | (0.93–1.11) | 0.61 |
| Middle Eastern (3) | 172 | (51.5) | 161 | (51.4) | 1.9 | 0.3910 | 0 | Fixed | 1.00 | (0.86–1.16) | 0.996 |
| Hispanic (2) | 207 | (40.7) | 394 | (40.1) | 0.4 | 0.5497 | 0 | Fixed | 1.06 | (0.93–1.20) | 0.39 |
| African (4) | 545 | (69.0) | 1057 | (73.3) | 19.2 | 0.0002 | 84.4 | Random | 0.64 | (0.38–1.06) | 0.083 |
| TT + CT (35) | 5582 | (57.2) | 8102 | (53.8) | 115.6 | <0.0001 | 70.6 | Random | 1.09 | (1.04–1.14) | 0.0008 |
| Caucasian (7) | 2923 | (57.4) | 4842 | (56.1) | 25.9 | 0.0002 | 76.9 | Random | 1.09 | (1.00–1.19) | 0.0427 |
| East Asian (13) | 1631 | (72.2) | 1537 | (65.5) | 42.6 | <0.0001 | 71.9 | Random | 1.13 | (1.04–1.21) | 0.0022 |
| South Asian (1) | 1 | (1.0) | 7 | (7.8) | - | - | - | - | 0.13 | - | - |
| Mixed (5) | 319 | (47.8) | 591 | (47.2) | 10.7 | 0.0295 | 62.8 | Random | 1.01 | (0.84–1.22) | 0.92 |
| Middle Eastern (3) | 162 | (48.5) | 152 | (48.6) | 1.8 | 0.3990 | 0 | Fixed | 1.00 | (0.85–1.17) | 0.996 |
| Hispanic (2) | 301 | (59.3) | 588 | (59.9) | 1.1 | 0.3013 | 6.4 | Fixed | 0.96 | (0.88–1.05) | 0.395 |
| African (4) | 245 | (31.0) | 385 | (26.7) | 14.9 | 0.0019 | 79.9 | Random | 1.35 | (0.93–1.94) | 0.11 |
| Subgroups | |||||||||||
| TT risk > 1 (7 countries) | 3001 | (43.8) | 3485 | (32.0) | |||||||
| TT (21) | 704 | (23.5) | 543 | (15.6) | 46.3 | 0.0007 | 56.8 | Random | 1.51 | (1.28–1.79) | <0.0001 |
| CT (21) | 1384 | (46.1) | 1601 | (45.9) | 25.7 | 0.1755 | 22.2 | Fixed | 0.99 | (0.94–1.05) | 0.8242 |
| CC (21) | 913 | (30.4) | 1341 | (38.5) | 66.6 | <0.0001 | 70.0 | Random | 0.76 | (0.66–0.87) | <0.0001 |
| TT + CT (21) | 2088 | (69.6) | 2144 | (61.5) | 58.3 | <0.0001 | 65.7 | Random | 1.14 | (1.07–1.21) | <0.0001 |
| TT risk < 1 (3 countries) | 3624 | (53.0) | 7182 | (66.0) | |||||||
| TT (11) | 373 | (10.3) | 781 | (10.9) | 6.4 | 0.7776 | 0 | Fixed | 0.95 | (0.84–1.07) | 0.37 |
| CT (11) | 1397 | (38.5) | 2833 | (39.4) | 17.8 | 0.0580 | 43.9 | Fixed | 0.98 | (0.929–1.03) | 0.34 |
| CC (11) | 1854 | (51.2) | 3568 | (49.7) | 15.2 | 0.1239 | 34.3 | Fixed | 1.03 | (0.99–1.07) | 0.13 |
| TT + CT (11) | 1770 | (48.8) | 3614 | (50.3) | 30.3 | 0.0008 | 67 | Random | 1.00 | (0.92–1.10) | 0.97 |
| TT risk varied (2 countries) | 219 | (3.2) | 215 | (2.0) | |||||||
| TT (2) | 0 | (0.0) | 0 | (0.0) | - | - | - | - | - | - | - |
| CT (2) | 61 | (27.9) | 61 | (28.4) | 4.3 | 0.0384 | 76.7 | Random | 0.50 | (0.06–4.08) | 0.50 |
| CC (2) | 158 | (72.1) | 154 | (71.6) | 6.0 | 0.0139 | 83.5 | Random | 1.00 | (0.74–1.33) | 0.98 |
| TT + CT (2) | 61 | (27.9) | 61 | (28.4) | 4.3 | 0.0384 | 76.7 | Random | 0.50 | (0.06–4.08) | 0.51 |
| MTHFR A1298C | All | Children | Parents | Mothers |
|---|---|---|---|---|
| 21 Studies (n Case/n Control) (2754/3419) | 13 Studies (1835/2003) | 8 Studies (919/1416) | 7 Studies (691/1165) | |
| Overall (21 Studies) | Risk Type: CC, CC + AC | Risk Type: CC, CC + AC | NS | NS |
| Subgroups | ||||
| Caucasian (5 Studies) | 5 Studies (838/1315) Protective: AC | 1 Study (229/251) -- | 4 Studies (609/1064) Protective: CC | 3 Studies (381/813) NS |
| East Asian (7 Studies) | 7 Studies (1290/1528) Risk Type: CC | 6 Studies (1137/1312) NS | 1 Study (153/216) -- | 1 Study (153/216) -- |
| South Asian (1 Study) | 1 Study (96/100) -- | 1 Study (96/100) -- | 0 -- | 0 -- |
| Mixed (2 Studies) | 2 Studies (104/64) NS | 1 Study (57/38) -- | 1 Study (47/26) -- | 1 Study (47/26) -- |
| Middle Eastern (2 Studies) | 2 Studies (206/192) Risk Type: CC | 2 Studies (206/192) Risk Type: CC | 0 -- | 0 -- |
| African (4 Studies) | 4 Studies (220/220) Risk Type: CC, CC + AC Protective: AA | 2 Studies (110/110) Risk Type: CC + AC Protective: AA | 2 Studies (110/110) Risk Type: CC + AC Protective: AA | 2 Studies (110/110) Risk Type: CC + AC Protective: AA |
| Partition Tree | Tukey Test | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | AICc | AP Death | Count | Mean | SD | Levels Compared | D | SED | Lower CI | Upper CI | p |
| TT % ct | 237.40 | 2, 3 | 16 | 9.58 | 8.58 | 4/2 | 5.42 | 4.69 | −6.13 | 16.97 | 0.49 |
| 4 | 18 | 14.45 | 6.19 | 4/3 | 4.75 | 2.74 | −1.99 | 11.49 | 0.21 | ||
| 3/2 | 0.67 | 4.82 | −11.2 | 12.53 | 0.99 | ||||||
| TT % CHD | 271.23 | 2, 3 | 16 | 13.95 | 12.54 | 4/2 | 10.86 | 7.69 | −8.06 | 29.78 | 0.35 |
| 4 | 18 | 21.57 | 11.86 | 4/3 | 6.87 | 4.49 | −4.17 | 17.91 | 0.29 | ||
| 3/2 | 3.99 | 7.89 | −15.44 | 23.42 | 0.87 | ||||||
| CT % ct | 271.28 | 3, 2 | 16 | 35.16 | 16.10 | 4/3 | 12.36 | 4.47 | 1.36 | 23.37 | 0.03 |
| 4 | 18 | 46.51 | 7.14 | 4/2 | 6.91 | 7.66 | −11.94 | 25.76 | 0.64 | ||
| 2/3 | 5.45 | 7.87 | −13.91 | 24.82 | 0.77 | ||||||
| CT % CHD | 271.16 | 2, 3 | 16 | 39.00 | 15.60 | 4/2 | 10.26 | 7.67 | −8.61 | 29.13 | 0.39 |
| 4 | 18 | 45.42 | 8.00 | 4/3 | 5.54 | 4.47 | −5.47 | 16.56 | 0.44 | ||
| 3/2 | 4.72 | 7.87 | −14.66 | 24.10 | 0.82 | ||||||
| TT + CT % ct | 289.28 | 2, 3 | 16 | 44.72 | 20.19 | 4/3 | 17.14 | 5.85 | 2.73 | 31.55 | 0.02 |
| 4 | 18 | 60.96 | 10.74 | 4/2 | 12.33 | 10.03 | −12.36 | 37.02 | 0.45 | ||
| 2/3 | 4.81 | 10.30 | −20.55 | 30.16 | 0.89 | ||||||
| TT + CT % | 300.01 | 2, 3 | 16 | 52.94 | 21.86 | 4/2 | 21.11 | 11.69 | −7.66 | 49.88 | 0.18 |
| CHD | 4 | 18 | 66.99 | 15.16 | 4/3 | 12.42 | 6.82 | −4.37 | 29.21 | 0.18 | |
| 3/2 | 8.69 | 12.01 | −20.86 | 38.23 | 0.75 | ||||||
| RR TT | 56.12 | 2, 3 | 13 | 1.30 | 0.46 | 4/2 | 0.24 | 0.42 | −0.80 | 1.28 | 0.84 |
| 4 | 18 | 1.50 | 0.61 | 4/3 | 0.20 | 0.22 | −0.33 | 0.73 | 0.63 | ||
| 3/2 | 0.04 | 0.43 | −1.03 | 1.11 | 0.996 | ||||||
| RR CT | 62.47 | 2, 3 | 16 | 1.23 | 0.81 | 3/2 | 0.68 | 0.35 | −0.18 | 1.54 | 0.14 |
| 4 | 18 | 0.98 | 0.15 | 3/4 | 0.37 | 0.20 | −0.11 | 0.86 | 0.16 | ||
| 4/2 | 0.31 | 0.34 | −0.53 | 1.14 | 0.64 | ||||||
| RR TT + CT | 57.95 | 2, 3 | 16 | 1.29 | 0.76 | 3/2 | 0.74 | 0.32 | −0.05 | 1.52 | 0.07 |
| 4 | 18 | 1.10 | 0.15 | 4/2 | 0.40 | 0.31 | −0.36 | 1.17 | 0.41 | ||
| 3/4 | 0.33 | 0.18 | −0.11 | 0.78 | 0.18 | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-L.; Yang, Y.-L.; Yu, C.H.; Shiao, S.P.K. Meta-Prediction of MTHFR Gene Polymorphism and Air Pollution on the Risks of Congenital Heart Defects Worldwide: A Transgenerational Analysis. Int. J. Environ. Res. Public Health 2018, 15, 1660. https://doi.org/10.3390/ijerph15081660
Yang H-L, Yang Y-L, Yu CH, Shiao SPK. Meta-Prediction of MTHFR Gene Polymorphism and Air Pollution on the Risks of Congenital Heart Defects Worldwide: A Transgenerational Analysis. International Journal of Environmental Research and Public Health. 2018; 15(8):1660. https://doi.org/10.3390/ijerph15081660
Chicago/Turabian StyleYang, Hsiao-Ling, Ya-Ling Yang, Chong Ho Yu, and S. Pamela K. Shiao. 2018. "Meta-Prediction of MTHFR Gene Polymorphism and Air Pollution on the Risks of Congenital Heart Defects Worldwide: A Transgenerational Analysis" International Journal of Environmental Research and Public Health 15, no. 8: 1660. https://doi.org/10.3390/ijerph15081660
APA StyleYang, H.-L., Yang, Y.-L., Yu, C. H., & Shiao, S. P. K. (2018). Meta-Prediction of MTHFR Gene Polymorphism and Air Pollution on the Risks of Congenital Heart Defects Worldwide: A Transgenerational Analysis. International Journal of Environmental Research and Public Health, 15(8), 1660. https://doi.org/10.3390/ijerph15081660






