Adsorption of Trace Estrogens in Ultrapure and Wastewater Treatment Plant Effluent by Magnetic Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of MGO
2.3. Adsorption Experiments in Estrogen-Spiked Ultrapure Water
2.4. Adsorption Experiments in WWTPs Effluent
2.5. HPLC-MS/MS Analysis for Estrogens
3. Results and Discussion
3.1. Characterization of Adsorbent
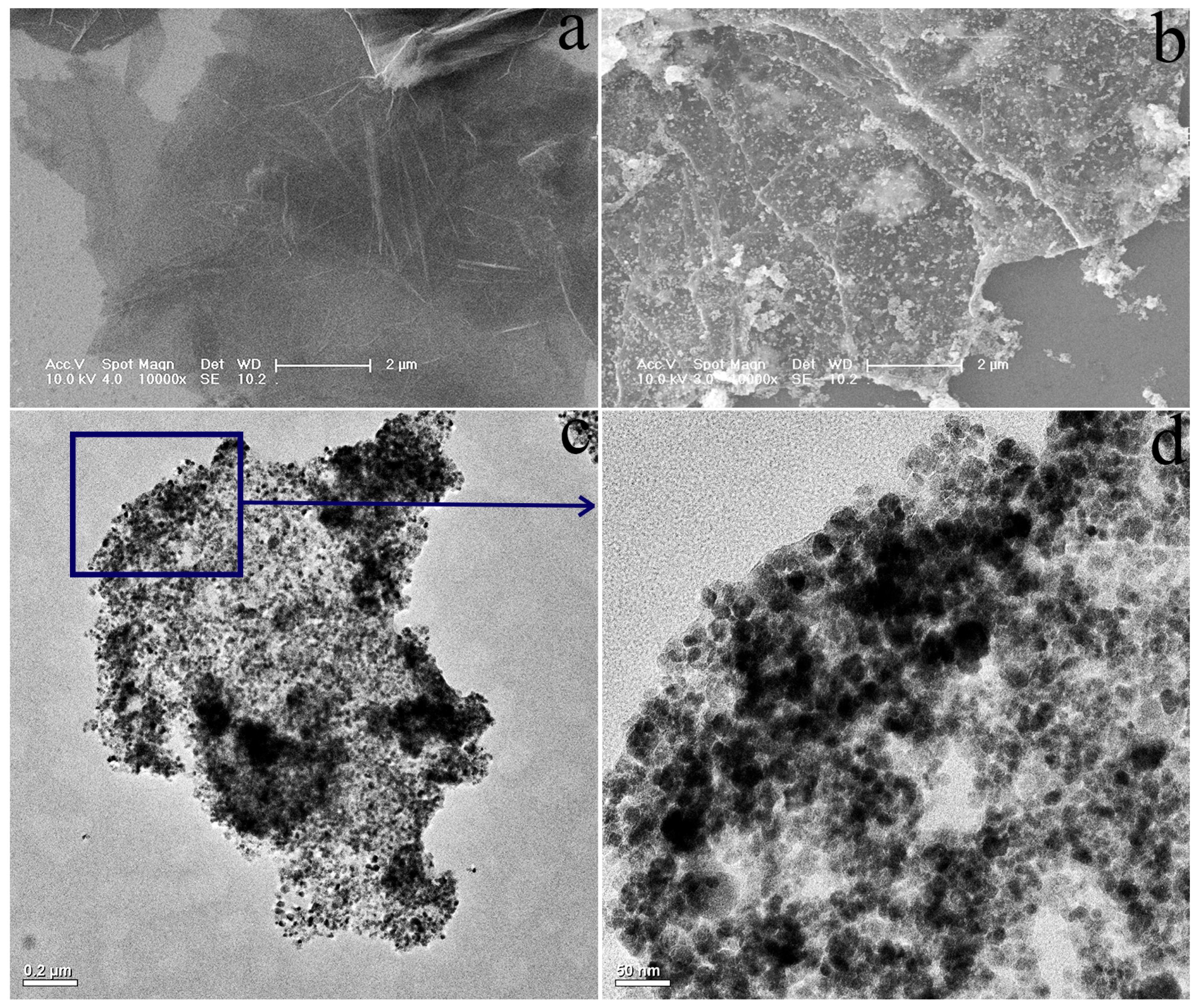
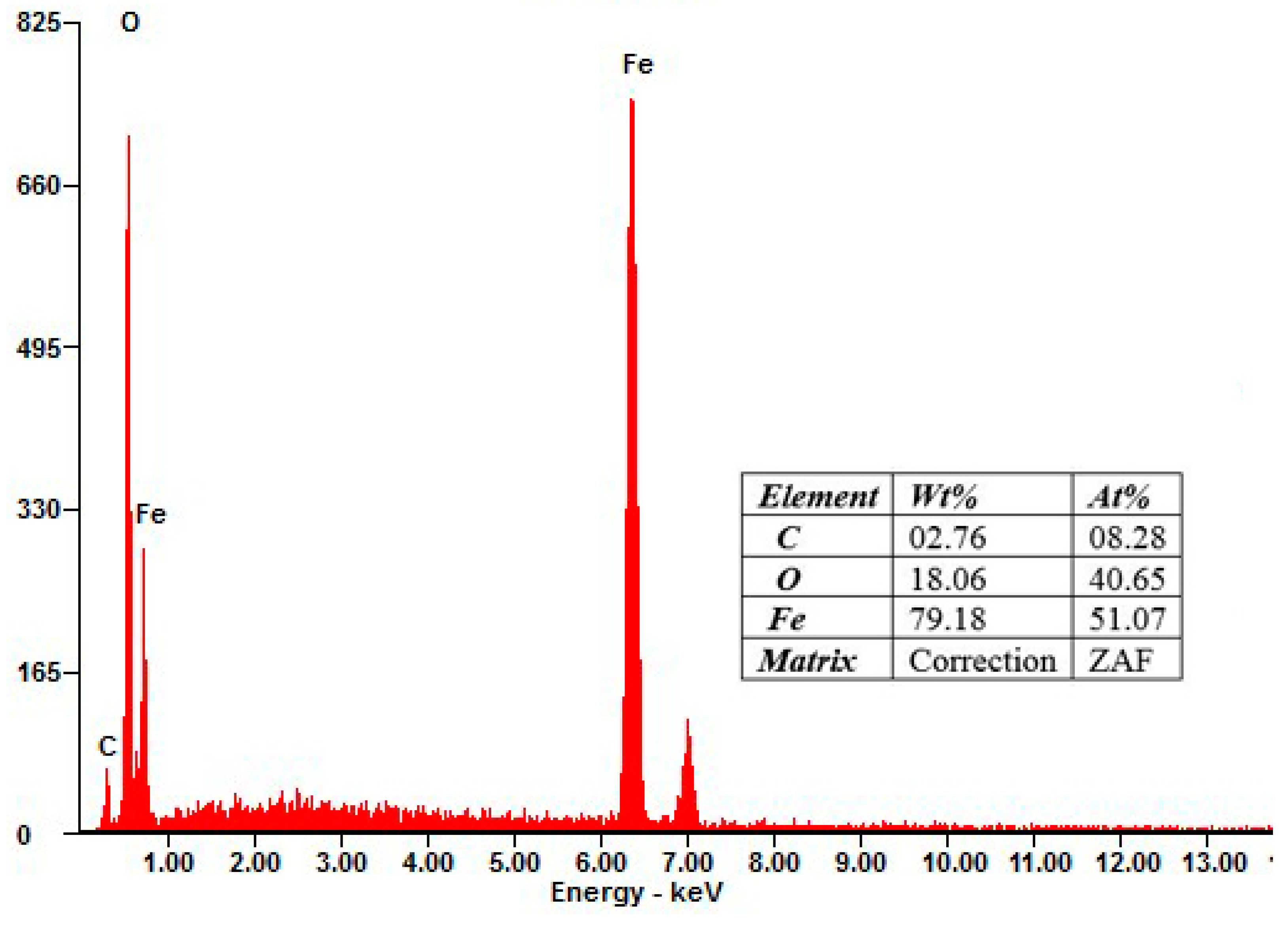
3.1.1. SEM
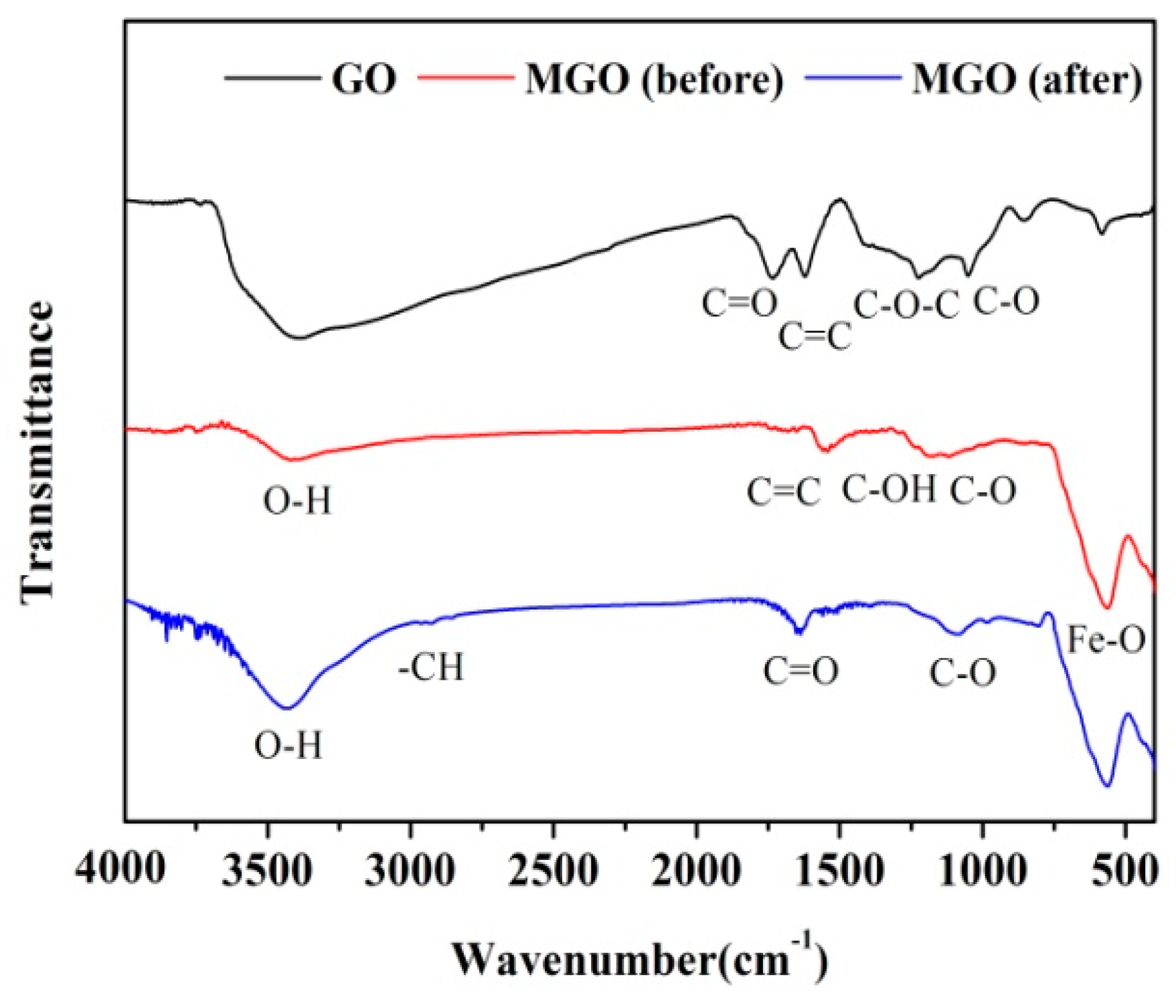
3.1.2. FTIR Spectroscopy
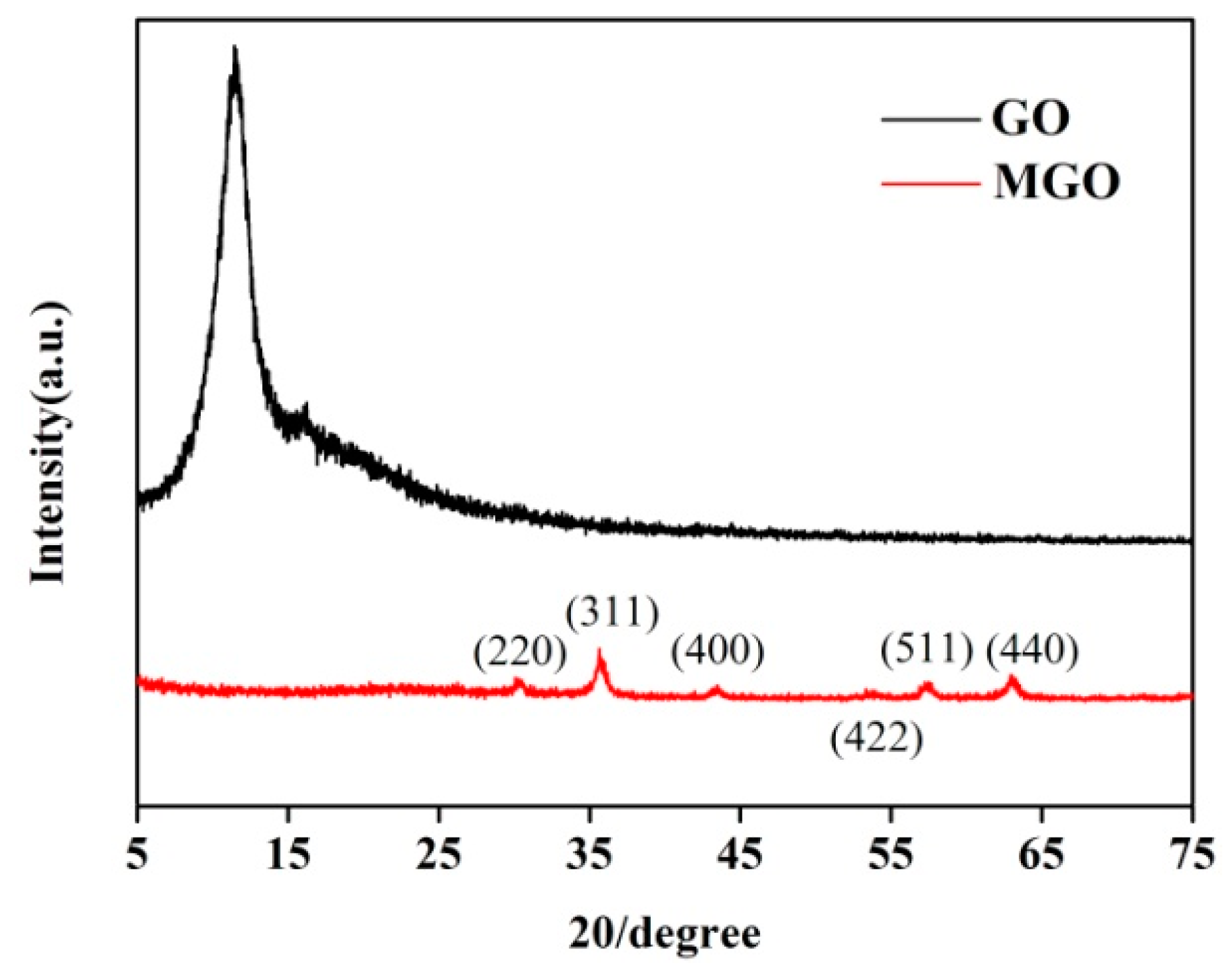
3.1.3. XRD
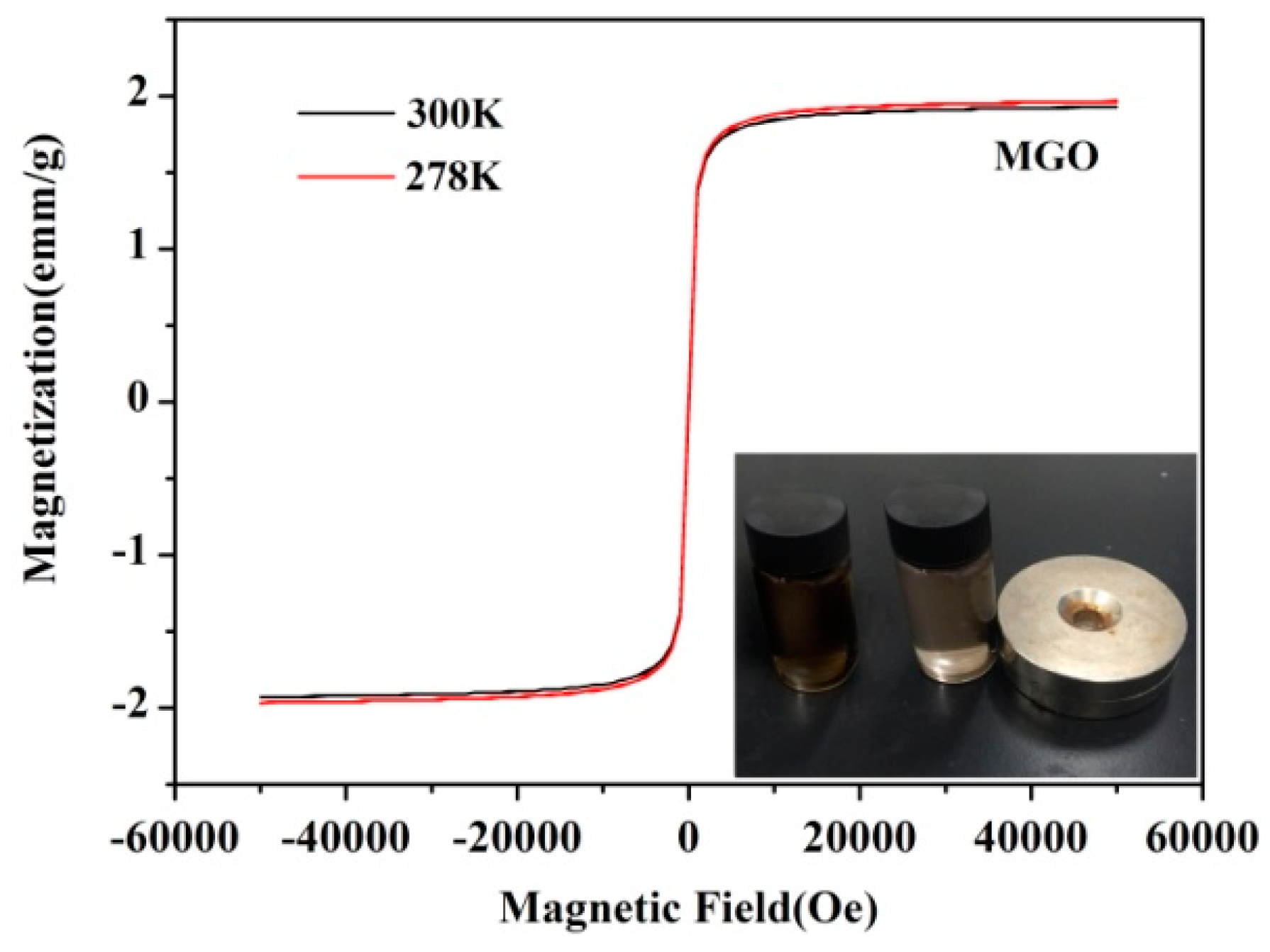
3.1.4. Magnetization
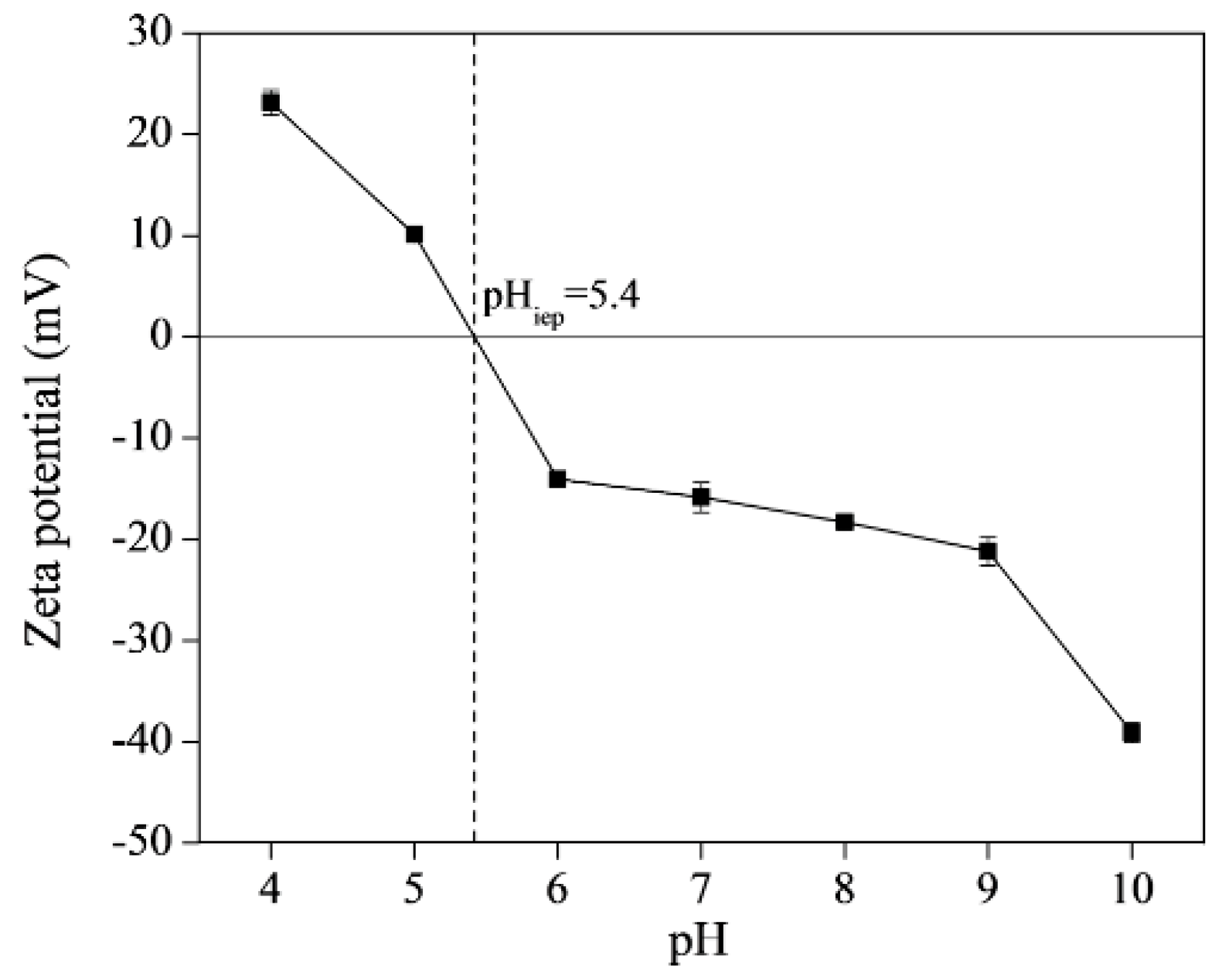
3.2. Effect of Initial Solution pH
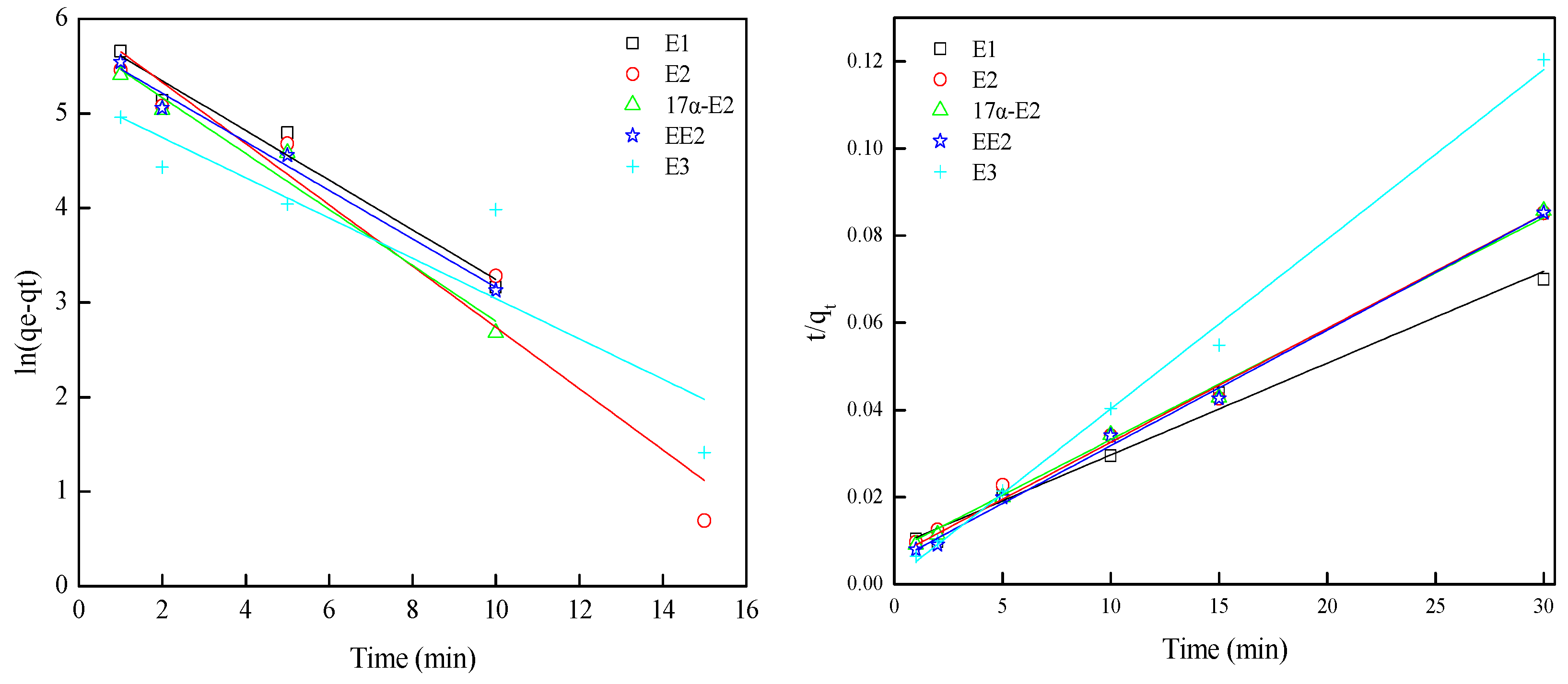
3.3. Adsorption Kinetics
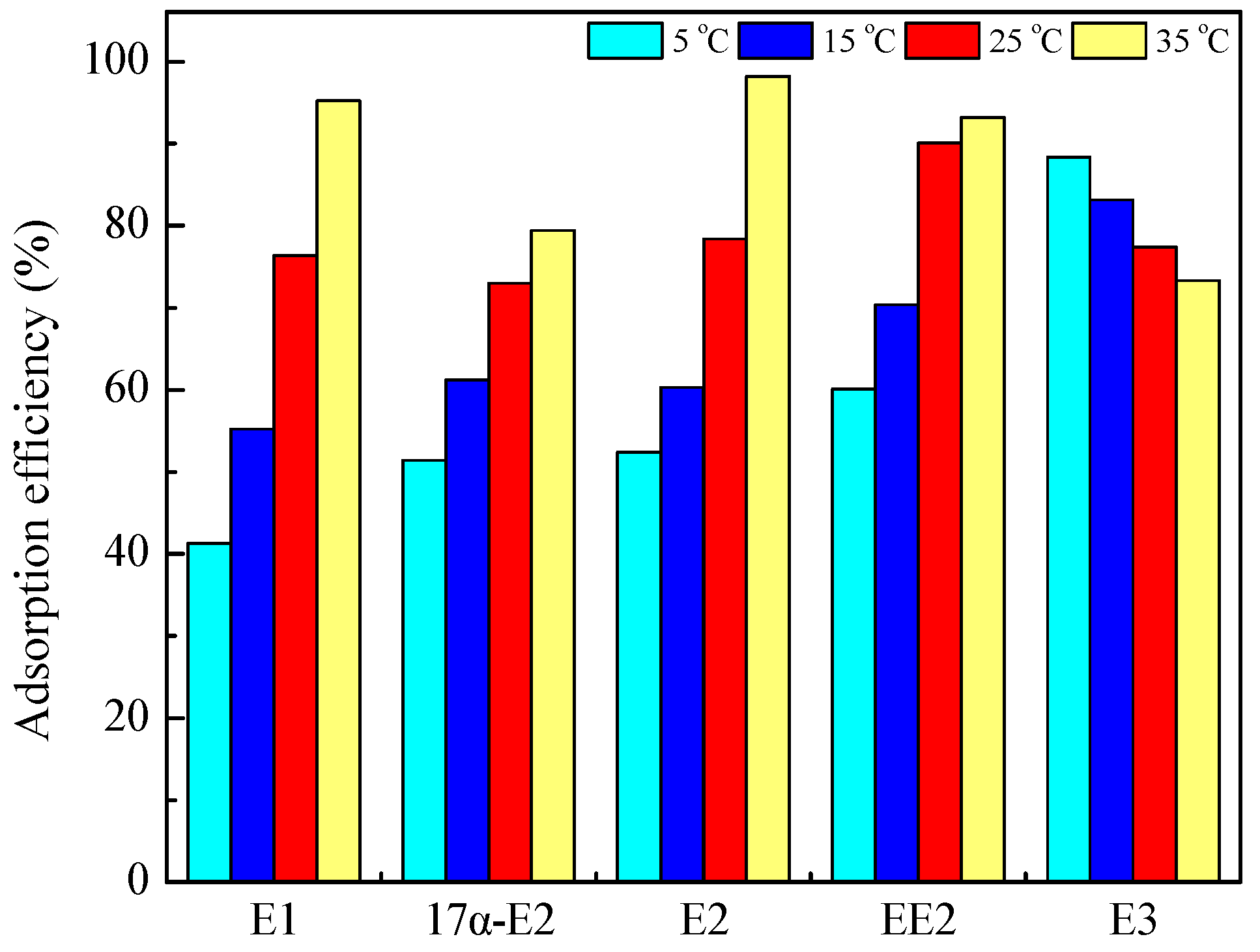
3.4. Effect of Temperature
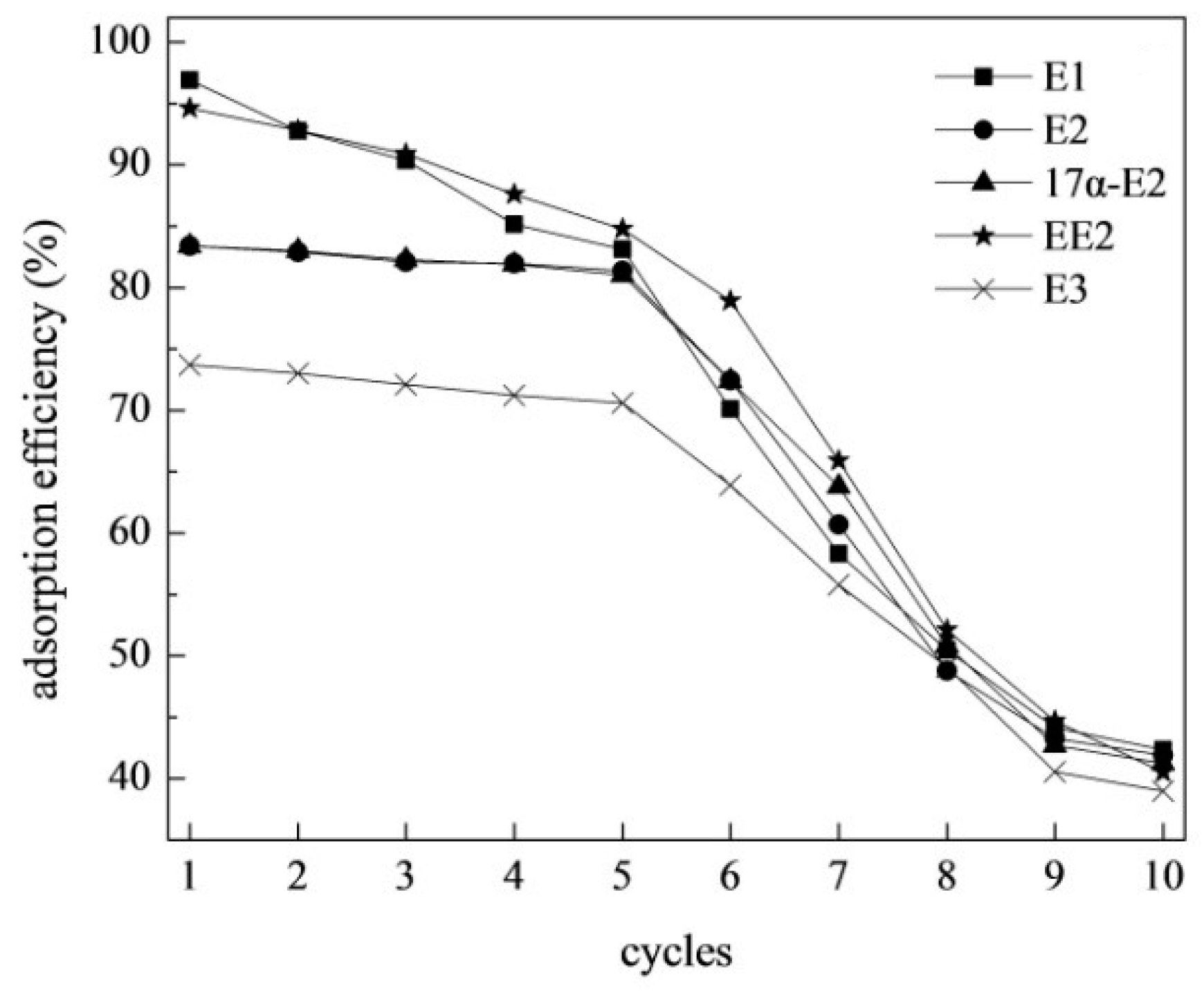
3.5. Regeneration and Reusability
3.6. Application to WWTP Effluent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- China Urban Construction Statistics Yearbook. 2016. Available online: http://www.mohurd.gov.cn/xytj/tjzljsxytjgb/jstjnj/index.html (accessed on 5 January 2018).
- Chen, W.; Lu, S.; Jiao, W.; Wang, M.; Chang, A.C. Reclaimed water: A safe irrigation water source? Environ. Dev. 2013, 8, 74–83. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, W.; Zhang, W.; Fan, Y.; Jiao, W. Wastewater reclamation and reuse in China: Opportunities and challenges. J. Environ. Sci. 2016, 39, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, W.; Cao, Z.; Fu, X.; Zhu, T. Occurrence of endocrine-disrupting compounds in reclaimed water from Tianjin, China. Anal. Bioanal. Chem. 2005, 383, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Li, Q.; Wang, X.C.; Wang, Y.; Wang, D.; Ngo, H.H. Micropollutants removal and health risk reduction in a water reclamation and ecological reuse system. Water Res. 2018, 138, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Sun, J.; Li, Y.; Lun, X.; Shan, D.; Nie, C.; Liu, M. 17α-Ethynylestradiol biodegradation in different river-based groundwater recharge modes with reclaimed water and degradation-associated community structure of bacteria and archaea. J. Environ. Sci. 2018, 64, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Estévez, E.; del Carmen Cabrera, M.; Molina-Díaz, A.; Robles-Molina, J.; del Pino Palacios-Díaz, M. Screening of emerging contaminants and priority substances (2008/105/EC) in reclaimed water for irrigation and groundwater in a volcanic aquifer (Gran Canaria, Canary Islands, Spain). Sci. Total Environ. 2012, 433, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, X.; Li, M.; Ma, Y.; Wang, J.; Liu, X. Occurrence and risk assessment of pharmaceuticals and personal care products and endocrine disrupting chemicals in reclaimed water and receiving groundwater in China. Ecotoxicol. Environ. Saf. 2015, 119, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Plahuta, M.; Tišler, T.; Toman, M.J.; Pintar, A. Toxic and endocrine disrupting effects of wastewater treatment plant influents and effluents on a freshwater isopod Asellus aquaticus (Isopoda, Crustacea). Chemosphere 2017, 174, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.F.; Praveena, S.M. Sources, mechanisms, and fate of steroid estrogens in wastewater treatment plants: A mini review. Environ. Monit. Assess. 2017, 189, 178. [Google Scholar] [CrossRef] [PubMed]
- Servos, M.R.; Bennie, D.T.; Burnison, B.K.; Jurkovic, A.; McInnis, R.; Neheli, T.; Schnell, A.; Seto, P.; Smyth, S.A.; Ternes, T.A. Distribution of estrogens, 17β-estradiol and estrone, in Canadian municipal wastewater treatment plants. Sci. Total Environ. 2005, 336, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Pavagadhi, S.; Tang, A.L.; Sathishkumar, M.; Loh, K.P.; Balasubramanian, R. Removal of microcystin-LR and microcystin-RR by graphene oxide: Adsorption and kinetic experiments. Water Res. 2013, 47, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Rodrigues, D.F. Investigation of acute effects of graphene oxide on wastewater microbial community: A case study. J. Hazard. Mater. 2013, 256–257, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Mazumdar, S.; Singh, S.K. Magnetic adsorbents for the treatment of water/wastewater—A review. J. Water Process Eng. 2015, 7, 244–265. [Google Scholar] [CrossRef]
- Altıntıg, E.; Altundag, H.; Tuzen, M.; Sarı, A. Effective removal of methylene blue from aqueous solutions using magnetic loaded activated carbon as novel adsorbent. Chem. Eng. Res. Des. 2017, 122, 151–163. [Google Scholar] [CrossRef]
- Sen, T.; Nomura, S.; Nishioka, H.; Sen, T. Synthesis and arsenic adsorption characteristics of a novel magnetic adsorbent. J. Environ. Conserv. Eng. 2017, 46, 156–162. [Google Scholar]
- Zhu, S.; Dong, G.; Yu, Y.; Yang, J.; Yang, W.; Fan, W.; Zhou, D.; Liu, J.; Zhang, L.; Huo, M. Hydrothermal synthesis of a magnetic adsorbent from wasted iron mud for effective removal of heavy metals from smelting wastewater. Environ. Sci. Pollut. Res. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dhoble, R.M.; Maddigapu, P.R.; Rayalu, S.S.; Bhole, A.; Dhoble, A.S.; Dhoble, S.R. Removal of arsenic(III) from water by magnetic binary oxide particles (MBOP): Experimental studies on fixed bed column. J. Hazard. Mater. 2017, 322, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Drenkova-Tuhtan, A.; Schneider, M.; Franzreb, M.; Meyer, C.; Gellermann, C.; Sextl, G.; Mandel, K.; Steinmetz, H. Pilot-scale removal and recovery of dissolved phosphate from secondary wastewater effluents with reusable ZnFeZr adsorbent@ Fe3O4/SiO2 particles with magnetic harvesting. Water Res. 2017, 109, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, S.; Li, J. Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]
- Deng, J.-H.; Zhang, X.-R.; Zeng, G.-M.; Gong, J.-L.; Niu, Q.-Y.; Liang, J. Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem. Eng. J. 2013, 226, 189–200. [Google Scholar] [CrossRef]
- Hu, X.-J.; Liu, Y.-G.; Wang, H.; Chen, A.-W.; Zeng, G.-M.; Liu, S.-M.; Guo, Y.-M.; Hu, X.; Li, T.-T.; Wang, Y.-Q.; et al. Removal of Cu(II) ions from aqueous solution using sulfonated magnetic graphene oxide composite. Sep. Purif. Technol. 2013, 108, 189–195. [Google Scholar] [CrossRef]
- Liu, M.; Chen, C.; Hu, J.; Wu, X.; Wang, X. Synthesis of Magnetite/Graphene Oxide Composite and Application for Cobalt(II) Removal. J. Phys. Chem. C 2011, 115, 25234–25240. [Google Scholar] [CrossRef]
- Bao, C.; Song, L.; Xing, W.; Yuan, B.; Wilkie, C.A.; Huang, J.; Guo, Y.; Hu, Y. Preparation of graphene by pressurized oxidation and multiplex reduction and its polymer nanocomposites by masterbatch-based melt blending. J. Mater. Chem. 2012, 22, 6088–6096. [Google Scholar] [CrossRef]
- Yang, X.; Chen, C.; Li, J.; Zhao, G.; Ren, X.; Wang, X. Graphene oxide-iron oxide and reduced graphene oxide-iron oxide hybrid materials for the removal of organic and inorganic pollutants. RSC Adv. 2012, 2, 8821. [Google Scholar] [CrossRef]
- Kumar, A.K.; Mohan, S.V.; Sarma, P. Sorptive removal of endocrine-disruptive compound (estriol, E3) from aqueous phase by batch and column studies: Kinetic and mechanistic evaluation. J. Hazard. Mater. 2009, 164, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Liu, Q.-H.; Qi, L.; Liu, H.-H.; Wang, H.-Y. Progress in research on manganese dioxide electrode materials for electrochemical capacitors. Chin. J. Anal. Chem. 2012, 40, 339–346. [Google Scholar] [CrossRef]
- Gabet-Giraud, V.; Miège, C.; Choubert, J.; Ruel, S.M.; Coquery, M. Occurrence and removal of estrogens and beta blockers by various processes in wastewater treatment plants. Sci. Total Environ. 2010, 408, 4257–4269. [Google Scholar] [CrossRef] [PubMed]
- Nakada, N.; Yasojima, M.; Okayasu, Y.; Komori, K.; Tanaka, H.; Suzuki, Y. Fate of oestrogenic compounds and identification of oestrogenicity in a wastewater treatment process. Water Sci. Technol. 2006, 53, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Qiang, Z.; Zhang, H.; Ben, W. Fate and seasonal variation of endocrine-disrupting chemicals in a sewage treatment plant with A/A/O process. Sep. Purif. Technol. 2012, 84, 9–15. [Google Scholar] [CrossRef]

| Compound | Precursor Ion | Product Ion | Declustering Potentials (V) | Collision Energy (eV) |
|---|---|---|---|---|
| E1 | 269.5 | 145.1 | −70 | −52 |
| E2 | 270.8 | 145.1 | −70 | −58 |
| 17α-E2 | 270.8 | 145.1 | −70 | −58 |
| EE2 | 294.9 | 145.1 | −60 | −53 |
| E3 | 286.6 | 145.1 | −70 | −58 |
| Kinetic Model | Estrogen | k1 (min−1) | k2 g/(μg∙min) | qe (cal) (µg/g) | qexp (µg/g) | r |
|---|---|---|---|---|---|---|
| pseudo-first-order | E1 | 0.436 | 633.9 | 387.6 | 0.94 | |
| E2 | 0.324 | 394.6 | 333.6 | 0.97 | ||
| 17α-E2 | 0.295 | 317.4 | 333.6 | 0.98 | ||
| EE2 | 0.470 | 625.7 | 378.4 | 0.93 | ||
| E3 | 0.213 | 176.4 | 294.8 | 0.88 | ||
| pseudo-second-order | E1 | 0.553 | 400.0 | 387.6 | 0.97 | |
| E2 | 1.001 | 386.1 | 333.6 | 0.99 | ||
| 17α-E2 | 1.112 | 375.9 | 333.6 | 0.99 | ||
| EE2 | 0.902 | 442.5 | 378.4 | 0.99 | ||
| E3 | 3.877 | 289.8 | 294.8 | 0.97 |
| Estrogens | Samples | Concentration (ng/L) | |
|---|---|---|---|
| Before Adsorption | After Adsorption | ||
| E1 | A | 31 | 1.5 |
| B | 56 | n.d. | |
| C | 38 | n.d. | |
| 17α-E2 | A | 27 | n.d. |
| B | 16 | 0.8 | |
| C | 21 | n.d. | |
| E2 | A | 39 | n.d. |
| B | 17 | n.d. | |
| C | 46 | n.d. | |
| EE2 | A | 25 | 2.3 |
| B | 13 | n.d. | |
| C | 27 | 1.1 | |
| E3 | A | 16 | 5.8 |
| B | 20 | 7.9 | |
| C | 14 | 4.8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Z.; Ying, Z.; Huo, M.; Yang, W. Adsorption of Trace Estrogens in Ultrapure and Wastewater Treatment Plant Effluent by Magnetic Graphene Oxide. Int. J. Environ. Res. Public Health 2018, 15, 1454. https://doi.org/10.3390/ijerph15071454
Wang X, Liu Z, Ying Z, Huo M, Yang W. Adsorption of Trace Estrogens in Ultrapure and Wastewater Treatment Plant Effluent by Magnetic Graphene Oxide. International Journal of Environmental Research and Public Health. 2018; 15(7):1454. https://doi.org/10.3390/ijerph15071454
Chicago/Turabian StyleWang, Xianze, Zhongmou Liu, Zhian Ying, Mingxin Huo, and Wu Yang. 2018. "Adsorption of Trace Estrogens in Ultrapure and Wastewater Treatment Plant Effluent by Magnetic Graphene Oxide" International Journal of Environmental Research and Public Health 15, no. 7: 1454. https://doi.org/10.3390/ijerph15071454
APA StyleWang, X., Liu, Z., Ying, Z., Huo, M., & Yang, W. (2018). Adsorption of Trace Estrogens in Ultrapure and Wastewater Treatment Plant Effluent by Magnetic Graphene Oxide. International Journal of Environmental Research and Public Health, 15(7), 1454. https://doi.org/10.3390/ijerph15071454





